Abstract
Endemic pemphigus foliaceus (EPF) is an autoimmune disease characterized by blister formation with a loss of cohesion and infiltration of inflammatory cells. We observed that supernatants of peripheral blood mononuclear cells from patients produced significantly more interleukin-1β (IL-1β) than those from stimulated healthy controls. Furthermore, a Th2 bias was observed in EPF patients when the IL-5/gamma interferon ratio was analyzed. These results indicate that cells from pemphigus patients react with a vigorous proinflammatory response.
Endemic pemphigus foliaceus (EPF), also known as “fogo selvagem,” is a chronic autoimmune disease characterized by acantholysis and the formation of intraepidermal blisters (23). Lesions appear on the face and can later spread, without reaching mucous tissues (19). In severe cases, it can spread to the scalp, face, dorsum, and arms and legs. The disease can be treated with the administration of glucocorticosteroids (6, 19) and other immunosuppressive drugs in more severe cases (3).
The rate of mortality caused by bullous skin diseases, including EPF, has been estimated to be about 10 to 40% and is mainly due to complications resulting from secondary infections (15). With the use of glucocorticosteroid therapy, the prognosis for this disease has been improved significantly.
EPF is associated with the presence of immunoglobulin G4 (IgG4) antibodies against antigens associated with the desmosomes (desmoglein 1) that promote an inflammatory reaction in the presence of lymphocytes, neutrophils, and eosinophils (7, 11). The lesions generate a decrease in the adhesion between the keratinocytes of the granular layer of the epidermis, with an increase in the intercellular space due to the reduction or the total disappearance of the desmosomes (10, 14, 19, 23, 24). The bullous skin breakup leads to superficial erosions, which result in diffuse erythema and the formation of crusts (19). Within the blister cavity, the cells observed in association with acantholysis have hyperchromatic nuclei and homogeneous cytoplasms.
The role of antibodies in the pathogenesis of the lesions was demonstrated by the injection of serum from patients with pemphigus into mice. These animals developed a disease that reproduced pemphigus lesions, indicating that the antibodies play an essential role in this disease (1).
The participation of the cell-mediated immune response in bullous skin disease has been shown. Peripheral blood mononuclear cells (PBMCs) from EPF patients proliferate after being stimulated with desmoglein epitopes. These cells exhibit a Th2 phenotype (17). Moreover, the development of bullous skin lesions after alpha interferon (IFN-α) therapy for hepatitis C or Kaposi's sarcoma (21, 22) suggests the involvement of cytokines in the disease.
In the study described here, we addressed other mediating factors that may be involved in the development of tissue lesions. We investigated the production of interleukin-1β (IL-1β), IL-4, IL-5, IL-10, IFN-γ, and tumor necrosis factor alpha (TNF-α) in PBMCs from EPF patients. The results show that IL-1β levels are significantly higher in EPF patients even during glucocorticosteroid therapy and that the cytokine balance favors Th2.
MATERIALS AND METHODS
Subjects.
Patients diagnosed with pemphigus foliaceus were invited to participate in this study. Ten patients with active lesions (9 with blisters and crusts and 1 with crusts only) and 15 control subjects were analyzed. The control subjects were matched with the patients by age and gender. The ages of the patients, who were receiving therapy with the glucocorticosteroid methylprednisolone, ranged from 21 to 88 years, and five were males.
Blood for PBMC purification was collected by venipuncture with a 20-ml heparinized syringe.
This study was approved by the Ethical Committee of the Faculdade de Medicina do TriÂngulo Mineiro, Minas Gerais, Brazil.
Cell culture.
PBMCs were isolated from heparinized blood by centrifugation at 400 × g for 20 min at room temperature in Ficoll-Paque (Sigma, St. Louis, Mo.), followed by three washes with RPMI (Gibco, Grand Island, N.Y.) medium. The pellets were resuspended in RPMI supplemented with 50 mM 2-mercaptoethanol (Sigma), 2 mM l-glutamine (Sigma), 40 μg of gentamicin per ml, and 5% fetal calf serum (Gibco) (complete medium).
For supernatant production, 2 × 106 cells per ml were cultured in a 24-well microplate in the presence of medium alone, 5 μg of phytohemagglutinin (PHA; Sigma) per ml, or 5 μg of lipopolysaccharide (LPS; Sigma) per ml. The plates were incubated at 37°C in a 5% CO2 atmosphere. At 48 and 120 h, the supernatants were collected, centrifuged, and stored at −70°C for analysis of cytokine production.
