Abstract
Fish acquire protective immunity against the ciliated protozoan parasite Ichthyophthirius multifiliis following sublethal infection or inoculation with I. multifiliis immobilization antigens (i-antigens). In both cases, parasite-immobilizing antibodies have been identified in sera and mucosal secretions. To investigate the kinetics of this immune response, antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) in the sera and cutaneous mucus of channel catfish (Ictalurus punctatus) that were either infected with parasites or given a single injection of purified i-antigen (5.0 μg/fish) in Freund's incomplete adjuvant. At 5 weeks, infected and inoculated fish had a mean serum (1:80 dilution) antibody absorbance (A405) value of 0.54 ± 0.17 and 0.35 ± 0.03, respectively, which were significantly higher (α = 0.05) than the pretreatment serum (1:80 dilution) antibody absorbance value of 0.24 ± 0.05. At 14 weeks, mean serum (1:80 dilution) ELISA absorbance values in the teo groups of fish increased to 0.79 ± 0.30 and 0.71 ± 0.24, respectively. In both groups of fish, antibody levels in cutaneous mucus (undiluted) were much lower than those in sera. Infected fish had detectable mucus (undiluted) antibody levels from 3 to 9 weeks, with the highest mean value (0.30 ± 0.07) occurring at 7 weeks. Although individual inoculated fish produced serum antibody absorbance values comparable to those seen in infected fish, the mean mucus antibody values in this group did not rise above pretreatment levels. I. multifiliis infection induced a transient mucosal antibody response that coincided with the resolution of infection. Whether elicited by infection or intraperitoneal injection of i-antigen, the serum and mucus antibody responses of channel catfish immunized against I. multifiliis did not occur synchronously.
The common parasitic ciliate Ichthyophthirius multifiliis is one of the most important protozoan pathogens of freshwater fish throughout the world. It has a serious impact on aquaculture due to its widespread distribution, indiscriminate host specificity, and high level of virulence. Disease outbreaks usually result in high mortality rates, primarily in intensively reared populations of fish. Fish that recover from natural or experimentally induced sublethal infections, however, become resistant to subsequent I. multifiliis challenge. Acquired immunity against this parasite has been well documented in numerous fish species, including channel catfish (Ictalurus punctatus), trout (Salmo gairdneri), and carp (Cyprinus carpio) (2, 13, 21, 32, 44). A long-term goal of our research has been to understand the mechanisms of this protective immunity in order to develop an effective vaccine against the parasite.
I. multifiliis-immune fish sera immobilize the live organism in vitro, and it has been postulated that immobilizing antibodies serve to block infection (5, 7, 8). Fish vaccinated against I. multifiliis by intraperitoneal injection of purified immobilization antigens (i-antigens; the surface proteins targeted by immobilizing antibodies) in Freund's complete adjuvant develop active protective immunity and produce antibodies against i-antigens (10, 16) in both the blood and the cutaneous mucus (4-6, 15, 45-47). Additionally, passive transfer to fish skin of immobilizing immunoglobulin G (IgG)-class murine monoclonal antibodies administered by intraperitoneal injection supports the concept that antibodies are a key component of the epithelial immune barrier (24). On the basis of this finding and on the basis of the observation that parasites rapidly leave the skin of I. multifiliis-immune fish, an antibody-mediated mechanism of cutaneous immunity has been proposed (8, 9, 12, 17).
Channel catfish, like other teleosts, produce a single class of immunoglobulin, namely, tetrameric IgM-like antibodies that occur in both the blood and mucus secretions (22, 28-30, 33, 34). Although there is experimental evidence supporting the existence of a separate mucosal immune system in fish, it is not known how mucus antibodies (which are indistinguishable in structure from serum antibodies) reach the surface epithelia of the gut, gills, and skin (1, 3, 11, 18-20, 25-28, 30, 31, 36-39, 41, 43). Ultimately, we would like to identify the sites of cutaneous mucus antibody induction and the mechanisms by which antibodies are transported to the skin. Toward this end, we are using channel catfish infected with I. multifiliis as a model to investigate the cutaneous immune response of fish to pathogens that invade the fish through epithelial tissues (17).
In this study, we used an enzyme-linked immunosorbent assay (ELISA) to compare over time the relative amounts of I. multifiliis-specific serum and cutaneous mucus antibodies elicited in naïve channel catfish following either surface infection or a single injection of purified i-antigen in Freund's incomplete adjuvant. We used incomplete adjuvant to enhance immunity without eliciting responses against bacterial components present in the complete adjuvant, as our intent was to compare only the antibody response elicited by the parasite. We found that mucosal antibodies were produced following either I. multifiliis infection or the injection of purified antigen and that in both cases their occurrence did not exactly coincide with serum antibody production. Our results suggest that parasite-specific antibodies in the cutaneous mucus of channel catfish do not arise by passive transfer or exudation from the blood.
MATERIALS AND METHODS
Parasite propagation.
The G5 I. multifiliis isolate used in this study has been characterized previously, and its propagation by passage on channel catfish has been described (14).
Purification of I. multifiliis protein antigens.
I. multifiliis i-antigen was purified from isolate G5 serotype D theront membrane proteins by previously published methods (23). Aliquots were flash frozen in liquid nitrogen and stored at −80°C. Aliquots were thawed to room temperature (RT) and diluted in 25 mM sodium acetate (pH 7.5) immediately before use in the ELISA procedure. Detergent-extracted membrane protein was further enriched for i-antigen by using a column on which a monoclonal antibody specific for I. multifiliis G5 i-antigen (G-361) was immobilized as described previously (23). The immunoaffinity-purified i-antigen was used to inject fish by the intraperitoneal route.
