Abstract
Immunodiffusion (ID) is the serologic test most frequently used for the diagnosis and posttherapy follow-up of patients with paracoccidioidomycosis (PCM). The ID test is highly specific (100%), but its sensitivity is relatively low (90%), leading to false-negative results. The aim of this study was to determine the profiles of antibodies in sera from patients with proven PCM and with negative results in the ID test (IDneg) versus positive results in the ID test (IDpos). We analyzed 46 sera from patients with active PCM for total immunoglobulin G (IgG) and IgG subclass responses to Paracoccidioides brasiliensis gp43 antigen (treated or not treated with sodium metaperiodate) by enzyme-linked immunosorbent assay and immunoblotting. Immunoblotting showed that both IDneg and IDpos sera recognized predominantly the gp43 fraction of the P. brasiliensis antigen used in the ID test. IDneg sera contain low-avidity antibodies, low levels of specific IgG (total) and IgG1, and high levels of IgG2 compared with IDpos sera. The antibodies present in IDneg sera were predominantly directed against carbohydrate epitopes, since treatment with sodium metaperiodate resulted in a significant decrease in antibody reactivity. These data suggest that the lack of reactivity of sera from PCM patients in the ID test may be related to the production of low-avidity IgG2 antibodies directed against carbohydrate epitopes.
Paracoccidioidomycosis (PCM) is the most prevalent systemic mycosis in Latin America. The disease, caused by the dimorphic fungus Paracoccidioides brasiliensis, has multiple clinical aspects, since the fungus is able to disseminate through the lymphatic system and bloodstream to any part of the host organism (6). There are two major clinical forms of PCM: juvenile form (JF) and adult form (AF). The JF equally affects young patients of both sexes and is characterized by systemic lymph node involvement, hepatosplenomegaly, and bone marrow dysfunction, thereby resembling a lymphoproliferative disease. The AF almost always affects adult males, who show a high frequency of pulmonary, skin, and visceral involvement (13).
The diagnosis of PCM is made by observation of the characteristic yeast cells in clinical materials (sputum, biopsy specimens, or scrapings of lesions) or cultures. However, due to the time and material required to perform these techniques, serologic tests have been developed to detect both antibodies (7, 19, 30, 31) and antigens (14, 15, 16, 22). Serologic tests are important tools when other procedures are not available as well as for posttherapy follow-up (21). The immunodiffusion (ID) test is one of the most widely used techniques due to the simplicity and low costs involved in its execution. The ID test is highly specific (about 100%), but false-negative results are not rare due to a sensitivity of 85 to 90% (8, 10, 11).
In a previous study, Mamoni et al. (20) showed that JF patients produce high levels of antibodies, predominantly IgG4 and IgE. The same profile was observed for AF patients with disseminated lesions (multifocal adult form), indicating that immunoglobulin G4 (IgG4) and IgE may be useful markers of disease severity and impairment of the protective immune response. On the other hand, patients with isolated lesions (unifocal adult form) produce low levels of antibodies, mainly of the IgG1 isotype (20). Isotype switching is in part controlled by cytokines present in the microenvironment where B cells differentiate. In fact, lymphocytes from JF patients responding to P. brasiliensis antigen produce high levels of interleukin 4 (IL-4) and IL-5 and low levels of gamma interferon, whereas cells from AF patients produce low levels of IL-4 and IL-5 and high levels of IL-12 (24).
Studies of the physicochemical nature of antigens and the antibodies that they elicit have revealed a restriction in the ability of IgG subclasses to be induced by certain antigens. It is well known that carbohydrate antigens elicit the production of IgG2 antibodies, whereas antiprotein antibodies are mainly IgG1, IgG3, and IgG4 (9, 27). A further feature of the humoral immune response is the avidity of antibody binding, which may be regarded as an estimate of the average affinity of polyclonal antibodies for a complex antigen (25). The avidity of antibody-antigen binding varies greatly according to the time of infection and the class of antibodies produced (12).
