Abstract
Between 1 June and 31 December 2002, 30,677 serum samples and 4,554 cerebrospinal fluid (CSF) samples were tested for West Nile virus (WNV)-specific immunoglobulin M (IgM) by an in-house enzyme-linked immunosorbent assay (ELISA); 1,481 serum samples (4.8%) and 345 CSF samples (7.6%) were positive for WNV IgM. Positive samples were forwarded to public health service laboratories (PHSLs) for further testing. PHSLs supplied results from their WNV IgM ELISAs for 654 samples; 633 (97%) were positive. PHSLs supplied WNV plaque reduction neutralization test results for 128 samples; 123 (96%) were positive. WNV IgM seroconversion and seroreversion trends were evaluated for 749 patients who each provided two serum samples that were tested during the study period. Of 574 patients whose first serum sample was IgM negative, 41 (7%) seroconverted (the second serum sample was IgM positive); of 175 patients whose first serum sample was IgM positive, 22 (13%) seroreverted (the second serum sample was IgM negative). The seroreversion rate was directly proportional to the time between serum sample collection; whereas only 1% of patients whose sera were collected <20 days apart showed seroreversion, 54% of patients whose sera were collected >60 days apart showed seroreversion. Conversion and reversion trends for CSF were evaluated for 68 patients. Of 54 patients whose first CSF specimen was IgM negative, 9 (17%) converted; none of 14 patients whose first CSF specimen was IgM positive reverted. Concomitant detection of WNV IgM in serum and CSF was assessed for 1,188 patients for whom paired serum and CSF specimens were available; for all 130 patients for whom IgM was detectable in CSF, IgM was also detectable in serum. These findings show that an in-house WNV IgM ELISA accurately identifies patients with WNV infection, document WNV IgM conversion and reversion trends, and demonstrate that WNV IgM detection in CSF is accompanied by WNV IgM detection in serum.
The 2002 West Nile Virus (WNV) season in the United States was characterized by a dramatic increase in the number of human WNV cases and fatalities compared to the numbers in prior years. More than 4,100 human cases and 280 fatalities occurred during 2002, whereas 66 cases and 9 fatalities occurred during 2001 (2, 3). Human cases were reported in 39 of the 48 contiguous states (plus the District of Columbia) in 2002 but in only 10 states in 2001 (2, 3).
Laboratory assays useful for the diagnosis of WNV infection include WNV RNA and WNV-specific immunoglobulin M (IgM) detection in serum or cerebrospinal fluid (CSF) (1, 5, 8). Although highly specific, WNV RNA detection in serum and CSF lacks sensitivity after the first few days of infection, most likely due to clearance of virus by WNV-specific antibodies (5, 8). WNV IgM detection, in contrast, shows good sensitivity; most WNV-infected individuals are seropositive for WNV IgM at presentation, and WNV IgM detection in CSF is diagnostic of WNV infection (5, 8). Due to the cross-reactivity of IgM among flaviviruses, however, specimens positive for WNV IgM should be tested by a confirmatory assay, such as the plaque reduction neutralization test (PRNT), to ensure WNV specificity (4, 6, 7).
We recently described a sensitive in-house WNV IgM enzyme-linked immunosorbent assay (ELISA) that was modified after the 2001 WNV season to improve its specificity (9). The 2002 WNV season offered the opportunity to determine the accuracy of this improved assay for the identification of WNV infections. As in 2001, specimens reactive by our in-house WNV IgM ELISA were forwarded to state public health service laboratories (PHSLs) for additional testing (9). This report presents the findings for specimens tested by our in-house WNV IgM ELISA and the additional assays performed at PHSLs. In addition, the large number of results for WNV IgM generated afforded us the opportunity to evaluate longitudinal trends in WNV IgM detection, as well as the concordance of the results for serum-CSF pairs, for a large number of individual patients.
MATERIALS AND METHODS
Specimens.
Human serum or CSF specimens were submitted to our facility for WNV IgM testing by referring laboratories between 1 June and 31 December 2002. None of the specimens were accompanied by information regarding the symptom onset date or the clinical presentation of the patient. Referring laboratories submitting a sample that tested positive for WNV IgM were contacted to determine the patient's state of residence. WNV IgM-positive samples were then forwarded to the appropriate state PHSL for additional WNV antibody testing; these tests included the PHSL WNV IgM ELISA and the WNV PRNT (4, 6, 7, 10).
In-house WNV IgM ELISA.