Production of anti-catfish Ig antibody.
Ig was purified from pooled channel catfish (I. punctatus) sera with an IgM purification column (ImmunoPure-IgM; Pierce, Rockford, Ill.) by the protocol recommended by the manufacturer. Fractions containing >0.5 μg of protein, determined by measurement of the absorbance (A280), were pooled and desalted with dextran columns (Pierce). A domestic, mixed-breed goat (Capra hircus) was injected intramuscularly three times over the course of 3 months with 1.0 μg of purified catfish Ig dissolved in 1.0 ml of phosphate-buffered saline (PBS; 136 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.2]) mixed 1:1 in Freund's incomplete adjuvant (40). Blood was periodically collected from the jugular vein by using a sterile needle and syringe and was stored at 4°C. The collected serum was aliquoted and stored at −20°C. Goat Ig was purified from sera with a Protein A/G column (Pierce). The goat antibody was biotinylated with a Sulfo-NHS-Biotinylation kit (Pierce), and percent biotinylation was calculated by a competitive binding assay incorporating 2,4′-hydroxy-azonbenzene benzoic acid and avidin, according to the instructions of the manufacturer.
Experimental animals.
Channel catfish fingerlings (mean weight, 18.5 ± 1.2 g) were obtained from The University of Georgia Agricultural Research Foundation's Aquaculture Facility, Athens. These specific-pathogen-free fish were raised from formalin-treated eggs in containment and fed a commercial game fish ration (Purina, St. Louis, Mo.) once a day. During the experiment, the fish were observed daily, and every other day water quality was monitored for pH and NO2 with standard test kits. Sodium bicarbonate (Fisher Scientific, Fair Lawn, N.J.) was added as needed to maintain water alkalinity. The fish were randomly assigned to treatment groups in 12 glass aquaria (volume, 76 liters) on a self-contained, recirculating biofiltered system at a density of 10 fish per aquarium and two aquaria per group. The tanks received water from a common source and shared a single filter. The water level was lowered in individual tanks during I. multifiliis infection and formalin treatments to isolate specific groups of fish. Fish immunized by I. multifiliis infection were kept in isolated aquaria with individual filter units until the infection was eliminated, at which time the fish were returned to their respective tanks. The water temperature ranged from 16 to 20°C during a 2-month acclimatization period. The water temperatures ranged from 20 to 24°C during the 14-week time course of the experiment.
Immunization of fish with I. multifiliis protein.
Fish were anesthetized with tricaine methane-sulfonate (100 to 200 mg/liter; MS-222; Argent Chemicals, Redmond, Wash.) dissolved in water that had been buffered with equal amounts of sodium bicarbonate (Fisher). Each fish received 5.0 μg of affinity-purified i-antigen diluted in 25 μl of PBS and mixed 1:1 with Freund's incomplete adjuvant. A 50-μl volume was injected into the peritoneal cavity of each fish at the ventral surface midline by using a 1-ml tuberculin syringe (Monoject; Sherwood Medical Company, St. Louis, Mo.) fitted with a 23-gauge by 1-in. needle (Becton Dickinson & Co., Franklin Lakes, N.J.).
Exposure of fish to I. multifiliis parasites.
Twenty catfish were exposed to I. multifiliis theronts (isolate G5, serotype D) maintained by passage on channel catfish (14). Unanesthetized fish were placed 10 at a time in 2-liter plastic beakers filled with charcoal-filtered water (200 ml/fish) containing a known number of theronts at room temperature for 1 h. The fish were initially exposed to the theronts at a ratio of 5,000 theronts/fish and were again exposed 2 weeks later at a ratio of 11,000 theronts/fish. Numerous parasites were observed on all fish 4 days after the second exposure. Formalin (formaldehyde, 37% solution; J. T. Baker, Phillipsburg, N.J.) was added to each tank at 0.26 ml/liter on the 5th day and every other day for 3 additional days. Complete water changes were performed twice a day starting on the evening of the 5th day and continuing for the duration of treatment. One fish died during treatment. The remaining fish appeared to be free of parasites 3 weeks after the infection was first observed.
Cutaneous mucus and serum collection.
Cutaneous mucus and blood samples collected from the fish 2 weeks prior to initiation of the experiment served as negative control (i.e., pretreatment) samples. After treatment, cutaneous mucus and serum samples were collected from the same fish at eight time points spanning a 14-week period. Four immunized fish and four I. multifiliis-exposed fish were sampled at weeks 1, 2, 3, and 5. Ten immunized fish and 10 I. multifiliis-exposed fish were sampled at weeks 7, 9, 11, and 14. Fish were randomly selected from each tank, anesthetized, and weighed. Mucus was collected prior to blood collection to prevent possible cross contamination of samples. After sampling, the tail fin of each fish was clipped for identification purposes to avoid sampling of the same fish on subsequent dates.
Blood (200 to 500 μl/fish) was collected from the caudal sinus by using a 23-gauge by 1-in. needle and a 1.0-ml syringe. Blood was transferred to 1.5-ml polypropylene tubes and was refrigerated overnight (4 to 6°C for 12 to 15 h) to maximize clot retraction. On the next day the tubes were centrifuged (10,000 × g for 10 min) in a tabletop microcentrifuge (Marathon 13K/M; Fisher), and the serum was transferred to 1.5-ml tubes for storage (−20°C). Mucus was processed as described below and stored at −80°C.
Detection of I. multifiliis-specific antibodies by ELISA.