Considering that all of the above-mentioned aspects may interfere with the results of a serologic test, in the present study we analyzed the antibody profiles of sera from patients with proven PCM and with negative results in the ID test with respect to isotype composition and avidity.
MATERIALS AND METHODS
Sera.
In a previous study, Blotta et al. described a specificity of 100% and a sensitivity of 87% for ID tests performed (3). For this study, we selected 28 serum samples from IDneg PCM patients and 18 from IDpos PCM patients (9 serum samples from patients with the JF and 9 serum samples from patients with the AF). The diagnosis was confirmed by histopathological examinations of skin or of ganglionic or pulmonary biopsy specimens or by the finding of the fungus in scrapings of skin lesions, in material obtained from lymph nodes, or in sputum (direct examination). All 28 IDneg serum samples were from patients with the unifocal (pulmonary) AF of the disease.
ID test.
The ID test was performed with a crude P. brasiliensis exoantigen as previously described (8).
Preparation of CFA.
P. brasiliensis B-339 was grown on Sabouraud glucose agar at 35°C for 3 days. The fungal growth from three randomly selected tubes (about 300 mg [wet weight]) was collected by gently scraping the surface. The cell mass was suspended in 1 ml of 0.15 M phosphate-buffered saline (PBS) (pH 7.4), mixed for 30 s in a Vortex mixer, and immediately centrifuged at 10,000 × g in an Eppendorf tabletop centrifuge for 60 s. The resulting supernatant fluid contained cell-free antigen (CFA). The protein concentration in CFA was determined by the method of Bradford (5).
Preparation of P. brasiliensis gp43 antigen.
Purified gp43 was obtained by affinity chromatography of the crude exoantigen of P. brasiliensis B-339 on Affigel-10 (Bio-Rad, Hercules, Calif.) coupled with anti-gp43 monoclonal antibody (7). gp43 was eluted from the column with 0.1 M glycine-HCl (pH 2.8), immediately neutralized with 2 M Tris (pH 9.0), concentrated in an Amicon 10K apparatus, and analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The protein content was measured by the method of Bradford (5).
SDS-PAGE.
Crude exoantigen or CFA obtained as described above was mixed with reducing sample buffer, containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 10% 2-mercaptoethanol, and 0.05% bromophenol blue. The polypeptides were separated by SDS-PAGE on 10% acrylamide gels in a Mini Protean II apparatus (Bio-Rad). Protein standards (Sigma, St. Louis, Mo.) with the following molecular masses were used: triosephosphate isomerase, 26.6 kDa; lactic dehydrogenase, 36.5 kDa; ovalbumin, 45 kDa; pyruvate kinase, 58 kDa; fructose-6-phosphate kinase, 84 kDa; and β-galactosidase, 116 kDa.
Immunoblotting for IgG and IgG subclasses.
Immunoblotting was performed as previously described (2), with a few modifications. Briefly, after electrophoresis, proteins were transferred to nitrocellulose paper (NCP) by using a Mini Trans-Blot transfer cell (Bio-Rad). Prior to immunological staining, the free sites on the NCP were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% bovine serum albumin (BSA) and 0.05% Tween 20 (TBS-BSA-T) (pH 7.3) for 2 h at room temperature. The NCP was sliced vertically, and the strips were incubated individually for 1 h at room temperature with sera obtained from patients and diluted 1:200 in TBS-BSA-T. The strips were washed six times for 10 min each time with TBS-BSA-T and then incubated with peroxidase- anti-human IgG (1:1,000 dilution) (A-8667; Sigma) in TBS-BSA-T for 1 h. The strips were washed again and incubated with the substrate (3,3′-diaminobenzidine · 4HCl [Sigma] at 0.5 mg/ml and 10 μl of H2O2 in PBS). After color development, the strips were rinsed extensively with distilled water.
