Abstract
Patients with antibody deficiency disorders are highly susceptible to microbial infections. Intravenous (i.v.) immunoglobulin concentrates were originally developed as replacement therapy for such patients. The present study assesses the measles virus neutralizing antibody titers and the antibody-dependent cell-mediated cytotoxicity (ADCC) capacities against Epstein-Barr virus (EBV)-infected cells of immunoglobulin G (IgG) preparations produced for i.v. use (i.v. IgG). The level of neutralizing antibodies against measles virus was determined by a syncytium neutralization test with Vero cells as targets. The measles virus neutralizing antibody titers of the i.v. IgG preparations were >3 × 102 and were an average of 1.0 log higher than the titers in pooled plasma from healthy subjects. The two IgG preparations tested showed similar ADCC activities against EBV-infected Raji cells, being active at concentrations of 3 mg/ml or higher. i.v. IgG bound to Raji cells but not to the EBV-negative Ramos cells, as evaluated by flow cytometry. Our in vitro findings may provide further support for the use of i.v. IgG for the prevention and treatment of infections caused by specific viral pathogens.
Characterization of the specific antimicrobial function of intravenous immunoglobulin G (i.v. IgG) preparations against particular microbial pathogens can assist in determining their therapeutic potential for specific infectious diseases. i.v. IgGs have been reported to contain antibodies directed against several viruses (24). However, the functionality of such antibodies against viral infections remains to be fully characterized.
Measles virus (MV) causes an acute disease that still kills more than 1 million children in the less well developed world every year (29). The severity of measles in the young is mainly due to secondary infections (2, 9) as a result of immune suppression. The mechanism of immune suppression is due to apoptosis of infected hemopoietic cells (13) and interference with dendritic and T-cell functions (16).
Measurable parameters of the immune response to MV infection include neutralization by antibody, antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent complement-mediated lysis, and cytotoxic T-lymphocyte activity (14, 15). The neutralizing antibody titer correlates well with protection from MV infection (6, 15).
NK cell activity is crucial against infection by Epstein-Barr virus (EBV). Low NK cell cytotoxic activity is linked with increased human sensitivity to severe disseminating herpesvirus group infections, including those caused by herpes simplex virus (3, 7) and EBV (22, 26). ADCC is thought to play a major role in controlling the spread of EBV in an infected individual. The viral membrane glycoprotein gp350/220, which is expressed at the surface of the virus-producing cell, was identified as a target for ADCC reactions (21). Sera from EBV-positive individuals provide antibodies for EBV-specific ADCC reactions (21). In individuals affected by X-linked lymphoproliferative disease, both the spontaneous NK cell cytotoxicity against EBV-infected cells and also EBV-infected cell lysis induced via CD16 are blocked (5, 8, 28, 30, 32, 34). These individuals have a severe mononucleosis when they are infected with EBV. The gene modified in these patients codes for an NK cell coreceptor that is crucial for activation of cytotoxicity in NK and CD8+ T cells. This demonstrates the importance that cytotoxic responses have for the control of EBV infections.
i.v. IgG preparations contain significant levels of anti-MV and anti-EBV antibodies (24). However, the functionality of these antiviral antibodies has not been fully characterized. In the present work we have investigated the capacity of i.v. IgG to neutralize MV infectivity and to activate ADCC activity against an EBV-transformed cell line. The results indicate that i.v. IgG preparations contain a full capacity to neutralize MV and are also able to activate ADCC on lymphocyte preparations against the EBV-infected cell line Raji.
MATERIALS AND METHODS
Reagents.
Sorbitol (5%; pH 5 to 6) and human albumin (20%) were provided by Instituto Grifols S.A. (Parets, Spain). Dulbecco's phosphate-buffered saline (DPBS) and phosphate-buffered saline (PBS) were from Gibco-Invitrogen (Barcelona, Spain). Dulbecco's modified Eagle's medium (DMEM), penicillin, streptomycin, fetal calf serum (FCS), and bovine serum albumin were purchased from Sigma-Aldrich (Madrid, Spain). FCS was decomplemented at 56°C for 30 min. Lymphoprep (Ficoll) was from Reactiva (Barcelona, Spain). Recombinant human interleukin 2 was from Glaxo (Geneva, Switzerland) and was a generous gift from M. Nabholz (Institut Suisse de Recherche Expérimentale sur le Cancer, Lausanne, Switzerland). The PKH67-GL green fluorescent cell tracker was from Sigma-Aldrich.