Detergent-extracted I. multifiliis membrane protein (3.0 to 5.0 μg/ml) diluted in 25 mM sodium acetate (pH 7.5) was applied to polyvinyl chloride 96-well plates (Falcon 353912FB; Becton Dickinson) at 100 μl per well. The plates were incubated overnight at 4°C, washed once with 200 μl of PBS-0.05% Tween (PBST) per well, and blocked with 1% normal goat serum (Sigma, St. Louis, Mo.) diluted in PBST (1 h at RT). Catfish serum samples diluted in PBS (1:80) and undiluted mucus samples were plated in triplicate wells at 100 μl/well. Triplicate control wells on each plate contained diluted sera from I. multifiliis-exposed and nonexposed catfish. Mucus assays also included wells of pooled mucus from nonexposed fish. The plates were incubated for 2 to 3 h at RT or overnight at 4°C and washed five times with PBST.
The biotinylated goat anti-catfish Ig antibody was applied at 0.25 to 0.50 μg/well. The plates were incubated for 1 h at RT and were washed five times with PBST. Application of the primary antibody was followed by application of streptavidin-alkaline phosphatase conjugate (E-2636 ExtraAvidin; Sigma) diluted in PBS (1:50,000). The enzyme substrate p-nitrophenyl phosphate diethanolamine (Bio-Rad, Hercules, Calif.) was added, and the absorbance (A405) values were determined on a kinetic microplate reader (model V-max; Molecular Devices Corp., Sunnyvale, Calif.) at 30 min and 1 h.
Cutaneous mucus collection.
A preliminary study was conducted to determine the standard error of the mucus collection method. Cutaneous mucus was collected from five adult channel catfish (weight, ∼1.4 to 2.3 kg each) obtained from The University of Georgia Agricultural Research Foundation's Aquaculture Facility. Fish were anesthetized with tricaine methane-sulfonate (100 to 200 mg/liter; MS-222; Argent Chemicals) dissolved in water that had been buffered with equal amounts of sodium bicarbonate (Fisher). Each fish was placed on a flat surface covered with Saran Wrap (S. C. Johnson and Son, Inc., Racine, Wis.). Mucus was collected from the fish by gently wiping both sides of the fish with pieces of cotton (Johnson & Johnson, Skillman, N.J.). Care was taken to not remove the entire mucus layer or abrade the skin during collection. Saturated cotton pieces were combined and placed in 50-ml conical centrifuge tubes (Corning, Corning, N.Y.) containing just enough PBS (approximately 10 ml/tube) to prevent drying. The cotton was pressed against the side of the tube with a spatula and discarded. The liquid remaining in each tube was pooled, the absorbance (A280) was recorded, and aliquots were stored frozen at −80°C. This pooled mucus sample (A280 = 0.6288) served as starting material for the following experiment.
Pieces of cotton (1.23 ± 0.33 g each) were placed in plastic weighing boats containing 1 to 2 ml of the pooled mucus sample. After 5 to 10 s, when the pieces were uniformly saturated, they were weighed again and individually inserted into glass tubes (12 by 75 mm; VWR Scientific Products, Buffalo Grove, Ill.) containing 500 μl of PBS/tube. The mucus samples were processed as described above, and the contents were transferred to individual 1.5-ml snap-lid polypropylene tubes (Fisher) and centrifuged at maximum speed (setting = 12) for 10 min in a tabletop microcentrifuge (Marathon 13K/M; Fisher). Supernatants were collected and stored at −80°C. The absorbance (A280) values of the processed mucus samples and original pooled mucus were compared, and the standard error was calculated.
SDS-PAGE and Western blotting.
One-dimensional sodium-dodecyl-sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) of I. multifiliis antigens and Western blotting were performed by previously published protocols (23). Purified i-antigen or detergent-extracted membrane proteins (0.5 μg per lane) were electrophoresed on 4% stacking and 12% resolving polyacrylamide gels containing 1% SDS in the stacking gel, with or without β-mercaptoethanol (Sigma) added to the loading buffer. Protein bands were visualized by staining with silver nitrate (Bio-Rad) according to the instructions of the manufacturer.
I. multifiliis membrane proteins resolved by SDS-PAGE were transferred to a polyvinylidene difluoride membrane (pore size, 0.45 μm; Pierce), blocked with 3% bovine serum albumin (fraction V; Sigma), and incubated overnight at 4°C with I. multifiliis-exposed fish serum diluted 1:100 in PBST (Sigma). After the blots were rinsed in PBST, they were incubated with 2.0 mg of biotinylated goat anti-catfish Ig antibody per ml diluted in PBST-1% bovine serum albumin at RT for 2 h and washed. ExtraAvidin (1:50,000; Sigma) in PBST was added, and the blots were incubated for 30 min at RT and washed again. The substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (1-Step; Pierce) was added, and development was stopped by rinsing the blots in water after 30 min of incubation at RT.
Statistical analyses.
Mean ± standard deviation absorbance values for the ELISAs were determined for the tank and the treatment groups. Significance (α = 0.05) was assigned by overlapping of resulting confidence intervals. Normality was determined by the Shapiro-Wilkes test. ELISA values for the serum and mucus samples, fish weights, and mucus absorbance (A280) values were analyzed for correlation by scatterplot matrix analysis and linear regression. A 0.95 confidence interval was used for calculations of the standard errors for the mucus samples. Prism software (version 3.02; GraphPad Software, San Diego, Calif.) and Jump software (JMP IN Version 3.2.6; SAS Institute, Inc., Wadsworth Publishing, Belmont, Calif.) were used to perform these analyses.