For the IgG subclass determination, after incubation with sera diluted 1:50 for 2 h, the strips were washed as described above. Then, antisubclass antibodies (anti-human IgG1 [I9388], IgG2 [I5635], IgG3 [I9763], or IgG4 [I9888]; Sigma) diluted 1:2,000 were added. After 1 h of incubation at 37°C, the strips were washed. Then, sheep anti-mouse IgG- peroxidase (1:1,000 dilution) (A6782; Sigma) was added. The subsequent steps were the same as those described for IgG.
ELISA for the determination of specific IgG.
For the IgG enzyme-linked immunosorbent assay (ELISA), 96-well plates (Greiner, Kremsmünster, Austria) were coated with P. brasiliensis gp43 at 2 μg/ml. The assays were performed as described previously (20). The samples were run in duplicate. The cutoff was determined to be the mean plus 2 standard deviations of the absorbance obtained with sera from 23 healthy individuals. Samples were considered positive when the absorbances were higher than 0.320 at a 1:800 serum dilution. The IgG titer was established as the highest dilution of serum with an absorbance higher than the cutoff.
ELISAs for the determination of IgG subclasses.
Ninety-six-well plates were coated with P. brasiliensis gp43 at 2 μg/ml in 0.1 M carbonate buffer (pH 9.6) overnight at 4°C. The plates were washed with PBS- 0.05% Tween 20 (PBS-T). The remaining binding sites were blocked with PBS-T- 5% nonfat dry milk for 2 h at 37°C. After three washes with PBS-T, serum samples (1:50 dilution) were added in duplicate, and the plates were incubated for 2 h at 37°C. The wells were washed as described above, and antisubclass antibodies (anti-human IgG1, IgG2, IgG3, or IgG4; Sigma) were added. After 1 h of incubation at 37°C, the plates were washed again, and sheep anti-mouse IgG- peroxidase (1:1,000 dilution) was added. After 1 h of incubation at 37°C and three washes with PBS-T, the substrate solution (o-phenylenediamine in 0.1 M citrate-phosphate buffer [pH 5.0] and 10 μl of 30% H2O2) was added, and the reaction was stopped by the addition of 2 N H2SO4. The optical densities (ODs) were read with an ELISA reader (SLT Spectra; SLT Instruments, Salzburg, Austria) at 492 nm. Results were expressed as the absorbance index (AI), a numerical value calculated by dividing the net absorbance of each test serum sample by the net absorbance of a positive reference serum pool on each plate and multiplying the resulting value by 100. The AI is an arbitrary value that is linearly related to the antibody concentration and allows the comparison of sera tested on different plates and in different experiments (20). The reference serum pool was composed of a mixture of four sera: two from JF and two from AF patients with PCM. The same pool was used throughout the study.
Treatment of gp43 with sodium metaperiodate.
The treatment of gp43 with sodium metaperiodate was previously described by Puccia et al. (26), who showed the elimination of cross-reactions with samples from patients with other mycoses due to the removal of periodate-sensitive carbohydrate epitopes containing galactosyl residues. After overnight coating with gp43 (2 μg/ml) at 4°C, half of the ELISA plate was treated with 40 mM sodium metaperiodate in 1 M citrate buffer (pH 4.5) for 30 min in the dark. Individual serum samples were added to the corresponding wells on both the metaperiodate-treated and the untreated halves of the plate. The subsequent steps were the same as those described above. The titers of the samples were determined, and the decrease in the absorbance of metaperiodate-treated samples was calculated by dividing the result for the metaperiodate-treated well by the result for the untreated well and multiplying the resulting value by 100.
Determination of avidity of anti-gp43 antibodies.
The avidity of anti-gp43 antibodies was estimated from their capacity to remain bound to the antigen in the presence of 6.0 M urea and was measured as described by Jenum et al. (17). Each serum sample was analyzed in a twofold dilution range (rows A and B, starting at 1:200). After incubation, row A was washed with PBS-T containing 6 M urea, whereas row B was washed with washing solution not containing urea. The subsequent steps of the reaction were the same as those of the standard ELISA. For each serum sample, two end-point titers (rows A and B) were calculated with the following formula: titer = dilutionx−1 × 10n; in this equation, dilutionx is the highest dilution giving an OD of >0.32 (cutoff) and n is equal to log2 × [(ODx − 0.32)/(ODx − ODy)], where ODx is the OD at dilutionx and ODy is the OD at the next higher dilution from dilutionx. The IgG avidity was calculated with the following formula: avidity = (titer in row A/titer in row B) × 100.