i.v. IgG preparations.
i.v. IgG was obtained by Instituto Grífols S.A. (Parets, Spain) through a purification procedure which yields unmodified IgG with a level of purity close to 100% (99.6% ± 0.3%). The i.v. IgG preparations (batches 111690, 201591, 201691, and 208191) contained protein at a concentration of 50 g/liter and were used in water containing 5% sorbitol as a stabilizer. A biological reference preparation (BRP; batch 2) with human IgGs was used as a control and was obtained from the European Directorate for the Quality of Medicines (EPH0990000; LGC Promochem, Barcelona, Spain). BRP was dissolved in water at a concentration of 50 g/liter and was kept at 4°C for 2 weeks.
Sera and plasma.
Plasma was prepared from 12 buffy coat preparations (Blood Bank, Hospital Clínic, Barcelona, Spain) after separation of blood cells by centrifugation, and the plasma was then pooled. This plasma pool was decomplemented by heat inactivation and then aliquoted and kept frozen at −20°C until use. The IgG and IgM contents of the plasma pool, as determined by immunonephelometry, were 8.611 and 1.113 g/liter, respectively. Positive control serum specimens positive for EBV and MV were purchased from Virion (Durviz, Valencia, Spain). The MV-positive serum sample contained 8.97 g of IgG per liter and 0.934 g of IgM per liter (as determined by immunonephelometry).
Labeling of target Raji and Ramos cells with fluorescent dye.
The human B-cell lines used in the ADCC assays were Raji cells (EBV genome positive; CCL-86; American Type Culture Collection) and Ramos cells (EBV genome negative; CRL-1596; American Type Culture Collection). The Raji and Ramos cells were grown in DMEM and RPMI, respectively, supplemented with 10% FCS (Sigma-Aldrich). Both cell lines were labeled with the green membrane dye PKH67-GL in accordance with the instructions of the manufacturer (Sigma-Aldrich). Target cells (2 × 107) were washed twice in DMEM and incubated with 2 ml of 2 μM PKH67-GL dye in Diluent C buffer solution (Sigma-Aldrich) at room temperature for 5 min. Labeling was stopped by incubation with 2 ml of FCS for 1 min and washing in DMEM-10% FCS. The extent of labeling was checked by flow cytometry. Labeled cells were aliquoted and kept under liquid nitrogen.
Preparation of effector lymphocytes.
Blood obtained from the buffy coat preparations was subjected to Ficoll separation to obtain mononuclear cells. Mononuclear cells were washed with DMEM and allowed to adhere to 75-cm2 plastic flasks (Nunc, Labclínics, Barcelona, Spain) (160 × 106 cells in 16 ml of medium) for 1 h at 37°C to eliminate monocytes because monocytes have been reported to inhibit NK cell activity in vitro (35, 36). Nonadherent cells (basically lymphocytes) were resuspended at 5 × 106 cells/ml in DMEM-5% FCS containing 20 U of interleukin 2 per ml (19), penicillin, and streptomycin and were incubated overnight at 37°C.
ADCC assay.
ADCC was measured by flow cytometry as described previously (18, 25). Peripheral blood lymphocytes (0.5 × 106 cells) were used as effectors and were mixed at a ratio of 50:1 with labeled target cells in a total volume of 200 μl of DMEM-2.5% FCS. Fifty microliters of the different i.v. IgG preparations (or plasma) was added to the cells. After the components were mixed, 200 μl was introduced into a U-bottom plate (Deltalab S.A., Rubi, Spain) and the cells were pelleted by a brief centrifugation (120 × g, 3 min). The cultures were incubated at 37°C in 5% CO2 for 2 h. Finally, the cells were placed on ice and propidium iodide was added to monitor cell death by flow cytometry. The mean fluorescence of triplicate wells was used in all cytotoxicity calculations. The maximum level of propidium iodide incorporation was determined in target cells lysed by three cycles of freezing and thawing. Cytotoxicity was expressed as the percentage of cell death among the PKH67-GL-positive target cells: (number of dead labeled target cells/[number of dead labeled target cells + number of live labeled target cells]) × 100. The percentage of dead target cells was corrected for spontaneous background cell death by subtracting the percentage of dead cells in control samples (PKH67-GL-labeled targets alone) from the percentage of dead cells in the test samples. As concentrated i.v. IgG contained 5% sorbitol as a stabilizer (no salt) and it was added to the cell suspension at one-fifth of the final volume, adequate amounts of 10× PBS were added to correct the 1/5 dilution effect.
