Abstract
Human leishmaniasis is a severe health problem in many countries around the world. Hence, a cheap, reliable, and accurate diagnostic test is required to fight this disease. Perhaps the direct agglutination test (DAT) meets these criteria, but antigen elaboration involves many difficulties. We have developed a new antigen elaboration method, the EasyDAT method, that avoids the problems associated with the DAT. In this study, we compared the traditional DAT antigen method with our EasyDAT antigen method by using canine sera. The sensitivities (100%) and specificities (98.7%) were the same for both methods; we therefore concluded that the EasyDAT Leishmania antigen method simplifies serologic diagnosis, making this method easier and cheaper to use.
Human visceral leishmaniasis, caused by a protozoan of the genus Leishmania, is a zoonotic disease whose main reservoirs are dogs. Visceral leishmaniasis is a potentially fatal disease that affects an estimated 500,000 people each year (9). Even though leishmaniasis is a serious public health problem in underdeveloped areas of the world, prevalence is quite low in the Mediterranean basin. Early diagnosis is the preferable way to fight this disease, which has been targeted by the World Health Organization.
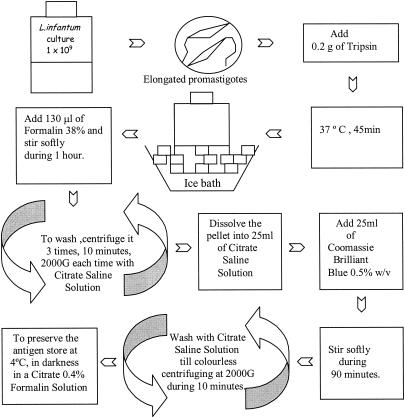
The main objective of this research was to develop a new protocol for antigen elaboration that avoids all the inconveniences of the traditional direct agglutination test (DAT) (3, 4) without compromising either the sensitivity or specificity. For EasyDAT antigen elaboration (Fig. 1), we cultured Leishmania infantum (MHOM/FR/78/LEM-75) at 26.4°C in half-liter flasks containing RPMI 1640 with l-glutamine and NaHCO3 (Sigma) and 5 mM HEPES, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 10% heat-inactivated fetal bovine serum, until the majority of the organisms had developed into the elongated promastigote forms. Promastigotes usually appear 3 to 5 days after culturing is begun, although this time varies between strains. The concentration of the culture was determined by counting the promastigotes in a Neubauer chamber and standardizing the concentration at 109 promastigotes/ml; 0.2 g of trypsin (at a 1:250 dilution with γ-irradiated porcine pancreas; Panreac) was added to the culture, which was maintained at 37°C for 45 min. After this time, the culture was placed in a frozen water bath to stop trypsinization. Then 130 μl of formalin (37 to 38% p/p ethanol stabilized; Panreac) was added. The culture was stirred gently for 1 h to fix the promastigotes properly. For harvesting, the culture was centrifuged (in 50-ml Falcon tubes at 2,000 × g for 10 min) two times in order to concentrate the promastigotes and one time with citrate saline solution to remove excess formalin. Finally, the pellet was dissolved in 25 ml of citrate saline solution. To stain the promastigotes, we used Coomassie brilliant blue (R-250; Merck) diluted to 0.5% (wt/vol); we added 25 ml of this solution to the fixed promastigotes, which produced a final color concentration of 0.25%. After stirring the mixture gently for 90 min, we harvested the promastigotes by centrifuging the mixture three times at 2,000 × g for 10 min, washing it each time in citrate saline solution. To store the EasyDAT Leishmania antigen produced, we dissolved the pellet in citrate saline solution with 0.4% formalin at a concentration of 50 × 106 promastigotes/ml and then preserved the solution, protected from light, at 4°C. It is important to emphasize that the entire procedure was performed at room temperature and that the process was not carried out under sterile conditions. The final antigen product was not contaminated since the original culture was sterile and the formalin, which was added 45 min after processing, prevented any subsequent contamination.
FIG. 1.
Steps for EasyDAT antigen elaboration.
An assay was performed to test the EasyDAT antigen and to demonstrate that the new process does not modify the sensitivity or specificity of the antigen. Since in Spain, as in other Mediterranean basin countries, canine leishmaniasis has a mean prevalence of 5 to 8% and reaches a prevalence of 30% in many areas (1, 7), the assay was carried out with specimens obtained from dogs, thereby avoiding the difficulties of obtaining a large number of samples from humans with leishmaniasis. A total of 289 canine serum samples were tested. The samples were divided into two groups. Group A included samples from 139 animals with positive lymph node aspiration, bone marrow examination, and/or parasite isolation in Novy-Nicolle-McNeal medium. Group B comprised samples from 150 dogs without symptoms and with negative smears and isolations.