Statistical methods.
The nonparametric Mann-Whitney or Kruskal-Wallis test was used to compare the variables between groups, and the nonparametric Wilcoxon test was used to compare the response of antibodies against the metaperiodate-treated antigen to that of antibodies against the untreated antigen. Significance was defined as a P value of ≤0.05.
RESULTS
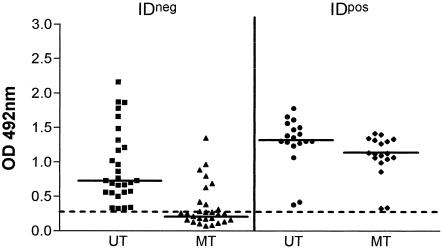
In ELISAs with gp43 from P. brasiliensis as the antigen, sera from IDneg patients were positive, although they had lower IgG titers than IDpos sera. Interestingly, when sera were tested with gp43 treated with sodium metaperiodate, the absorbance values of IDneg sera dropped significantly, reaching ODs below the cutoff (0.320). On the other hand, IDpos sera, although showing a significant decrease in absorbance values, maintained ODs above the cutoff (Fig. 1).
FIG. 1.
Metaperiodate-treated (MT) or untreated (UT) anti-gp43 IgG in IDneg and IDpos sera. Results are expressed as the absorbance at a 1:800 dilution. The horizontal bars represent the median, and the dotted line represents the cutoff (0.320). For the Wilcoxon test of UT IDneg versus MT IDneg sera, the P value was <0.0001; for UT IDpos versus MT IDpos sera, the P value was <0.0001.
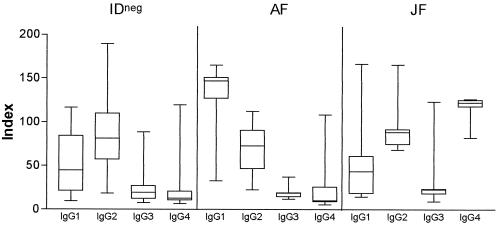
Sodium metaperiodate oxidizes carbohydrate epitopes, reducing the binding of antibodies directed against this portion of proteins. As IgG2 is preferentially produced against carbohydrate antigens, we decided to verify the profiles of IgG subclasses produced by the IDneg patients. As shown in Fig. 2, IDneg patients predominantly produced IgG2 antibodies against gp43, whereas IDpos patients produced more IgG1 (AF patients) or IgG2 and IgG4 (JF patients).
FIG. 2.
Levels of anti-P. brasiliensis gp43 IgG1, IgG2, IgG3, and IgG4 in sera from IDneg patients and IDpos patients (AF and JF of PCM). Results are expressed as the AI. The horizontal bars in the boxes represent the median, and the error bars represent the standard deviations.
When the IgG ELISA was performed with gp43 treated with sodium metaperiodate, we observed a large decrease in the absorbance values of the IDneg sera. Furthermore, the ELISAs for subclasses showed a decrease in IgG2 absorbance values in IDneg and AF (IDpos) patients, supporting the anticarbohydrate nature of these antibodies. Interestingly, besides the decrease in IgG2 reactivity, IDneg sera showed a remarkable decrease in IgG1 reactivity compared with the other groups (Table 1).
TABLE 1.
Effect of P. brasiliensis gp43 treatment with sodium metaperiodate on ELISA resultsa
| Serum sample | % Decrease in absorbance or AI for:
|
||||
|---|---|---|---|---|---|
| Total IgG | IgG1 | IgG2 | IgG3 | IgG4 | |
| IDneg | 56b | 56b | 63c | 42 | 25 |
| IDpos | |||||
| AF | 15 | 21 | 51c | 26 | 22 |
| JF | 15 | 24 | 23 | 34 | 9 |
Assays were performed with serum samples from IDneg patients and IDpos patients (AF and JF of PCM). The data represent the decrease in the absorbance at a 1:800 dilution (OD at 492 nm) for total IgG and the AI for IgG subclasses after treatment of P. brasiliensis gp43 with sodium metaperiodate.