The titers of EBV-specific IgGs (and IgMs in plasma) in samples were also determined by enzyme immunoassay (EIA; Virion Durviz) (20). The titer of EBV-specific antibody was the reciprocal of the serum dilution midway between the first dilution with a result less than or equal to the mean + 2 standard deviations (SDs) of the EIA value for negative controls for all dilutions combined and the dilution immediately before that. The titers of EBV-specific IgG were 190,665 for i.v. IgG, 275,302 for BRP, and 42,670 for plasma. The titer of EBV-specific IgM in plasma was 419.
Immunofluorescence.
The levels of binding of i.v. IgG and plasma samples to Raji and Ramos cells were determined. Half a million cells per sample were suspended in PBS-2% FCS on ice in a volume of 100 μl and incubated with 50 μl of i.v. IgG or plasma. After 30 min, the cells were washed twice and stained with 10 μl of anti-human IgG-fluorescein isothiocyanate (FITC), anti-human IgM-FITC, or isotype control monoclonal antibodies (Becton Dickinson, Madrid, Spain) in PBS-2% FCS on ice in a volume of 100 μl. After 30 min, the cells were washed once and analyzed by fluorescence-activated cell sorter analysis. The secondary antibody alone (anti-human IgG-FITC and anti-human IgM-FITC) was also added to the cells as controls.
Flow cytometry analysis.
Dye-labeled Raji cells (0.1 × 104 cells/experimental point) were analyzed on an Epics XL flow cytometer (Coulter Corporation, Miami, Fla.) within 1 h of the end of incubation with NK cells. Excitation of the sample was done with a 488-nm air-cooled argon-ion laser at 15 mW of power. The instrument was set up with the standard configuration (forward scatter and side scatter), and the green (525-nm) fluorescence for the PKH67-GL dye and the red (675-nm) fluorescence for propidium iodide were collected. Only green cells (Raji and Ramos cells) were considered in the analysis. The dead cells among the green cells were detected and counted for the analysis according to their levels of propidium iodide incorporation. Green fluorescence was represented as a logarithmic histogram. Optical alignment was based on the signal optimized from 10-nm fluorescent beads (Flowcheck; Epics Division, Coulter Corp.). Time versus fluorescence was used as a control for the stability of the instrument.
MV neutralization assay.
A syncytium inhibition assay was applied to measure neutralizing antibody titers (14). The Schwarz strain of MV was obtained as the Rimevax vaccine (SmithKline Beecham, Madrid, Spain). The vaccine, which contains an MV titer of 1,000 50% tissue culture infective doses, was resuspended in 0.6 ml of DMEM-5% FCS (Sigma-Aldrich) containing penicillin and streptomycin and was distributed in 30-μl aliquots in 96-well U-bottom plates (Deltalab S.A.). Tenfold serial dilutions (15 μl; 1:3 to 1:3,000) of i.v. IgG prepared in DMEM-5% albumin were incubated with the viral aliquots for 1 h at 37°C in 5% CO2 (14).
Vero cells (CCL-81; American Type Culture Collection) were grown in DMEM-5% FCS and were expanded the day before testing in 24- and 48-well plates. Cells at 70 to 80% confluence were washed with DPBS, and the virus-antibody cocktail (40 μl) was added to duplicate wells (24-well plates) or to four wells (48-well plates). The cells were incubated with the virus for 1 h at 37°C, the cocktail was aspirated, and the cells were washed with DPBS. The cells were then incubated with 0.5 ml of DMEM-5% FCS-penicillin-streptomycin at 37°C until syncytium formation could be determined by light microscopy (48 h). The end-point dilution was considered the first serum dilution that resulted in one or more syncytia (14). Titers were calculated by the method of Kärber (23) and are expressed as the final dilution of i.v. IgG present in the i.v. IgG-virus mixture at the 50% end-point dilution. The same results (50% neutralizing doses, 10−2.5 for i.v. IgG and BRP and 10−1 for plasma [23]) were obtained by using 24-well plates (duplicate wells for each dilution) and 48-well plates (four wells for each dilution).