Cytokine titration.
For cytokine titration, microplates (Nunc, Roskilde, Denmark) were sensitized overnight with anti-IL-1β monoclonal antibody (MAb; Genzyme, Cambridge, Mass.), anti-TNF-α MAb (Genzyme), anti-IL-4 MAb (Mabtech, Nacka, Sweden), anti-IL-5 MAb (Pharmingen, San Diego, Calif.), anti IL-10 MAb (Pharmingen), or anti-IFN-γ MAb (Mabtech). Nonspecific binding was prevented by incubating the plates with 3% bovine serum albumin (Sigma) in phosphate-buffered saline (PBS). The plates were incubated overnight with 100 μl of a 1:2 dilution of culture supernatant in PBS, 2% bovine serum albumin, and standard cytokines (Pharmingen and R&D, Minneapolis, Minn.). The plates were then washed four times with 0.05% Tween in PBS and incubated with rabbit anti-IL-1β antibody (Genzyme), rabbit anti-TNF-α antibody (Genzyme), biotinylated anti-IL-4 MAb (Mabtech), biotinylated anti-IL-5 MAb (Pharmingen), biotinylated anti-IL-10 MAb (Pharmingen), or biotinylated anti-IFN-γ MAb (Mabtec) for 4 h. The plates were then washed and incubated for 2 h with alkaline phosphatase-conjugated goat anti-rabbit IgG (Pierce, Rockford, Ill.) or alkaline phosphatase conjugated to streptavidin. Finally, the plates were washed four times, and enzymatic activity was developed by incubating the plates with p-nitrophenyl phosphate (Sigma). The absorbance at 405 nm was read in a microplate reader (Bio-Rad, Hercules, Calif.). The sensitivity of the tests was 10 to 30 pg/ml.
Statistical analysis.
Differences in cytokine levels between patients and controls were analyzed by the Mann-Whitney test, with significance set at a P value of <0.05. The balance between Th2 and Th1 cells was expressed by the ratio of IL-5 and IFN-γ levels, and differences between IL-5/IFN-γ ratios were analyzed by an unpaired t test, with significance set at a P value of <0.05.
RESULTS
Production of inflammatory cytokines.
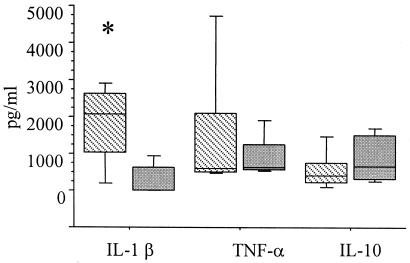
All patients included in this study presented with active lesions, and diagnosis was performed by clinical anatomicopathological examination. PBMCs derived from patients and healthy controls were stimulated with PHA or LPS for 48 or 120 h, and the supernatants were tested for the presence of regulatory and inflammatory cytokines. The results in Fig. 1 show that after LPS stimulation, the IL-1β level was significantly higher in the supernatants from pemphigus patients than in those from the healthy controls (P < 0.05). The levels of IL-10 after PHA stimulation did not differ between the patients and the controls. These results indicate a proinflammatory response by pemphigus patients even while they are receiving glucocorticosteroid therapy.
FIG. 1.
Production of cytokines involved in the inflammatory reaction after stimulation with LPS and PHA. PBMCs from patient (hatched bars) and control subjects (gray bars) were cultured in the presence of PHA or LPS for 48 h. The cytokine levels in the supernatants (in picograms per milliliter) were determined by ELISA. Horizontal lines represent median values, boxes represent 25th to 75th percentiles, and vertical lines represent 10th to 90th percentiles. Statistical significance between groups is indicated (*, P < 0.05, Mann-Whitney test).
Th1-Th2 cytokine balance.
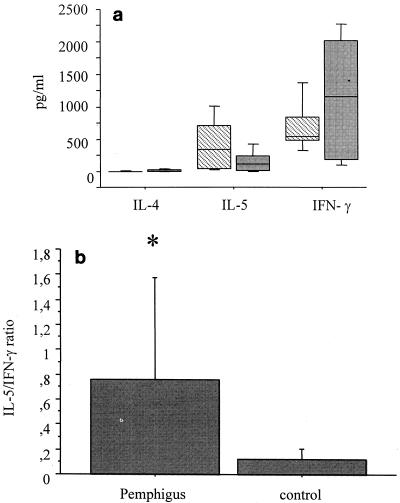
PBMCs derived from patients and healthy controls were stimulated with PHA for 48 or 120 h, and the supernatants were tested for the presence of regulatory cytokines. After PHA stimulation, the levels of IL-4, IL-5, and IFN-γ did not differ significantly between the patients and the healthy controls (Fig. 2a). The Th1-Th2 cytokine balance was analyzed by determination of the IL-5/IFN-γ ratio. As shown in Fig. 2b, patients had a cytokine balance that favored Th2, with the levels of Th2 cytokines being significantly higher for the patients than for the controls.