The P value was ≤0.05 for a comparison with the AF or JF group.
The P value was ≤0.05 for a comparison with the JF group.
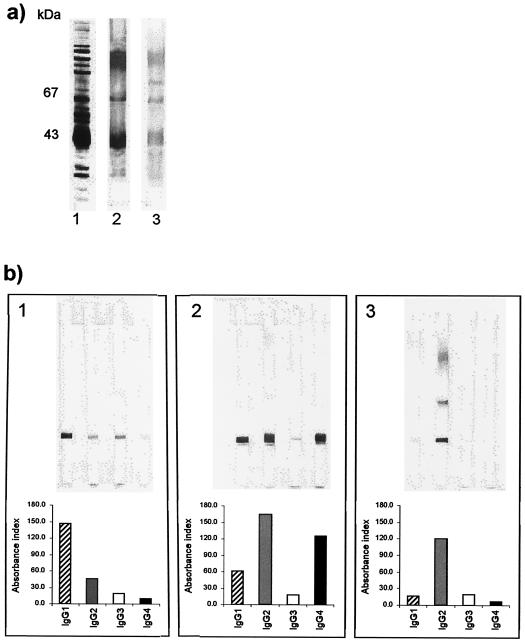
To determine whether the differences in the subclass responses measured by ELISAs were due to differences in the antigen fraction recognized, we examined the antibody response to a P. brasiliensis crude antigen or CFA. Western blot analysis revealed the same band patterns for all groups (Fig. 3a). The subclass immunoblots showed the same patterns of subclass distribution as those observed in the ELISAs, with all sera recognizing mainly the 43-kDa fraction (Fig. 3b).
FIG. 3.
Antibody response to a P. brasiliensis crude antigen or CFA. (a) Lane 1, SDS-PAGE of P. brasiliensis CFA (silver stain); lanes 2 and 3, IgG immunoblot of representative IDneg and IDpos sera. (b) Top: IgG subclass immunoblots of a representative serum sample from one patient in each group: 1, AF PCM; 2, JF PCM; and 3, IDneg. Bottom: respective ELISA results expressed as the AI.
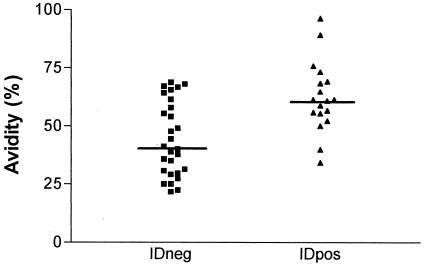
Additionally, IDpos and IDneg sera were tested for antibody avidity, since low avidity could be involved in a lack of reactivity in ID tests. We observed that the antibodies in IDneg sera had a lower avidity than those in IDpos sera (Fig. 4).
FIG. 4.
IgG avidity of IDneg and IDpos sera. The horizontal bars represent the median. In a comparison of IDneg versus IDpos sera, the P value was 0.0018.
DISCUSSION
ID is the most widely used serologic test for the diagnosis and follow-up of treatment of PCM. However, despite its low cost and simplicity, several studies have demonstrated the occurrence of false-negative results with this method (8, 10, 11).
Differences in the profiles of antibodies produced by patients with the JF and AF of PCM were previously described (20). IDneg patients, although presenting clinical symptoms of the AF of PCM, predominantly produce IgG2 against P. brasiliensis gp43, whereas IDpos patients produce mainly IgG1. gp43 is a glycoprotein of 43,000 Da that can be purified from the supernatant fluid of yeast cell cultures by affinity chromatography. This molecule specifically binds to the extracellular matrix protein laminin, influencing pathogenesis (18, 32). Moreover, gp43 is the immunodominant antigen in PCM, being recognized by almost all sera from patients with the disease (2).