As concentrated i.v. IgG (50 mg/ml) contained 5% sorbitol as a stabilizer (no salt) and it was added (15 μl) to the cell suspension at one-third of the final volume, adequate amounts of 10× PBS (1.7 μl) were added to this dilution to correct the 1/3 dilution effect.
The titers of MV-specific IgGs (and IgMs in plasma) in the samples were also determined by EIA (Virion Durviz) (20). The titer of MV-specific antibody was the reciprocal of the serum dilution midway between the first dilution with a result less than or equal to the mean + 2 SDs of the EIA value for negative controls at all dilutions combined and the dilution immediately before that. The titers of MV-specific IgG were 65,510 for i.v. IgG, 269,501 for BRP, 18,636 for plasma, and 39,286 for a convalescent-phase serum sample. The titers of MV-specific IgM in plasma and serum were 933 and 1,302, respectively.
RESULTS AND DISCUSSION
i.v. IgG samples provide ADCC activity against Raji cells.
i.v. IgG preparations have been reported to contain anti-EBV antibodies (24; this study [see “ADCC assay” in Materials and Methods]), and these antibodies are expected to have neutralizing, complement-fixing, or ADCC activities. To assess whether i.v. IgG preparations would confer ADCC activity against EBV-infected cells, the human EBV-positive cell line Raji (12) was used as a target for the ADCC assays. To quantify the extent to which Raji cells were killed by NK cells, the membranes of Raji cells were labeled with a green fluorescent dye (PKH67-GL) to distinguish them from effector NK cells. The death of green fluorescent target cells (Raji cells) was identified by propidium iodide staining and flow cytometry.
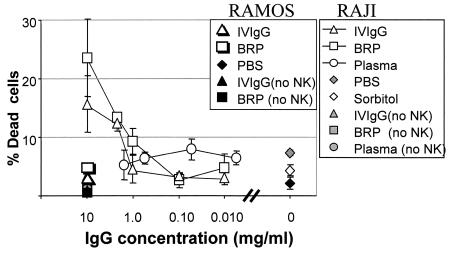
Lymphocytes (0.5 × 106 cells) containing NK cells were prepared from healthy donors by density centrifugation and were mixed at a ratio of 50:1 with labeled Raji and Ramos cells. i.v. IgG and plasma samples were added to the effector cell-target cell mixture, and the mixture was incubated at 37°C for a total of 2 h (Fig. 1).
FIG. 1.
ADCC activities against EBV-infected Raji cells provided by i.v. IgG preparations. The ADCC potentials of i.v. IgG and plasma samples against EBV-positive Raji cells and EBV-negative Ramos cells were tested. The mean ± SD percentage of specific cell death following incubation of the targets with blood lymphocytes is shown. The IgG concentrations in the i.v. IgG preparations and plasma samples are indicated. A plasma pool was prepared with plasma from 12 donors and had 8.611 g of IgG per liter. PBS and 5% sorbitol replaced i.v. IgGs in the samples. All experimental points were run in triplicate. The results of one representative experiment from a total of four that were conducted are shown. BRP with human IgGs was used as a control and was obtained from European Directorate for the Quality of Medicines.
When Raji cell targets and blood lymphocytes were mixed in the absence of i.v. IgG, the level of cytotoxicity ranged between 4 and 8% (Fig. 1, samples labeled PBS and sorbitol). i.v. IgG augmented Raji target cell lysis, increasing it two- to threefold. i.v. IgG increased the level of cytotoxicity when it was added at a concentration of 3 mg/ml or higher. The BRP reference preparation of i.v. IgG yielded the same results as the i.v. IgG preparation. Plasma samples did not show measurable ADCC activity under these conditions. The results of the experiment shown in Fig. 1 are representative of those of four additional experiments performed with the same batch of i.v. IgG. A total of four different i.v. IgG batches were tested, with analogous results obtained for all batches (data not shown).