FIG. 2.
Production of regulatory cytokines after stimulation with PHA. PBMCs from patients (hatched bars) and control subjects (gray bars) were cultured in the presence of PHA for 120 h. (a) The cytokine levels in the supernatants (in picograms per milliliter) were determined by ELISA. Horizontal lines represent median values, boxes represent 25th to 75th percentiles, and vertical lines represent 10th to 90th percentiles. (b) IL-5/IFN-γ ratios were obtained by dividing the IL-5 levels by the IFN-γ levels in cultured supernatants stimulated with PHA. Bars represent averages, and vertical lines represent standard deviations. Statistical significance between groups is indicated (*, P < 0.05, the Student t test).
DISCUSSION
The prevalence of antibodies against desmoglein 1 is higher among healthy individuals who live in areas where pemphigus foliaceus is endemic. The beginning of the disease is preceded by the continuous production of these types of antibodies (24). This suggests that mediating factors other than antibodies may be involved in disease development. Due to its endemic nature, it is suspected that some environmental factor has a role in the pathogenesis of the disease. Since some subjects live in areas of endemicity and do not develop skin lesions, the involvement of genetic factors may also contribute to the susceptibility to this disease. There is evidence that disease susceptibility is under the control of multiple genes (5) and is associated with HLA-DR1, which is a known susceptibility factor (20).
In this study, significantly higher levels of IL-1β were observed in EPF patients after stimulation of their PBMCs with LPS. IL-1β is a cytokine involved in inflammatory responses, and its presence may influence the lesion formation mechanism because of its ability to stimulate the expression of intercellular adhesion molecules and the production of chemotactic peptides, such as monocyte chemoattractant protein 1, by fibroblasts and epithelial cells (13, 16). Inflammatory mediators such as IL-1, prostaglandins, thromboxanes, and leukotrienes were previously observed in patients with bullous skin diseases. Moreover, the role of IL-1 has been demonstrated in vivo and in vitro (2). Furthermore, the development of lesions by injection of patient serum into mice was less intense in IL-1-knockout mice than in wild-type mice (8). These mediators are present at various concentrations, depending on the duration of blister development (10). Keratinocytes have been recognized as a source and target of a large variety of cytokines. Keratinocytes express type I and type II IL-1 receptors, and the presence of caspase 1 in keratinocytes has been observed under inflammatory conditions, suggesting its ability to activate IL-1 (12).
Although the levels of regulatory cytokines were not significantly different between the two groups evaluated in this study, IFN-γ levels were lower in EPF patients, while IL-5 levels were higher in these patients. A significant difference in the Th1-Th2 cytokine balance was observed when cytokine levels were analyzed on the basis of IL-5/IFN- γ ratios in supernatants after stimulation with PHA for 120 h. EPF patients produced cytokines with both the Th1 and the Th2 patterns, although a balance in favor of Th2-like cytokines was observed. Conversely, healthy controls had a balance in favor of Th1-like cytokines. This may reflect the importance of Th2 cytokines in the production of antibodies, especially IgG4, a Th2-related immunoglobulin isotype (9). Regulatory cytokines were previously observed to play a role in the production of cytokines with both the Th1 and the Th2 patterns in patients with pemphigus vulgaris, with the Th2 cytokine response being dominant (4, 18).
In conclusion, the results presented here support the hypothesis that patients with EPF present a Th2-like cytokine response and that other mediators, such as IL-1β, a proinflammatory cytokine, may participate in the tissue damage observed in patients with this disease. If the role of IL-1β is confirmed by further investigations, it may allow the introduction of new therapeutic approaches for the management of EPF patients.
Acknowledgments
This work was supported by FAPEMIG. G. Paschoini was supported by CNPq/PIBIC.