The most interesting result obtained in the present study was that antibodies produced by IDneg patients were predominantly directed against carbohydrate epitopes, since the treatment of gp43 with sodium metaperiodate resulted in a significant decrease in absorbance in the IgG ELISA. Periodate treatment can be used to identify antibody responses directed against carbohydrate-containing epitopes on various glycoconjugates by associating the oxidation of vicinal hydroxyl groups on sugars with dialdehydes, causing a loss of antibody recognition (4, 23).
It is known that IgG2 antibodies are preferentially directed against carbohydrate antigens, a fact confirmed here by the observation of the decrease in the reactivity of these antibodies against sodium metaperiodate-treated gp43. In IDpos sera from AF patients, a marked decrease in IgG2 but not in IgG1 levels was observed after this treatment. As a result, the total IgG ELISA values did not drop significantly, since IgG1 is predominant in these sera. In IDpos sera from JF patients, the levels of IgG2 were similar to those in IDneg sera; however, the decrease in reactivity after metaperiodate treatment was small, indicating that IgG2 antibodies were probably directed against noncarbohydrate epitopes. On the other hand, in IDneg sera, all IgG subclasses (IgG1 in particular) showed reduced reactivity against sodium-metaperiodate treated gp43, indicating that these sera produce antibodies predominantly directed against carbohydrate epitopes. Additionally, since IDneg sera did not react in ID tests but did react in ELISAs, it is possible that the carbohydrate epitopes in the gp43 molecule are exposed more when the antigen is coupled to a solid phase, whereas in solution they are unavailable for antibody binding (28).
Antigen treatment with sodium metaperiodate was previously used to improve the specificity of serologic tests for the diagnosis of several diseases, such as histoplasmosis (33), schistosomiasis (23), and PCM (30). The treatment of gp43 with sodium metaperiodate or the use of deglycosylated gp43 abolishes the cross-reactions caused by sera from patients with other systemic mycoses, such as hystoplasmosis or Jorge Lobos’ disease, in ELISAs (26, 31), since the carbohydrate motifs of gp43 are shared by numerous microorganisms. In the present study, sodium metaperiodate treatment was also able to abolish the cross-reactions caused by sera from patients with other pathologies (data not shown). These findings confirm the importance of the carbohydrate epitopes of gp43, which were responsible for the cross-reactions observed in the ELISAs.
We also analyzed the avidity of antibodies in both groups, since it is known that low-avidity antibodies may interfere with ID tests. We found that IDneg sera had a lower avidity than IDpos sera. As a rule, low-avidity antibodies predominate during acute-phase infection, whereas high-avidity ones are produced later (1, 25). PCM is a long-term chronic disease in which patients are constantly exposed to fungal antigens. In view of this fact, the presence of low-avidity antibodies in patients with PCM was unexpected but was explained by the predominance of IgG2. Low-avidity IgG2 has been described for malaria and other infections (12, 29).