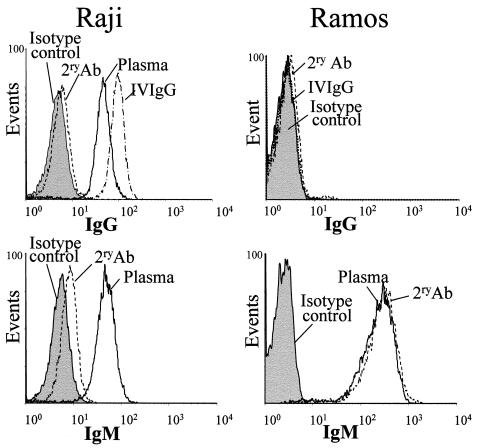
The presence in i.v. IgG preparations of antibodies specific for the whole EBV virus and for those EBV antigens expressed in an EBV-positive cell line (Raji cells) was demonstrated by EIA (see “ADCC assay” in Materials and Methods) and immunofluorescence studies, respectively. EBV-positive Raji cells and EBV-negative Ramos B cells were incubated with i.v. IgG and then stained with an FITC-labeled anti-human IgG antibody or isotype control. Raji cells but not Ramos cells were recognized by i.v. IgG (Fig. 2). Consequently, i.v. IgG did not increase the level of NK cell cytotoxicity against Ramos cells (Fig. 1). Thus, the assay specifically measured the ADCC that was directed against Raji cells by the EBV-specific antibody. These results are consistent with the reported activity of i.v. IgG in targeting Daudi cells, an EBV-positive cell line, for recognition by NK cells (31).
FIG. 2.
i.v. IgG binds to Raji cells but not to Ramos cells. Cells were incubated with i.v. IgG or plasma and stained with anti-human IgG-FITC (top panels), anti-human IgM-FITC (bottom panels), or the isotype control mouse IgG1-FITC (gray shading). 2ry Ab, cells incubated only with the FITC-conjugated secondary antibody. The results presented here are representative of those from two identical experiments.
The pool of plasma also contained Raji cell-specific IgGs (Fig. 2), although plasma did not provide ADCC activity against Raji cells (Fig. 1). IgM antibodies specific for Raji cells but not for Ramos cells were also observed in plasma (Fig. 2). Such IgMs could be competing with IgG for EBV antigens on Raji cells, thus explaining the lack of ADCC activity of plasma antibodies specific for EBV. Raji cells express a smaller amount of membrane IgM than Ramos cells (Fig. 2).
Raji and Vero cells become infected with the MV Schwarz strain and form syncytia.
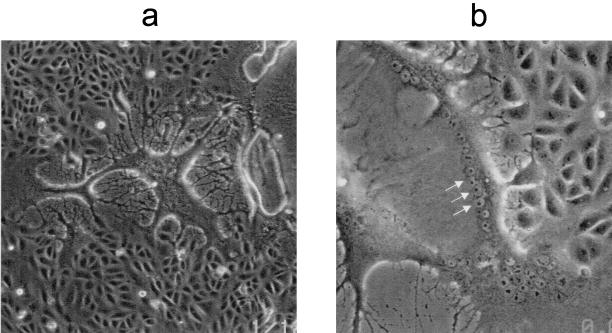
For security reasons, the Schwarz vaccine strain of MV was used in the present study. Since 1954, when MV was first isolated in tissue culture (11), continuous monkey cell lines (e.g., Vero) have commonly been used for MV isolation (4). Vero cells were infected with the Schwarz strain of MV, and at 48 h postinfection multiple syncytia appeared (Fig. 3). The syncytia rapidly degraded and disappeared, leaving empty space. At a higher magnification, multiple nuclei were visible inside the syncytia (Fig. 3b, arrows).
FIG. 3.
Syncytium formation in MV-infected Vero cells. Vero cells were infected with MV, and syncytium formation was observed 48 h postinfection (a). At higher magnification (b), multiple nuclei are visible inside syncytia (arrows). The results presented here are representative of those from five identical experiments.
Raji cells have previously been shown to be infected with MV strains AR and Edmonston (15, 17). After inoculation with the Schwarz strain of MV, Raji cells expressed the MV hemagglutinin protein on their surfaces and formed observable syncytia at 24 h (data not shown). A considerable cytopathic effect occurred after 3 to 7 days of infection. In some studies Raji cells infected with MV became susceptible to ADCC activity (15). In our hands, uninfected Raji cells were good targets for ADCC (Fig. 1) due to the expression of EBV antigens. This fact precluded the study of ADCC activity against MV-infected Raji cells.