All serum samples were tested with the DAT traditional antigen (5) and with the EasyDAT antigen method. The EasyDAT results showed 100% sensitivity (139 positives and no negatives out of 139 samples determined to be positive by parasitological methods [group A]), 98.7% specificity (148 negatives and 2 positives out of 150 samples determined to be negative by parasitological methods [group B]), a positive predictive value of 98.5%, a negative predictive value of 100%, and an efficacy of 99.3%. The cutoff titer was established as 1/800 to obtain identical titers for both procedures. Moreover, we tested the same serum samples again every month to evaluate the durability of the EasyDAT antigen. It has been established that the antigen remains durable for 6 months, a result similar to that for the traditional DAT antigen.
Since visceral leishmaniasis occurs mainly in areas of the world where health services are poorly developed, research has focused on the development of a simple, cheap, and reliable diagnostic test for the disease (2, 3, 6). The DAT has been recommended for use under field conditions (8) but presents severe problems in terms of reproducibility of results, which depends on antigen elaboration (3). The EasyDAT antigen method shows the same sensitivity, specificity, and durability as the traditional DAT antigen method but offers the additional advantages of cost reduction, standardization of the quantities of trypsin and formalin needed relative to the number of promastigotes (if the weight/volume ratio of the traditional protocol is used), reduction of the antigen elaboration time to only 5 h, and centrifugation without refrigeration. This newly developed procedure makes antigen elaboration easier and thus makes the diagnostic test more accessible in less-developed areas of the world.
REFERENCES
- 1.Abranches, P., G. Santos Gomes, N. Rachamin, L. Campino, L. F. Schnur, and C. L. Jaffe. 1991. An experimental model for canine visceral leishmaniasis. Parasite Immunol. 13:537-550. [DOI] [PubMed] [Google Scholar]
- 2.Boelaert, M., S. el Safi, D. Jaquet, A. de Muynck, P. van der Stuyft, and D. Le Ray. 1999. Operational validation of the direct agglutination test for diagnosis of visceral leishmaniasis. Am. J. Trop. Med. Hyg. 60:129-134. [DOI] [PubMed] [Google Scholar]
- 3.Boelaert, M., S. el Safi, H. Mousa, J. Githure, P. Mbati, V. L. Gurubacharya, J. Shrestha, D. Jacquet, A. de Muynck, D. Le Ray, and P. Van der Stuyft. 1999. Multicentre evaluation of repeatability and reproducibility of the direct agglutination test for visceral leishmaniasis. Trop. Med. Int. Health 4:31-37. [DOI] [PubMed] [Google Scholar]
- 4.el Harith, A., R. J. Slappendel, I. Reiter, F. van Knapen, P. de Korte, E. Huigen, and A. H. J. Kolk. 1989. Application of a direct agglutination test for detection of specific anti-Leishmania antibodies in the canine reservoir. J. Clin. Microbiol. 27:2252-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.el Harith, A., A. Kolk, P. A. Kager, J. Leeuwenburg, F. J. Faber, R. Muigai, S. Kingu, and J. J. Laarman. 1987. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans. R. Soc. Trop. Med. Hyg. 81:603-606. [DOI] [PubMed] [Google Scholar]
- 6.Masum, M. A., D. A. Evans, D. M. Minter, and A. Harith. 1995. Visceral leishmaniasis in Bangladesh: the value of DAT as a diagnostic tool. Trans. R. Soc. Trop. Med. Hyg. 89:185-186. [DOI] [PubMed] [Google Scholar]
- 7.Morillas, F., F. Sanchez Rabasco, J. Ocaña, J. Martin-Sanchez, J. Ocaña-Wihelmi, C. Acedo, and M. C. Sanchiz-Marin. 1996. Leishmaniasis in the focus of the Axarquia region, Malaga province, southern Spain: a survey of the human, dog, and vector. Parasitol. Res. 82:569-570. [DOI] [PubMed] [Google Scholar]
- 8.Singla, N., G. S. Singh, S. Sundar, and V. K. Vinayak. 1993. Evaluation of the direct agglutination test as an immunodiagnostic tool for kala-azar in India. Trans. R. Soc. Trop. Med. Hyg. 87:276-278. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. 1997. Tropical disease research: progress 1995-1996. Thirteenth Programme Report UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. World Health Organization, Geneva, Switzerland.