RESULTS
i-antigens of I. multifiliis G5.
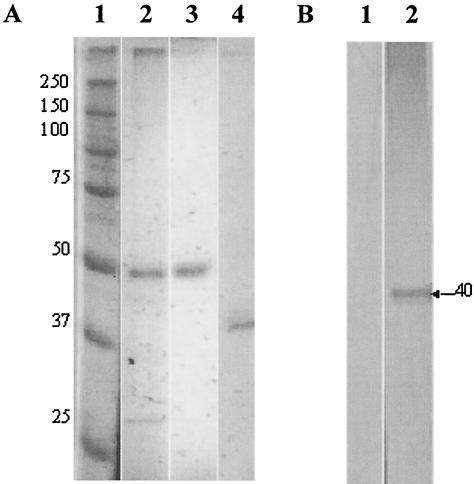
Detergent-extracted membrane proteins contained a prominent protein with an estimated molecular mass of either 55 or 35 kDa when the proteins were run on SDS-polyacrylamide gels under reducing or nonreducing conditions, respectively. The protein was identified as the i-antigen of isolate G5 by immunoaffinity chromatography with the immobilizing mouse monoclonal antibody G3-61, which was previously produced and characterized in our laboratory (23) (Fig. 1A). Sera from I. multifiliis-exposed channel catfish recognized only unreduced i-antigen on Western blots (Fig. 1B). These results confirm those of previous studies that fish polyclonal antibodies bind to conformational epitopes of i-antigens (45).
FIG. 1.
Analysis of I. multifiliis i-antigen by SDS-PAGE and Western blotting. (A) SDS-PAGE of I. multifiliis G5 serotype D i-antigen. Proteins were resolved on a 12% gel under either reducing or nonreducing conditions and stained with silver nitrate. Lane 1, prestained, reduced protein markers (sizes are indicated in kilodaltons); lane 2, reduced I. multifiliis membrane proteins showing a prominent band of ∼50 kDa (0.5 μg); lane 3, reduced immunoaffinity-purified i-antigen (0.5 μg); lane 4, unreduced affinity-purified i-antigen (0.5 μg). Note that the unreduced i-antigen has a molecular mass of ∼40 kDa. (B) Western blotting analysis of i-antigen with sera from I. multifiliis-immunized channel catfish. Affinity-purified i-antigens were electrophoresed on 12% gels under either reducing or nonreducing conditions and transferred to a polyvinylidene difluoride membrane. The blotted proteins were probed with I. multifiliis-immune channel catfish sera. Bound channel catfish antibody was detected with goat anti-catfish Ig and rabbit anti-goat Ig conjugated to alkaline phosphatase. Antibody signals were developed in nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate substrate. Lane 1, reduced i-antigen (0.5 μg); lane 2, unreduced i-antigen (0.5 μg).
Systemic antibody response.
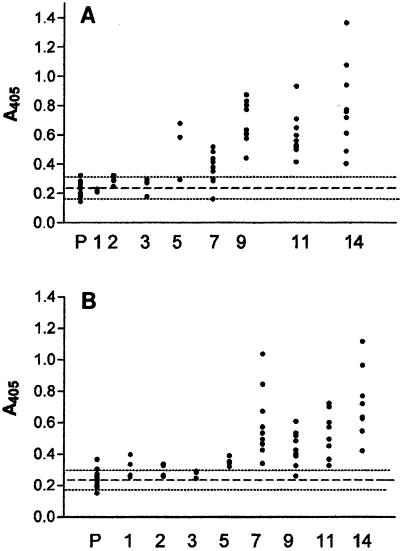
The sera of naïve channel catfish that survived infection with I. multifiliis had a mean (1:80 dilution) antibody absorbance value at 5 weeks of 0.54 ± 0.17 (optical density at 405 nm [OD405]), which was higher than that for preexposure sera (1:80 dilution), and the absorbance continued to increase to a maximum mean value of 0.79 ± 0.30 at 14 weeks. The mean serum antibody levels between the two replicates in this group (10 fish per replicate) were statistically similar (except at week 9). This result demonstrates that surface infection with I. multifiliis produced a relatively uniform serological response in the 20 outbred channel catfish used in this experiment. The mean absorbance values for serum between the two replicates of infected fish remained within 0.29 OD405 units throughout the sampling period (Fig. 2A).
FIG. 2.
I. multifiliis-specific antibody titers in sera measured by ELISA. (A) Channel catfish immunized by surface exposure to 11,000 theronts; (B) channel catfish immunized by intraperitoneal injection of 5.0 μg of immunoaffinity purified i-antigen in Freund's incomplete adjuvant per fish. The fish were sampled prior to infection or injection (P) and at 1, 2, 3, 5, 7, 9, 11, and 14 weeks posttreatment, as indicated on the x axis. Dots represent the absorbance values for individual fish serum samples (1:80 dilution). The dashed lines represent the pretreatment mean (0.24 ± 0.05), and the dotted lines represent the upper and lower confidence interval limits (α = 0.05) for both treatment groups (A and B).
A single intraperitoneal injection of purified i-antigen in incomplete Freund's adjuvant produced a serological response in outbred channel catfish comparable to that observed in infected fish (Fig. 2B). Both replicates of inoculated animals (10 per replicate) produced i-antigen-specific serum antibodies at 5 weeks postinjection, with a mean (1:80 dilution) absorbance value of 0.35 ± 0.03, which was significantly higher than the mean absorbance value for the pretreatment serum samples (1:80 dilution) of 0.24 ± 0.05. The serum antibody response continued to increase to a maximum mean (1:80 dilution) value of 0.71 ± 0.24 at 14 weeks. The mean absorbance values for the two replicates remained within 0.22 OD405 units throughout the sampling period (Table 1).