The WNV IgM capture ELISA incorporating background subtraction was performed as described previously (9), with minor modifications as indicated. Extensive in-house validation studies demonstrated that the modifications did not affect the quality of the results (data not shown). Briefly, diluted serum or CSF was incubated for 1.5 h in duplicate microtiter wells coated with rabbit anti-human IgM (heavy chain specific). After the wells were washed, one well received WNV antigen (supernatant from Vero cells infected with WNV by a proprietary procedure), and the other well received specimen diluent (rather than uninfected Vero cell supernatant, as originally described). After 3 h at room temperature (rather than overnight at 4°C, as originally described), the wells then sequentially received flavivirus monoclonal antibody 6B6-C1, horseradish peroxidase-conjugated goat anti-mouse IgG, enzyme substrate, and stop solution, as originally described, with appropriate washes between steps (9). All assays included positive control, negative control, and calibrator sera (9). The absorbance at 450 nm was measured with an ELISA reader. For each specimen, the absorbance value of the well receiving specimen diluent was subtracted from the absorbance value of the well receiving WNV antigen. This corrected absorbance value was then used to calculate the index value, defined as the corrected absorbance value for the specimen divided by the corrected absorbance value for the calibrator serum sample. A positive result was defined as an index value ≥2.0 (rather than ≥1.0, as originally described); this modification was based on prior studies showing improved specificity, with little impact on sensitivity, when an index of 2.0 is used to distinguish a positive result from a negative result (9).
Analysis of results for individual patients.
The database of WNV IgM ELISA results was examined to identify patients for whom two serum or two CSF specimens were submitted during the study period. Specimens with different collection dates and showing agreement in patient name, patient birth date, and submitting laboratory were considered different specimens collected from the same patient. Similarly, identification of paired serum and CSF specimens from the same patient required that the name, birth date, and submitting laboratory match, and both specimens had to be collected within a 24-h period. Differences among proportions were evaluated by contingency table analysis.
RESULTS
Seasonal findings.
Table 1 summarizes the results obtained by the in-house WNV IgM ELISA during the 2002 WNV season in the United States. More than 35,000 specimens were tested, and WNV IgM was detected in 5% of the specimens. The proportion of IgM-positive specimens was somewhat higher for CSF specimens than for serum specimens. Samples with positive WNV IgM results were sent to PHSLs for additional testing; PHSLs supplied their results for nearly 40% of these specimens (see Table 3 for details).
TABLE 1.
Summary of WNV IgM results for the 2002 season
| Test or result | No. (%) of specimensa
|
||
|---|---|---|---|
| Serum | CSF | Total | |
| Tested using in-house WNV IgM ELISA | 30,677 | 4,554 | 35,231 |
| In-house WNV IgM ELISA positive | 1,481 (4.8) | 345 (7.6) | 1,826 (5.2) |
| PHSL WNV IgM ELISA and/or PRNT result supplied | 624 (42.1) | 75 (21.7) | 699 (38.2) |
Percentages represent the percentage of the preceding number in the same column.
TABLE 3.
PHSL results in relation to in-house WNV IgM ELISA index values
| Sample | In-house index | No. (%a) of specimens
|
|||||
|---|---|---|---|---|---|---|---|
| PHSL WNV IgM ELISA result
|
PHSL WNV PRNT result
|
||||||
| Negative | Positive | Total | Negative | Positive | Total | ||
| Serum | 2.0-2.99 | 9 | 70 (89) | 79 | 1 | 12 (92) | 13 |
| 3.0-3.99 | 6 | 98 (94) | 104 | 1 | 31 (97) | 32 | |
| 4.0-4.99 | 4 | 127 (97) | 131 | 1 | 32 (97) | 33 | |
| 5.0-5.99 | 2 | 91 (98) | 93 | 0 | 14 (100) | 14 | |
| 6.0-6.99 | 0 | 66 (100) | 66 | 0 | 15 (100) | 15 | |
| ≥7.0 | 0 | 110 (100) | 110 | 1 | 14 (93) | 15 | |
| Serum total | 21 | 562 (96) | 583 | 4 | 118 (97) | 122 | |
| CSF | 2.0-2.99 | 0 | 8 (100) | 8 | 0 | 2 (100) | 2 |
| 3.0-3.99 | 0 | 8 (100) | 8 | 1 | 1 (50) | 2 | |
| 4.0-4.99 | 0 | 9 (100) | 9 | 0 | 0 | 0 | |
| 5.0-5.99 | 0 | 8 (100) | 8 | 0 | 0 | 0 | |
| 6.0-6.99 | 0 | 20 (100) | 20 | 0 | 1 (100) | 1 | |
| ≥7.0 | 0 | 18 (100) | 18 | 0 | 1 (100) | 1 | |
| CSF total | 0 | 71 (100) | 71 | 1 | 5 (83) | 6 | |
| Grand total | 21 | 633 (97) | 654 | 5 | 123 (96) | 128 | |
Percentage of total.