In conclusion, we observed that the lack of reactivity in ID tests of sera from patients with proven PCM may be related to the production of low titers of low-avidity IgG2 antibodies directed against carbohydrate epitopes. To avoid this problem, some authors have proposed the combined use of techniques with high specificities, such as ID, and with high sensitivities, such as ELISAs, with cross-reactions being abolished by the use of antigens treated with sodium metaperiodate or in a deglycosylated form (26, 31). However, our results showed that for some patients who preferentially produce low-avidity anti-P. brasiliensis IgG2 (IDneg patients), sodium metaperiodate treatment also abolishes reactivity in ELISAs, thus impairing assay sensitivity.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
REFERENCES
- 1.Bachmann, M. F., U. Kalinke, A. Althage, G. Freer, C. Burkhart, H. P. Roost, M. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024-2027. [DOI] [PubMed] [Google Scholar]
- 2.Blotta, M. H. S. L., and Z. P. Camargo. 1993. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationship with clinical forms of paracoccidioidomycosis. J. Clin. Microbiol. 31:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blotta, M. H. S. L., R. L. Mamoni, S. J. Oliveira, S. Nouér, P. M. O. Papaiordanou, A. Goveia, and Z. P. Camargo. 1999. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in southeast region. Am. J. Trop. Med. Hyg. 61:390-394. [DOI] [PubMed] [Google Scholar]
- 4.Bobbit, J. M. 1956. Periodate oxidation of carbohydrates. Adv. Carbohydr. Chem. Biochem. 11:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brummer, E., E. Castaneda, and A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo, Z. P., J. L. Gesztesi, E. C. Saraiva, C. P. Taborda, A. P. Vicentini, and J. D. Lopes. 1994. Monoclonal antibody capture enzyme immunoassay for detection of Paracoccidioides brasiliensis antibodies in paracoccidioidomycosis. J. Clin. Microbiol. 32:2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo, Z. P., C. Unterkircher, S. P. Campoy, and L. R. Travassos. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J. Clin. Microbiol. 26:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cataldo, F., and D. Paternostro. 1990. Le sottoclassi delle IgG e loro significato clinico. Minerva Pediatr. 42:509-514. [PubMed] [Google Scholar]
- 10.Del Negro, G. M. B., G. Benard, C. M. Assis, M. S. M. Vidal, N. M. Garcia, C. Otani, M. A. Shikanaia-Yasuda, and C. S. Lacaz. 1995. Lack of reactivity of paracoccidioidomycosis sera in the double immunodiffusion test with the gp43 antigen: report of two cases. J. Med. Vet. Mycol. 33:113-116. [DOI] [PubMed] [Google Scholar]
- 11.Del Negro, G. M. B., N. M. Garcia, E. G. Rodrigues, I. N. Cano, M. S. M. V. Aguiar, V. S. Lírio, and C. S. Lacaz. 1991. The sensitivity, specificity and efficiency values of some serological tests used in the diagnosis of paracoccidioidomycosis. Rev. Inst. Med. Trop. São Paulo 33:277-280. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira, M. U., E. A. S. Kimura, J. M. de Souza, and A. M. Katzin. 1996. The isotype composition and avidity of naturally acquired anti-Plasmodium falciparum antibodies: differential patterns in clinically immune Africans and Amazonian patients. Am. J. Trop. Med. Hyg. 55:315-323. [DOI] [PubMed] [Google Scholar]
- 13.Franco, M., M. R. Montenegro, R. P. Mendes, S. A. Marques, N. L. Dillon, and N. G. S. Mota. 1987. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev. Soc. Bras. Med. Trop. 20:129-132. [DOI] [PubMed] [Google Scholar]
- 14.Freitas da Silva, G., and M. C. Roque Barreira. 1992. Antigenemia in paracoccidioidomycosis. J. Clin. Microbiol. 30:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia, N. M., G. M. Del Negro, H. P. Martins, and C. S. Lacaz. 1987. Detection of paracoccidioidomycosis circulating antigen by the immunoelectroosmophoresis-immunodiffusion technique. Preliminary report. Rev. Inst. Med. Trop. São Paulo 29:327-328. [DOI] [PubMed] [Google Scholar]