TABLE 1.
Mean diluted ELISA absorbance values for serum (1:80) and undiluted cutaneous mucus following I. multifiis infection or inoculation of i-antigen
| Compartment and mode of immunization | ELISA value (A405) at wk:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | 1 | 2 | 3 | 5 | 7 | 9 | 11 | 14 | |
| Seruma | |||||||||
| i-antigen inoculation | 0.24 | 0.22 | 0.29 | 0.26 | 0.54 | 0.37 | 0.68 | 0.60 | 0.79 |
| Infection | 0.24 | 0.31 | 0.29 | 0.27 | 0.35 | 0.59 | 0.43 | 0.51 | 0.71 |
| Mucusc | |||||||||
| i-antigen inoculation | 0.19 | 0.24 | 0.24 | 0.24 | 0.21 | 0.21 | 0.26 | 0.23 | 0.19 |
| Infection | 0.23 | 0.22 | 0.24 | 0.28 | 0.27 | 0.30 | 0.26 | 0.19 | 0.18 |
Diluted 1:80.
P, pretreatment.
Undiluted.
Cutaneous antibody responses.
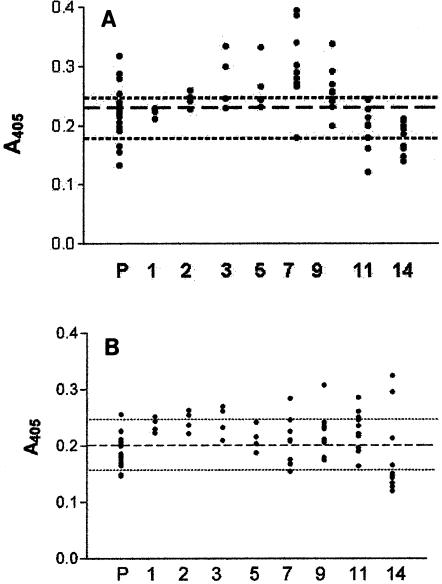
Compared to the systemic response, cutaneous mucus antibody levels were low in both the infected and the inoculated groups of fish (Fig. 3). This result was expected, as relatively low mucus antibody concentrations have previously been reported in fish immunized against I. multifiliis and other antigens (1, 26, 45, 48). Our mucus collection method was reproducible, with a relatively low standard error of 0.175. As a result of the dilution factor introduced by mucus collection and processing, the absorbance values obtained in the ELISA were estimated to be approximately 40% less than those representative of the actual amount of parasite-specific antibody present in the tissues. Histological evaluation of paraffin-embedded skin sections showed that the collection method did not significantly damage the skin and abraded only the outermost layer of the epidermis (data not shown).
FIG. 3.
I. multifiliis-specific antibody titers in cutaneous mucus measured by ELISA. (A) Channel catfish immunized by surface exposure to 11,000 theronts; (B) channel catfish immunized by intraperitoneal injection of 5.0 μg of immunoaffinity purified i-antigen in Freund's incomplete adjuvant per fish. Fish were sampled prior to infection or injection (P) and at 1, 2, 3, 5, 7, 9, 11, and 14 weeks posttreatment, as indicated on the x axis. Dots represent the absorbance values for individual fish mucus samples (undiluted). The dashed line represents the pretreatment mean for the fish prior to infection (0.23 ± 0.05) (A) or injection (0.19 ± 0.05) (B). Dotted lines represent the upper and the lower confidence interval limits for both treatment groups (0.21 ± 0.05; α = 0.05).
The low concentrations of parasite-specific antibody in mucus as well as variations in the immune responses among individual fish made the significance of changes in mean mucus absorbance values difficult to assess (Table 1). Nevertheless, a few individuals from each of the two treatment groups had undiluted mucus antibody levels that were higher than the pretreatment mean value of 0.21 ± 0.04. An ELISA absorbance value of 0.396 OD405 units was detected in a single injected fish at 14 weeks, and one infected fish had a value of 0.395 OD405 units at 7 weeks. All the infected fish had mean undiluted mucus antibody levels above 0.21 ± 0.04 from 3 to 9 weeks, with a peak value of 0.30 ± 0.07 at 7 weeks. Wide standard deviations at each time point, however, essentially rendered these changes statistically insignificant (Fig. 3). One replicate group of injected fish had individual mucus absorbance values above the overall pretreatment mean for mucus of 0.21 ± 0.04 at 2 and 3 weeks after injection, but the mean values between replicates for this same period were not statistically significantly different from one another or from background values. Within the same replicate group of injected fish, two individuals had relatively high antibody levels of 0.325 and 0.396, respectively, at 14 weeks, but the replicate mean value for that time point was low (0.22 ± 0.08) (Fig. 3B). Absorbance values for mucus from this treatment group were not significantly different from the pretreatment absorbance values throughout the sampling period.
Comparison of systemic and mucosal antibody responses.
Correlation analyses were performed for 148 matched samples by using serum antibody ELISA values, mucus antibody ELISA values, fish weight, and total mucus protein (Table 2). Fish weight was used as a relative measure of fish size. Total mucus protein was estimated by measurement of the absorbance (A280). A low positive correlation (0.337) was observed between the titer in serum and fish weight. This result was expected, as the fish continued to grow over the course of the experiment. A low positive correlation (0.034) was also observed between the titer in mucus and the OD280 for mucus. All other analyses had slightly negative values (−0.076 to −0.24). Correlation analyses performed with a subset of samples (n = 20) determined that fish with the highest serum antibody levels did not have correspondingly high mucus antibody levels (correlation coefficient, −0.116) (data not shown).