A monthly breakdown of the in-house WNV IgM ELISA results is shown in Table 2. September 2002 was the peak month for the number of specimens tested; similar trends were observed for both serum specimens and CSF specimens. Likewise, September was the peak month for the proportions of sera and CSF testing positive for WNV IgM.
TABLE 2.
In-house WNV IgM ELISA results by month for the 2002 WNV season
| Mo | Serum specimens
|
CSF specimens
|
||
|---|---|---|---|---|
| No. tested | No. (%) positive | No. tested | No. (%) positive | |
| June | 339 | 2 (0.6) | 76 | 0 (0) |
| July | 693 | 17 (2.5) | 187 | 10 (5.3) |
| August | 6,762 | 206 (3.0) | 952 | 62 (6.5) |
| September | 13,050 | 818 (6.3) | 1,646 | 211 (12.8) |
| October | 7,482 | 370 (4.9) | 1,086 | 53 (4.9) |
| November | 1,777 | 57 (3.2) | 421 | 8 (1.9) |
| December | 574 | 11 (1.9) | 186 | 1 (0.5) |
PHSL results for specimens positive by in-house WNV IgM ELISA.
PHSL results for forwarded WNV IgM-positive specimens are presented in Table 3 as a function of the in-house WNV IgM ELISA index values. PHSLs supplied the results of their WNV IgM ELISAs for 654 specimens and the results of their PRNTs for 128 specimens. Both PHSL ELISA and PRNT results were supplied for 83 samples (81 serum samples, 2 CSF samples), only ELISA results were supplied for 571 samples (502 serum samples, 69 CSF samples), and only PRNT results were supplied for 45 samples (41 serum samples, 4 CSF samples). Overall, 97% of specimens positive for WNV IgM by the in-house ELISA were also positive for WNV IgM by the PHSL WNV IgM ELISA. For serum, the overall rate of concordance between the in-house and PHSL ELISA results was 96%; when the results were analyzed in relation to the in-house WNV IgM index values, the rate of concordance increased as the in-house ELISA index value increased. For CSF, 100% concordance between the in-house ELISA results and the PHSL ELISA results was observed.
Table 3 also shows the PHSL PRNT results as a function of the in-house WNV IgM ELISA index values. Overall, 96% of specimens positive for WNV IgM by the in-house ELISA also exhibited positive WNV PRNT results. For serum, the concordance was 97%; for CSF, the concordance was 83%, reflecting a single specimen with discordant results. This CSF with a discordant result was positive by the PHSL WNV IgM ELISA, as well as the in-house WNV IgM ELISA.
Longitudinal trends in WNV IgM detection for individual patients.
The in-house WNV IgM ELISA results for 749 patients for whom two serum specimens were tested during the study period are shown in Table 4. Of 574 patients whose first serum sample was WNV IgM negative, 41 serocoverted (the second serum sample was WNV IgM positive). Of 175 patients whose first serum sample was WNV IgM positive, 22 seroreverted (the second serum sample was WNV IgM negative). The median number of days between serum collection dates was markedly higher for the seroreversion group than for the other groups (52 versus 13 to 16 days). Of the 22 patients showing WNV IgM seroreversion, 9 showed concomitant WNV IgG seroconversion (the first serum sample was IgG negative, the second serum sample was WNV IgG positive). Of the 13 seroreverting patients whose first serum sample was WNV IgG positive, the second serum sample from 6 patients showed increased WNV IgG levels, the second serum sample from 6 patients showed unchanging or lower (but still detectable) WNV IgG levels, and the second serum sample from 1 patient had no detectable WNV IgG (data not shown).
TABLE 4.