Human total IgGs neutralize syncytium formation in Vero cells.
Vaccine strains of MV infect cells expressing CD46 (10, 27). This is a major receptor for MV entry into cells, and antibodies binding to CD46 or the viral ligand for CD46 can block infection by vaccine virus strains (1, 33).
The results of syncytium inhibition assays obtained with sera from healthy individuals have previously shown antibody log titers that ranged from 1.0 to 3.7 (14), with an important contribution of IgGs (14). Therefore, it was expected that pooled IgG preparations would contain significant MV-neutralizing capacities (6).
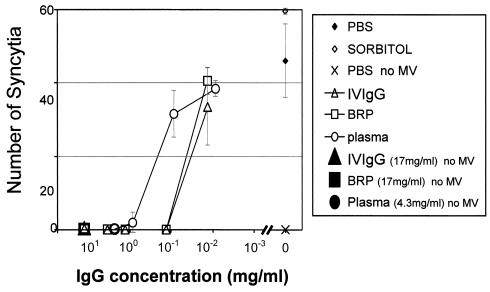
To measure the neutralizing potential of i.v. IgG preparations against infectivity by MV, a test of inhibition of syncytium formation was used (14). Virus was incubated with different concentrations of antibodies, and the infection capacity of the virus was quantified by counting the number of syncytia that formed in Vero cells (the syncytia in Vero cells were easier to count than those in Raji cells). Figure 4 shows that i.v. IgGs have a full capacity to neutralize MV when the i.v. IgG preparations were diluted down to 0.17 mg/ml. This results in a syncytium-neutralizing titer of 316. Figure 4 shows the results of a representative experiment of a total of four that were performed with the same i.v. IgG batch. Three additional i.v. IgG batches were tested in 48-well plates with four wells for each dilution in duplicate experiments. The syncytium-neutralizing titer obtained for all batches was the same (titer, 316). The BRP of i.v. IgG yielded the same results as i.v. IgG. However, plasma showed a lower neutralizing capacity, with a titer of 10. A positive control serum sample yielded the same neutralizing titer as plasma. This suggests that i.v. IgG has a higher protective potential against MV than serum.
FIG. 4.
Syncytium-neutralizing capacity of i.v. IgG. Vero cells were incubated with MV-specific i.v. IgGs or an MV-plasma cocktail in duplicate wells (24-well plates) for 1 h, as explained in Materials and Methods. Shown are the mean ± SD numbers of syncytia counted in independent wells of duplicate samples. PBS and sorbitol indicate Vero cells incubated with a cocktail of MV and PBS and 5% sorbitol, respectively. The results of one representative experiment of a total of four that were performed are shown. BRP with human IgGs was used as a control and was obtained from the European Directorate for the Quality of Medicines.
The neutralizing antibody titer measured by the plaque neutralization test is considered protective if the titer is >120 (6). On average, titers obtained by the syncytium inhibition assay are fivefold lower than those measured by the plaque neutralization test (14). Therefore, the titer obtained by the syncytium inhibition assay (>3 × 102) (Fig. 4) suggests that i.v. IgG could be diluted up to 10-fold (5 × [3 × 102]/10, which is >120) before losing its protective capacity against MV.
In conclusion, the i.v. IgG preparations tested contain a full capacity to neutralize the infectivity of MV. Furthermore, the ADCC assays revealed the presence of antibodies in i.v. IgG preparations with the capacity to provide ADCC activity against EBV-infected cells.
Acknowledgments
This research was supported by grant 00/0024-03 from the Fondo de Investigación Sanitaria (to E.E.), an Instituto Grífols grant (to E.E., M.R., and S.V.), and a grant from the University of Barcelona (to I.P.).
We thank the team from the Serveis Cientifico Tecnics de la Universitat de Barcelona (Barcelona, Spain) for expert assistance with flow cytometry analysis.
M.C. and I.P. contributed equally to this work.