TABLE 2.
Correlation analyses of fish weight, mucus total protein concentration, and I. multifiliis-specific antibodies in mucus and sera as determined by ELISA
| Parameter | Correlation coefficientsa
|
||
|---|---|---|---|
| Fish wt (g) | Mucus protein (OD280) | Mucus antibodies (OD405) | |
| Mucus protein | −0.076 | 0.304 | |
| Mucus antibodies | −0.155 | 0.304 | |
| Serum antibodies | 0.337 | −0.240 | −0.194 |
Correlations between row and column variables.
In summary, the primary systemic antibody responses of channel catfish immunized against I. multifiliis by infection or injection resulted in similar antibody levels in the blood. Cutaneous mucus antibody levels were much lower. The highest mean mucus antibody values occurred at 7 weeks in infected fish, but these returned to pretreatment levels by 14 weeks. These results suggest that I. multifiliis infection induces a transient mucosal immune response that coincides with the resolution of infection and appears to occur independently of serum antibody production.
DISCUSSION
Channel catfish immunized either by a single injection of I. multifiliis i-antigen emulsified in incomplete Freund's adjuvant or by infection developed primary serum antibody responses at 5 weeks after injection or infection that continued to increase through 14 weeks. The parasite-specific antibody levels were much lower in the cutaneous mucus than in the serum. Fish inoculated with i-antigen produced lower levels of parasite-specific mucus antibody than fish exposed to I. multifiliis. Furthermore, in both cases (injection and infection), the mucus antibody concentrations did not increase concomitantly with the serum antibody concentrations, suggesting that cutaneous antibodies do not arise by passive diffusion from the blood.
Elucidation of the mechanisms of antibody production in fish skin would further an understanding of mucosal immunity in fish, as well as have practical application for the development of a vaccine against I. multifiliis (7, 17). While the systemic and mucosal antibody responses of fish appear to occur separately (1, 35-39, 42, 45, 48), the actual mechanisms and sites of cutaneous antibody induction, production, and secretion have yet to be determined (18, 36, 47). Antibodies detected in the mucus of the plaice (Pleuronectes platessa L.), ayu (Plectoglossus altivelis), sheepshead (Archosargus probatocephalus), and channel catfish are physically and immunologically identical to those isolated from the blood yet do not appear to arise by transduction (19, 20, 22, 26, 30, 34). For example, experimental evidence from studies with the sheepshead (a common marine fish) showed that antibodies isolated from the blood, subsequently labeled with I125, and injected intravenously back into the same fish were not detected in the cutaneous mucus or bile (25). The results of immune transfer studies with channel catfish and immobilizing mouse IgM-class monoclonal antibodies or sera from I. multifiliis-immune fish suggest that a physiological barrier blocks the passage of large antibody molecules (at least as large as 750 kDa) from peripheral blood to the skin (24). Such findings suggest that cutaneous antibodies are produced locally in the skin. Our research supports this possibility, but definitive proof awaits the demonstration of antibody-secreting lymphocytes in the skin.
In this study, experimental injection or infection of channel catfish resulted in primary systemic antibody responses that were similar in duration and magnitude, indicating that the i-antigen by itself stimulates a serum antibody response comparable to that observed following infection. The intraperitoneal injection of antigen did not stimulate as great a mucosal response as that generated by active infection, however. We know from previous work that fish inoculated with purified i-antigen without adjuvant are not protected against parasite challenge (J. L. Wang and H. W. Dickerson, unpublished data). Fish injected intraperitoneally with either i-antigen combined with Freund's complete adjuvant or live theronts, however, become solidly immune to subsequent I. multifiliis challenge (4). Thus, stimulation by parasite activity or adjuvant seems to play a critical role in eliciting acquired protective immunity against the parasite. How this relates to the production of mucus antibodies is under investigation in this laboratory.
Acknowledgments
This work was supported by Mentored Clinical Scientist Award AI01429 from NIAID, National Institutes of Health.
We thank Natalia Guseva for providing the purified i- antigen used to inject the fish and Jane Noe for excellent technical support in the care and maintenance of fish and parasite cultures.
Members of the Statistic Department, The University of Georgia, Athens, participated in experimental design and analysis.