In-house WNV IgM ELISA results for patients for whom two serum or CSF specimens were submitted during the study period
| Specimen types | In-house IgM ELISA result
|
No. of specimens | Median no. of days (range) between specimen collection | |
|---|---|---|---|---|
| First specimen | Second specimen | |||
| Serum-serum | Negative | Negative | 533 | 13 (1-138) |
| Negative | Positive | 41 | 14 (1-86) | |
| Positive | Negative | 22 | 52 (17-111) | |
| Positive | Positive | 153 | 16 (1-67) | |
| CSF-CSF | Negative | Negative | 45 | 6 (1-15) |
| Negative | Positive | 9 | 6 (2-16) | |
| Positive | Positive | 14 | 5 (1-25) | |
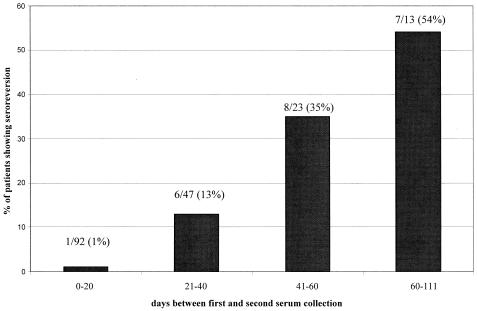
Figure 1 presents a detailed analysis of the relationship between seroreversion rates and the time between serum collection among all 175 patients for whom two serum samples were tested during the study period and whose first serum sample was WNV IgM positive. The proportion of patients showing seroreversion was directly proportional to the time between serum sample collection; only 1% of patients whose two serum specimens were collected less than 20 days apart showed seroreversion, whereas 54% of patients whose two serum specimens were collected more than 60 days apart showed seroreversion. This difference among proportions was statistically significant (P < 0.001, contingency table analysis). Stepwise increases in the proportion of patients showing seroreversion were observed for intermediate time categories (collection times, 20 to 60 days apart).
FIG. 1.
Relationship between seroreversion rate and time between serum sample collection. Serum samples from all patients for whom two serum samples were tested during the study period and whose first serum sample was WNV IgM positive by the in-house ELISA were included in the analysis. The values above each histogram present the number of patients exhibiting seroreversion/total number of patients for the indicated time category (and the resulting percentage).
Conversion and reversion trends for patients for whom two CSF specimens were tested during the study period are shown in Table 4. Of 54 patients whose first CSF specimen was WNV IgM negative, 9 converted. Of the 14 patients whose first CSF specimen was WNV IgM positive, none reverted. The median time between CSF collection dates was essentially the same for the conversion group as for the groups showing no change in CSF WNV IgM status.
WNV IgM detection in serum-CSF pairs from individual patients.
Paired serum and CSF samples were tested for nearly 1,200 patients during the study period (Table 5). The vast majority of pairs (>85%) were negative for WNV IgM, and a small proportion of pairs (<4%) had detectable WNV IgM in serum but not in CSF. Of particular note, however, was the observation that among all serum-CSF pairs in which WNV IgM was detectable in CSF, WNV IgM was also detectable in serum.
TABLE 5.
In-house WNV IgM ELISA results for serum-CSF pairs
| Result for serum | Result for CSF | No. (% of total) |
|---|---|---|
| Negative | Negative | 1,015 (85.4) |
| Positive | Negative | 43 (3.6) |
| Positive | Positive | 130 (10.9) |
DISCUSSION
Following the 2001 WNV season, our in-house WNV IgM ELISA was modified so that nonspecific binding of flavivirus monoclonal antibody and/or conjugated goat anti-mouse IgG was detected by a background subtraction approach (9). This modified WNV IgM ELISA was used to test more than 35,000 specimens during the 2002 WNV season. As shown in this report, >95% of specimens testing positive for WNV IgM by our modified ELISA also tested positive for WNV IgM by the WNV IgM ELISA performed by state PHSLs. Explanations for the small number of discordant results observed remain unclear; possibilities include differences in the WNV antigen preparations used in our in-house IgM ELISA (native WNV antigen) compared with those used in the PHSL ELISAs (recombinant viral particulate antigen [4]), differences in the expression of results (an index for the in-house ELISA versus the patient sample absorbance value/negative control absorbance value ratio for the PHSL ELISA [6, 7]), and operator error at either our facility or the PHSLs. However, considering the large number of specimens tested and the number of PHSL facilities supplying ELISA results (n = 16), the high level of agreement between the in-house ELISA results and the PHSL ELISA results is remarkable. Similarly, nearly all in-house ELISA-positive specimens that were tested by PRNT were PRNT positive, further attesting to the accuracy of the in-house WNV IgM ELISA.
The extraordinarily large number of specimens analyzed by the in-house WNV IgM ELISA during the 2002 season afforded us the opportunity to evaluate changes in WNV IgM levels over time for more than 800 patients (749 for whom pairs of serum specimens were available, 68 for whom pairs of CSF specimens were available). We documented seroconversion from WNV IgM-negative status to WNV IgM-positive status for a small proportion of patients, with a median time of 2 weeks between serum collection dates. Similarly, the CSF of a minor proportion of patients showed conversion from WNV IgM-negative status to WNV IgM-positive status. This finding indicates that a small proportion of patients with WNV infection do not have detectable levels of WNV IgM at the time of presentation.