REFERENCES
- 1.Bartz, R., R. Firsching, B. Rima, V. ter Meulen, and J. Schneider-Schaulies. 1998. Differential receptor usage by measles virus strains. J. Gen. Virol. 79:1015-1025. [DOI] [PubMed] [Google Scholar]
- 2.Beckford, A. P., R. O. Kaschula, and C. Stephen. 1985. Factors associated with fatal cases of measles. A retrospective autopsy study. S. Afr. Med. J. 68:858-863. [PubMed] [Google Scholar]
- 3.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731-1735. [DOI] [PubMed] [Google Scholar]
- 4.Borges, M. B., G. F. Mann, and S. Freire Mda. 1996. Biological characterization of clones derived from the Edmonston strain of measles virus in comparison with Schwarz and CAM-70 vaccine strains. Mem. Inst. Oswaldo Cruz 91:507-513. [DOI] [PubMed] [Google Scholar]
- 5.Bottino, C., M. Falco, S. Parolini, E. Marcenaro, R. Augugliaro, S. Sivori, E. Landi, R. Biassoni, L. D. Notarangelo, L. Moretta, and A. Moretta. 2001. NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 194:235-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, R. T., L. E. Markowitz, P. Albrecht, J. A. Stewart, L. M. Mofenson, S. R. Preblud, and W. A. Orenstein. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 7.Ching, C., and C. Lopez. 1979. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect. Immun. 26:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey, A. J., R. A. Brooksbank, O. Brandau, T. Oohashi, G. R. Howell, J. M. Bye, A. P. Cahn, J. Durham, P. Heath, P. Wray, R. Pavitt, J. Wilkinson, M. Leversha, E. Huckle, C. J. Shaw-Smith, A. Dunham, S. Rhodes, V. Schuster, G. Porta, L. Yin, P. Serafini, B. Sylla, M. Zollo, B. Franco, D. R. Bentley, et al. 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20:129-135. [DOI] [PubMed] [Google Scholar]
- 9.Coovadia, H. M., A. Wesley, and P. Brain. 1978. Immunological events in acute measles influencing outcome. Arch. Dis. Child. 53:861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 11.Enders, J. F., and T. C. Peebles. 1954. Propagation in tissue cultures of cytopathic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 86:277-286. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, M. A., B. G. Achong, Y. M. Barr, B. Zajac, G. Henle, and W. Henle. 1966. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J. Natl. Cancer Inst. 37:547-559. [PubMed] [Google Scholar]
- 13.Esolen, L. M., S. W. Park, J. M. Hardwick, and D. E. Griffin. 1995. Apoptosis as a cause of death in measles virus-infected cells. J. Virol. 69:3955-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forthal, D. N., G. Landucci, A. Habis, M. Zartarian, J. Katz, and J. G. Tilles. 1994. Measles virus-specific functional antibody responses and viremia during acute measles. J. Infect. Dis. 169:1377-1380. [DOI] [PubMed] [Google Scholar]
- 15.Forthal, D. N., G. Landucci, J. Katz, and J. G. Tilles. 1993. Comparison of measles virus-specific antibodies with antibody-dependent cellular cytotoxicity and neutralizing functions. J. Infect. Dis. 168:1020-1023. [DOI] [PubMed] [Google Scholar]
- 16.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grote, D., S. J. Russell, T. I. Cornu, R. Cattaneo, R. Vile, G. A. Poland, and A. K. Fielding. 2001. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 97:3746-3754. [DOI] [PubMed] [Google Scholar]
- 18.Hatam, L., S. Schuval, and V. R. Bonagura. 1994. Flow cytometric analysis of natural killer cell function as a clinical assay. Cytometry 16:59-68. [DOI] [PubMed] [Google Scholar]
- 19.Henney, C. S., K. Kuribayashi, D. E. Kern, and S. Gillis. 1981. Interleukin-2 augments natural killer cell activity. Nature 291:335-338. [DOI] [PubMed] [Google Scholar]