REFERENCES
- 1.Ainsworth, A. J., C. D. Rice, and L. Xue. 1995. Immune responses of channel catfish, Ictalurus punctatus (Rafinesque), after oral or intraperitoneal vaccination with particulate or soluble Edwardsiella ictaluri antigen. J. Fish Dis. 18:397-409. [Google Scholar]
- 2.Beckert, H., and R. Allison. 1964. Some host responses of the white catfish to Ichthyophthirius multifiliis (Fouquet). Proc. S. E. Assoc. Game Fish Comm. 18:438-441. [Google Scholar]
- 3.Bradshaw, C. M., A. S. Richard, and M. M. Sigel. 1971. IgM antibodies in fish mucus. Proc. Soc. Exp. Biol. Med. 136:1122-1124. [DOI] [PubMed] [Google Scholar]
- 4.Burkart, M. A., T. G. Clark, and H. W. Dickerson. 1990. Immunization of channel catfish, Ictalurus punctatus Rafinesque, against Ichthyophthirius multifiliis (Fouquet): killed versus live vaccines. J. Fish Dis. 13:401-410. [Google Scholar]
- 5.Clark, T. G., H. W. Dickerson, J. B. Gratzek, and R. C. Findley. 1987. In vitro response of Ichthyophthirius multifiliis to sera from immune catfish. J. Fish Biol. 31:203-208. [Google Scholar]
- 6.Clark, T. G., H. W. Dickerson, and R. C. Findley. 1988. Immune response of channel catfish to ciliary antigens of Ichthyophthirius multifiliis. Dev. Comp. Immunol. 12:581-594. [DOI] [PubMed] [Google Scholar]
- 7.Clark, T. G., T. L. Lin, and H. W. Dickerson. 1995. Surface immobilization antigens of Ichthyophthirius multifiliis: their role in protective immunity. Annu. Rev. Fish Dis. 5:113-131. [Google Scholar]
- 8.Clark, T. G., T. L. Lin, and H. W. Dickerson. 1996. Surface antigen cross-linking triggers forced exit of a protozoan parasite from its host. Proc. Natl. Acad. Sci. USA 93:6825-6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, T. G., and H. W. Dickerson. 1997. Antibody-mediated effects on parasite behavior: evidence of a novel mechanism of immunity against a parasitic protist. Parasitol. Today 13:477-480. [DOI] [PubMed] [Google Scholar]
- 10.Clark, T. G., Y. Gao, J. Gaertig, X. Wang, and G. Cheng. 2001. The I-antigens of Ichthyophthirius multifiliis are GPI-anchored proteins. J. Eukaryot. Microbiol. 48:332-337. [DOI] [PubMed] [Google Scholar]
- 11.Cobb, C. S., M. G. Levy, and E. J. Noga. 1998. Acquired immunity to amyloodiniosis is associated with an antibody response. Dis. Aquat. Organisms 34:125-133. [DOI] [PubMed] [Google Scholar]
- 12.Cross, M. L., and R. A. Matthews. 1992. Ichthyophthiriasis in carp, Cyprinus carpio L.: fate of parasites in immune fish. J. Fish Dis. 15:497-505. [Google Scholar]
- 13.Cross, M. L., and R. A. Matthews. 1993. Localized leukocyte response to Ichthyophthirius multifiliis establishment in immune carp, Cyprinus carpio L. Vet. Immunol. Immunopathol. 38:341-358. [DOI] [PubMed] [Google Scholar]
- 14.Dickerson, H. W., D. L. Dawe, J. B. Gratzek, J. Brown, and S. W. Pyle. 1981. Induction of Ichthyophthirius multifiliis Fouquet infections in channel catfish Ictalurus punctatus Rafinesque: standardization of the procedure. Dev. Biol. Stand. 49:331-336. [Google Scholar]
- 15.Dickerson, H. W., A. L. Lohr, and J. B. Gratzek. 1985. Experimental intraperitoneal infection of the channel catfish, Ictalurus punctatus (Rafinsque), with Ichthyophthirius multifiliis (Fouquet). J. Fish Dis. 8:139-142. [Google Scholar]
- 16.Dickerson, H. W., T. G. Clark, and R. C. Findly. 1989. Ichthyophthirius multifiliis has membrane-associated immobilization antigens. J. Protozool. 36:159-164. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson, H. W., and T. G. Clark. 1998. Ichthyophthirius multifiliis: a model of cutaneous infection and immunity in fishes. Immunol. Rev. 166:377-384. [DOI] [PubMed] [Google Scholar]
- 18.Diconza, J. J., and W. J. Halliday. 1971. Relationship of catfish serum antibodies to immunoglobulin in mucus secretions. Aust. J. Exp. Biol. Med. Sci. 49:517-519. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher, T. C., and P. T. Grant. 1969. Immunoglobulin in the serum and mucus of the plaice (Pleuronectes platessa). Biochem. J. 115:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher, T. C., and A. White. 1973. Antibody production in the plaice (Pleuronectes platessa L.) after oral and parenteral immunization with Vibrio anguillarum antigens. Aquaculture 1:417-428. [Google Scholar]
- 21.Hines, R. S., and D. T. Spira. 1974. Ichthyophthiriasis in the mirror carp Cyprinus carpio (L.). V. Acquired immunity. J. Fish Biol. 6:373-378. [Google Scholar]
- 22.Itami, T., Y. Takahashi, T. Oamoto, and K. Kubono. 1988. Purification and characterization of immunoglobulin in skin mucus and serum of ayu. Nippon Suisan Gakkaishi 54:1611-1617. [Google Scholar]
- 23.Lin, T. L., and H. W. Dickerson. 1992. Purification and partial characterization of immobilization antigens from Ichthyophthirius multifiliis. J. Protozool. 39:457-463. [DOI] [PubMed] [Google Scholar]
- 24.Lin, T. L., T. G. Clark, and H. W. Dickerson. 1996. Passive immunization of channel catfish (Ictalurus punctatus) against the ciliated protozoan parasite Ichthyophthirius multifiliis by the use of murine monoclonal antibodies. Infect. Immun. 64:4085-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobb, C. J., and L. W. Clem. 1981. The metabolic relationships of the immunoglobulins in fish serum, cutaneous mucus and bile. J. Immunol. 127:1525-1529. [PubMed] [Google Scholar]