A smaller but perhaps more interesting group of patients showed seroreversion, reflecting the disappearance of WNV IgM over time. The observed changes in WNV IgG levels for the WNV IgM seroreversion group indicated that loss of detectable WNV IgM reflected isotype switching typical of the immune response to infection. Detailed analysis of seroreversion among patients from whom two serum specimens were collected and whose first serum specimen was WNV IgM positive revealed stepwise increases in the seroreversion rate as the time between serum collection dates increased. Seroreversion was documented in over half of patients whose two serum specimens were collected more than 60 days apart.
Our data regarding IgM seroreversion are particularly interesting in light of the findings of Roehrig et al. (10), who demonstrated that WNV IgM remained detectable for up to 500 days after disease onset in 7 of 12 WNV-infected patients with encephalitis. Our study differs from that described by Roehrig et al. (10) in several aspects: (i) the maximal time between specimen collection was 111 days in our study, whereas it was 500 days in the study of Roehrig et al. (10); (ii) our group of patients is assumed to represent a mixture of WNV fever patients and WNV encephalitis patients; and (iii) disease onset dates for patients are lacking. Although these differences limit our ability to directly compare our results to those of Roehrig et al. (10), our findings suggest that WNV IgM seroreversion within 7 months after infection is not uncommon among a heterogeneous group of WNV-infected patients. Integral to our hypothesis is the assumption that the patients evaluated in our study became infected during the 2002 season (rather than the 2001 season) and thus had been infected for a maximum of 7 months. In support of this assumption, 20 of 22 patients exhibiting WNV IgM seroreversion were from states that did not report human WNV cases until 2002 (2). Similarly, we assume that sera submitted to a reference laboratory for WNV IgM testing in 2002 were most likely collected from patients experiencing symptoms associated with recent WNV infection. Further support for frequent WNV IgM seroreversion within a few months after disease onset comes from studies of patients infected with WNV during a 1996 epidemic in Romania. Tardei et al. (11) found that approximately 45% of infected patients no longer had detectable WNV IgM in convalescent-phase sera collected more than 2 months after the onset of illness.
We also had the opportunity to assess WNV IgM levels in paired serum and CSF specimens from nearly 1,200 patients. The most notable finding from this analysis was that all patients with detectable WNV IgM in CSF also had detectable WNV IgM in serum. Thus, WNV infection follows the expected pattern of intrathecal IgM production only in conjunction with extrathecal IgM production (5, 8). Put in more practical terms, patients with detectable WNV IgM in their CSF should be expected to have detectable WNV IgM in their sera; a WNV IgM-positive result for CSF and a WNV IgM-negative result for serum should thus be viewed with suspicion, and testing should be repeated with additional specimens.
Acknowledgments
We thank PHSL staff members from the following states for supplying WNV antibody results: California, Florida, Illinois, Kentucky, Louisiana, Maryland, Michigan, Mississippi, Missouri, Nebraska, New York, Ohio, Pennsylvania, Tennessee, Texas, and Wisconsin. We also thank Robert Lanciotti (Centers for Disease Control and Prevention, Ft. Collins, Colo.) for valuable discussions. Jane Filamor, Susan Vogeli, and Maryam Saber-Tehrani provided expert technical assistance.
REFERENCES
- 1.Briese, T., X.-Y. Jia, C. Huang, L. J. Grady, and W. I. Lipkin. 1999. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet 354:1261-1262. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2002. West Nile Virus Activity—United States, 2001. Morb. Mortal. Wkly. Rep. 51:497-501. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2003. West Nile Virus update current case count. [Online.] http://www.cdc.gov/od/oc/media/wncount.htm. Accessed 21 April 2003.
- 4.Davis, B. S., C.-J. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marfin, A. A., and D. J. Gubler. 2001. West Nile encephalitis: an emerging disease in the United States. Clin. Infect. Dis. 33:1713-1719. [DOI] [PubMed] [Google Scholar]
- 6.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen, L. R., and A. A. Marfin. 2002. West Nile virus: a primer for the clinician. Ann. Intern. Med. 137:173-179. [DOI] [PubMed] [Google Scholar]
- 9.Prince, H. E., and W. R. Hogrefe. 2003. Performance characteristics of an in-house assay system used to detect West Nile virus (WNV)-specific immunoglobulin M during the 2001 WNV season in the United States. Clin. Diagn. Lab. Immunol. 10:177-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roehrig, J. T., D. Nash, B. Maldin, A. Labowitz, D. A. Martin, R. S. Lanciotti, and G. L. Campbell. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile Virus encephalitis cases. Emerg. Infect. Dis. 9:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardei, G., S. Ruta, V. Chitu, T. F. Tsai, and C. Cernescu. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J. Clin. Microbiol. 38:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]