- 16.Gomez, B. L., J. I. Figueroa, A. J. Hamilton, B. Ortiz, M. A. Robledo, R. J. Hay, and A. Restrepo. 1997. Use of monoclonal antibodies in diagnosis of paracoccidioidomycosis: new strategies for detection of circulating antigens. J. Clin. Microbiol. 35:3278-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenum, P. A., B. Stray-Pedersen, and A. G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes, J. D., M. C. Moura-Campos, A. P. Vicentini, J. L. Gesztesi, W. de Souza, and Z. P. Camargo. 1994. Characterization of glycoprotein gp43, the major laminin-binding protein of Paracoccidioides brasiliensis. Braz. J. Med. Biol. Res. 27:2309-2313. [PubMed] [Google Scholar]
- 19.Mamoni, R. L., C. L. Rossi, Z. P. Camargo, and M. H. S. L. Blotta. 2001. Capture enzyme-linked immunosorbent assay to detect specific immunoglobulin E in sera of patients with paracoccidioidomycosis. Am. J. Trop. Med. Hyg. 65:237-241. [DOI] [PubMed] [Google Scholar]
- 20.Mamoni, R. L., S. A. Nouer, S. J. Oliveira, C. C. Musatti, C. L. Rossi, Z. P. Camargo, and M. H. S. L. Blotta. 2002. Enhanced production of specific IgG4, IgE, IgA and TGF-β in sera from patients with the juvenile form of paracoccidioidomycosis. Med. Mycol. 40:153-159. [DOI] [PubMed] [Google Scholar]
- 21.Martins, R., S. Marques, M. Alves, D. Fecchio, and M. F. Franco. 1997. Serological follow-up of patients with paracoccidioidomycosis treated with itraconazole using dot-blot, ELISA and Western-blot. Rev. Inst. Med. Trop. São Paulo 39:261-269. [DOI] [PubMed] [Google Scholar]
- 22.Mendes-Giannini, M. J., J. P. Bueno, M. A. Shikanai-Yasuda, A. W. Ferreira, and A. Masuda. 1989. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J. Clin. Microbiol. 27:2842-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noya, B. A., C. Colmenares, H. Lanz, M. A. Carcchiolo, S. Losada, and O. Noya. 2000. Schistosoma mansoni: immunodiagnosis is improved by sodium metaperiodate, which reduces cross-reactivity due to glycosylated epitopes of soluble egg antigen. Exp. Parasitol. 95:106-112. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira, S. J., R. L. Mamoni, C. C. Musatti, P. M. O. Papaiordanou, and M. H. S. L. Blotta. 2002. Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: comparison with infected and non-infected controls. Microbes Infect. 4:139-144. [DOI] [PubMed] [Google Scholar]
- 25.Persson, M. A. A., S. E. Brown, M. W. Steward, L. Hammarström, C. I. E. Smith, C. R. Howard, M. Wahl, B. Rynnel-Dagöö, G. Lefranc, and A. O. Carbonara. 1988. IgG subclass-associated affinity differences of specific antibodies in humans. J. Immunol. 140:3875-3879. [PubMed] [Google Scholar]
- 26.Puccia, R., and L. R. Travassos. 1991. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J. Clin. Microbiol. 29:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snapper, C. M., and J. J. Mond. 1993. Towards a comprehensive view of immunoglobulin class switching. Immunol. Today 14:15-17. [DOI] [PubMed] [Google Scholar]
- 28.Soderquist, M. E., and A. G. Walton. 1980. Structural changes in proteins adsorbed on polymer surfaces. J. Colloid Interface Sci. 75:386-397. [Google Scholar]
- 29.Sterla, S., H. Sato, and A. Nieto. 1999. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 21:27-34. [DOI] [PubMed] [Google Scholar]
- 30.Taborda, C. P., and Z. P. Camargo. 1993. Diagnosis of paracoccidioidomycosis by passive haemagglutination assay of antibody using a purified and specific antigen—gp43. J. Med. Vet. Mycol. 31:155-160. [DOI] [PubMed] [Google Scholar]
- 31.Taborda, C. P., and Z. P. Camargo. 1994. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 32:554-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicentini, A. P., J. L. Gesztesi, M. F. Franco, W. de Souza, J. Z. de Moraes, L. R. Travassos, and J. D. Lopes. 1994. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect. Immun. 62:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zancope-Oliveira, R. M., S. L. Bragg, E. Reiss, B. Wanke, and M. Peralta. 1994. Effects of histoplasmin M antigen chemical and enzymatic deglycosylation on cross-reactivity in the enzyme-linked immunoelectrotransfer blot method. Clin. Diagn. Lab. Immunol. 1:390-393. [DOI] [PMC free article] [PubMed] [Google Scholar]