- 26.Lobb, C. J., and L. W. Clem. 1981. Phylogeny of immunoglobulin structure and function. XI. Secretory immunoglobulins in the cutaneous mucus of the sheepshead, Archosargus probatocephalus. Dev. Comp. Immunol. 5:587-596. [DOI] [PubMed] [Google Scholar]
- 27.Lobb, C. J., and L. W. Clem. 1981. Phylogeny of immunoglobulin structure and function. XII. Secretory immunoglobulins in the bile of the marine teleost Archosargus probatocephalus. Mol. Immunol. 18:615-619. [DOI] [PubMed] [Google Scholar]
- 28.Lobb, C. J., and L. W. Clem. 1983. Distinctive subpopulations of catfish serum antibodies and immunoglobulin. Mol. Immunol. 20:811-818. [DOI] [PubMed] [Google Scholar]
- 29.Lobb, C. J. 1985. Covalent structure and affinity of channel catfish anti-dinitrophenyl antibodies. Mol. Immunol. 22:993-999. [DOI] [PubMed] [Google Scholar]
- 30.Lobb, C. J. 1987. Secretory immunity induced in catfish, Ictalurus punctatus, following bath immunization. Dev. Comp. Immunol. 11:727-738. [DOI] [PubMed] [Google Scholar]
- 31.Lumsden, J. S., V. E. Osterland, P. J. Byrne, and H. W. Ferguson. 1993. Detection of a distinct gill-surface antibody response following horizontal infection and bath challenge of brook trout Salvelinus fontinalis with Flavobacterium branchiophilum, the causative agent of bacterial gill disease. Dis. Aquat. Organisms 16:21-27. [Google Scholar]
- 32.McCallum, H. I. 1986. Acquired resistance of black mollies Poecilia latipinna to infection by Ichthyophthirius multifiliis. Parasitology 93:251-261. [DOI] [PubMed] [Google Scholar]
- 33.Ourth, D. D. 1980. Secretory IgM, lysozyme and lymphocytes in the skin mucus of the channel catfish, Ictalurus punctatus. Dev. Comp. Immunol. 4:65-74. [DOI] [PubMed] [Google Scholar]
- 34.Ourth, D. D. 1986. Purification and quantification of channel catfish (Ictalurus punctatus) immunoglobulin M. J. Appl. Ichthyol. 3:140-143. [Google Scholar]
- 35.Peleterio, M. C., and R. H. Richards. 1988. Immunocytochemical studies on immunoglobulin-containing cells in the epidermis of rainbow trout, Salmo gairdneri Richardson: influence of bath immunization. J. Fish Biol. 32:845-858. [Google Scholar]
- 36.Rombout, J. W., L. J. Blok, C. H. Lamers, and E. Egberts. 1986. Immunization of carp (Cyprinus carpio) with a Vibrio anguillarum bacterin: indications for a common mucosal immune system. Dev. Comp. Immunol. 10:341-351. [DOI] [PubMed] [Google Scholar]
- 37.Rombout, J. H., N. Taverne, M. van de Kamp, and A. J. Taverne-Theile. 1993. Differences in mucus and serum immunoglobulin of carp (Cyprinus carpio L.). Dev. Comp. Immunol. 17:309-317. [DOI] [PubMed] [Google Scholar]
- 38.Rombout, J. H., A. J. Taverne-Thiele, and M. I. Villena. 1993. The gut-associated lymphoid tissue (GALT) of carp (Cyprinus carpio L.): an immunocytochemical analysis. Dev. Comp. Immunol. 17:55-66. [DOI] [PubMed] [Google Scholar]
- 39.Rombout, J. H., A. A. van den Burg, C. T. van den Burg, P. Witte, and E. Egberts. 1989. Immunological importance of the second gut segment of carp. III. Systemic and/or mucosal immune responses after immunization with soluble or particulate antigen. J. Fish Biol. 35:179-186. [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Smith, S. A., M. G. Levy, and E. J. Noga. 1992. Development of an enzyme-linked immunosorbent assay (ELISA) for the detection of antibody to the parasitic dinoflagellate Amyloodinium ocellatum in Oreochromis aureus. Vet. Parasitol. 42:145-155. [DOI] [PubMed] [Google Scholar]
- 42.St. Louis-Cormier, E. A., C. K. Osterland, and P. D. Anderson. 1984. Evidence for a cutaneous secretory immune system in rainbow trout (Salmo gairdneri). Dev. Comp. Immunol. 8:71-80. [DOI] [PubMed] [Google Scholar]
- 43.Van Muiswinkel, W. 1995. The piscine immune system: innate and acquired immunity In P. T. K. Woo (ed.), Fish diseases and disorders, Vol. 1. CAB International, Wallingford, United Kingdom.
- 44.Wahli, T., and W. Meier. 1985. Ichthyophthiriasis in trout: an investigation of natural defense mechanisms. In A. E. Ellis (ed.), Fish and shellfish pathology. Academic Press, London, United Kingdom.
- 45.Wang, X., and H. W. Dickerson. 2002. Surface immobilization antigen of the parasitic ciliate Ichthyophthirius multifiliis elicits protective immunity in channel catfish (Ictalurus punctatus). Clin. Diagn. Lab. Immunol. 9:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, C. 1995. Ph.D. thesis. The University of Georgia, Athens.
- 47.Xu, D. H., P. H. Klesius, and R. A. Shelby. 2002. Cutaneous antibodies in excised skin from channel catfish Ictalurus punctatus Rafinesque immune to Ichthyophthirius multifiliis. J. Fish Dis. 25:45-52. [DOI] [PubMed] [Google Scholar]
- 48.Zillberg, D., and P. H. Klesius. 1997. Quantification of immunoglobulin in the serum and mucus of channel catfish at different ages and following infection with Edwardsiella ictaluri. Vet. Immunol. Immunopathol. 58:171-180. [DOI] [PubMed] [Google Scholar]