- 20.Inoue, N., J. Kuranari, S. Harada, H. Nakajima, M. Ohbayashi, Y. Nakamura, N. Miyasaka, K. Ezawa, F. Ban, and K. Yanagi. 1992. Use of enzyme-linked immunosorbent assays with chimeric fusion proteins to titrate antibodies against Epstein-Barr virus nuclear antigen 1. J. Clin. Microbiol. 30:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jilg, W., C. Bogedain, H. Mairhofer, S. Y. Gu, and H. Wolf. 1994. The Epstein-Barr virus-encoded glycoprotein gp 110 (BALF 4) can serve as a target for antibody-dependent cell-mediated cytotoxicity (ADCC). Virology 202:974-977. [DOI] [PubMed] [Google Scholar]
- 22.Joncas, J., Y. Monczak, F. Ghibu, C. Alfieri, A. Bonin, G. Ahronheim, and G. Rivard. 1989. Brief report: killer cell defect and persistent immunological abnormalities in two patients with chronic active Epstein-Barr virus infection. J. Med. Virol. 28:110-117. [DOI] [PubMed] [Google Scholar]
- 23.Kärber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 24.Krause, I., R. Wu, Y. Sherer, M. Patanik, J. B. Peter, and Y. Shoenfeld. 2002. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations—a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfusion Med. 12:133-139. [DOI] [PubMed] [Google Scholar]
- 25.Lee-MacAry, A. E., E. L. Ross, D. Davies, R. Laylor, J. Honeychurch, M. J. Glennie, D. Snary, and R. W. Wilkinson. 2001. Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J. Immunol. Methods 252:83-92. [DOI] [PubMed] [Google Scholar]
- 26.Merino, F., W. Henle, and P. Ramirez-Duque. 1986. Chronic active Epstein-Barr virus infection in patients with Chediak-Higashi syndrome. J. Clin. Immunol. 6:299-305. [DOI] [PubMed] [Google Scholar]
- 27.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols, K. E., D. P. Harkin, S. Levitz, M. Krainer, K. A. Kolquist, C. Genovese, A. Bernard, M. Ferguson, L. Zuo, E. Snyder, A. J. Buckler, C. Wise, J. Ashley, M. Lovett, M. B. Valentine, A. T. Look, W. Gerald, D. E. Housman, and D. A. Haber. 1998. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA 95:13765-13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein, W. A., P. M. Strebel, M. Papania, R. W. Sutter, W. J. Bellini, and S. L. Cochi. 2000. Measles eradication: is it in our future? Am. J. Public Health 90:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parolini, S., C. Bottino, M. Falco, R. Augugliaro, S. Giliani, R. Franceschini, H. D. Ochs, H. Wolf, J. Y. Bonnefoy, R. Biassoni, L. Moretta, L. D. Notarangelo, and A. Moretta. 2000. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J. Exp. Med. 192:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz, J. E., J. Y. Kwak, L. Baum, A. Gilman-Sachs, K. D. Beaman, Y. B. Kim, and A. E. Beer. 1996. Effect of intravenous immunoglobulin G on natural killer cell cytotoxicity in vitro in women with recurrent spontaneous abortion. J. Reprod. Immunol. 31:125-141. [DOI] [PubMed] [Google Scholar]
- 32.Sayos, J., C. Wu, M. Morra, N. Wang, X. Zhang, D. Allen, S. van Schaik, L. Notarangelo, R. Geha, M. G. Roncarolo, H. Oettgen, J. E. De Vries, G. Aversa, and C. Terhorst. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395:462-469. [DOI] [PubMed] [Google Scholar]
- 33.Schneider-Schaulies, J., J. J. Schnorr, U. Brinckmann, L. M. Dunster, K. Baczko, U. G. Liebert, S. Schneider-Schaulies, and V. ter Meulen. 1995. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc. Natl. Acad. Sci. USA 92:3943-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seemayer, T. A., T. G. Gross, R. M. Egeler, S. J. Pirruccello, J. R. Davis, C. M. Kelly, M. Okano, A. Lanyi, and J. Sumegi. 1995. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr. Res. 38:471-478. [DOI] [PubMed] [Google Scholar]
- 35.van den Bosch, G., F. Preijers, A. Vreugdenhil, J. Hendriks, F. Maas, and T. De Witte. 1995. Granulocyte-macrophage colony-stimulating factor (GM-CSF) counteracts the inhibiting effect of monocytes on natural killer (NK) cells. Clin. Exp. Immunol. 101:515-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang, J., and D. Zucker-Franklin. 1984. Modulation of natural killer (NK) cells by autologous neutrophils and monocytes. Cell. Immunol. 86:171-182. [DOI] [PubMed] [Google Scholar]