REFERENCES
- 1.Amagai, M., T. Nishikawa, H. C. Nousari, G. J. Anhalt, and T. Hashimoto. 1998. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J. Clin. Investig. 102:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhol, K. C., K. Desai, S. Kumari, J. E. Colon, and A. R. Ahmed. 2001. Pemphigus vulgaris: the role of IL-1 and IL-1 receptor antagonist in pathogenesis and effects of intravenous immunoglobulin on their production. Clin. Immunol. 100:172-180. [DOI] [PubMed] [Google Scholar]
- 3.Bystryn, J. C. 1984. Adjuvant therapy of pemphigus. Arch. Dermatol. 120:941-951. [PubMed] [Google Scholar]
- 4.Caproni, M., B. Giomi, C. Cardinali, E. Salvatori, E. Pestelli, A. D'Agata, B. Bianchi, P. Toto, C. Feliciani, and P. Fabbri. 2000. Further support for a role for Th2-like cytokines in blister formation of pemphigus. Clin. Immunol. 98:264-271. [DOI] [PubMed] [Google Scholar]
- 5.Cerna, M., M. Fernandez-Vina, H. Friedman, J. R. Moraes, M. E. Moraes, L. Diaz, and P. Stastny. 1993. Genetic markers for susceptibility to endemic Brazilian pemphigus foliaceus (fogo selvagem) in Xavante Indians. Tissue Antigens 42:138-140. [DOI] [PubMed] [Google Scholar]
- 6.da Cunha, D. F., S. F. da Cunha, J. P. Monteiro, T. P. Ferreira, J. A. dos Santos, R. A. Furtado, R. S. Marssaro, R. A. Muniz, and R. A. da Silva Gomes. 2000. Nutritonal evaluation of pemphigus foliaceus patients on long-term glucocorticoid therapy. Rev. Inst. Med. Trop. São Paulo 42:23-26. [DOI] [PubMed] [Google Scholar]
- 7.D'Auria, L., M. Pietravalle, A. Mastroianni, C. Ferraro, A. Mussi, C. Bonifati, B. Giacalone, and F. Ameglio. 1998. IL-5 levels in the serum and blister fluid of patients with bullous pemphigoid: correlations with eosinophil cationic protein, RANTES, IgE and disease severity. Arch. Dermatol. Res. 290:25-27. [DOI] [PubMed] [Google Scholar]
- 8.Feliciani, C., P. Toto, P. Amerio, S. M. Pour, G. Coscione, P. Amerio, G. Shivji, B. Wang, and D. N. Sauder. 1999. In vitro and in vivo expression of interleukin-1alpha and tumor necrosis factor alpha mRNA in pemphigus vulgaris: interleukin-1alpha and tumor necrosis factor-alpha are involved in acantholysis. J. Investig. Dermatol. 114:71-77. [DOI] [PubMed] [Google Scholar]
- 9.Gascan, H., J. F. Gauchat, M. G. Roncarolo, H. Yssel, H. Spits, and J. E. de Vries. 1991. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J. Exp. Med. 173:747-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grando, S. A. 1989. Fixation of pemphigus vulgaris and foliaceus antibodies in shedding snake epidermis. Dermatologica 178:8-11. [DOI] [PubMed] [Google Scholar]
- 11.Grando, S. A., B. T. Glukhenky, G. N. Drannik, E. V. Epshtein, A. P. Kostromin, and T. A. Korostash. 1989. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch. Dermatol. 125:925-930. [PubMed] [Google Scholar]
- 12.Grone, A. 2002. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 88:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Groves, R. W., E. Ross, J. N. Barker, J. S. Ross, R. D. Camp, and D. M. MacDonald. 1992. Effect of in vivo interleukin-1 on adhesion molecule expression in normal human skin. J. Investig. Dermatol. 98:384-387. [DOI] [PubMed] [Google Scholar]
- 14.Ishii, K., M. Amagai, R. P. Hall, T. Hashimoto, A. Takayanagi, S. Gamou, N. Shimizu, and T. Nishikawa. 1997. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J. Immunol. 159:2010-2017. [PubMed] [Google Scholar]
- 15.Joly, P. 1999. Autoimmune bullous skin diseases. Rev. Med. Interne 20:26-38. (In French.) [DOI] [PubMed]
- 16.Larsen, C. G., C. O. Zachariae, J. J. Oppenheim, and K. Matsushima. 1989. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem. Biophys. Res. Commun. 160:1403-1408. [DOI] [PubMed] [Google Scholar]
- 17.Lin, M. S., C. L. Fu, V. Aoki, G. Hans-Filho, E. A. Rivitti, J. R. Moraes, M. E. Moraes, A. M. Lazaro, G. J. Giudice, P. Stastny, and L. A. Diaz. 2000. Desmoglein-1-specific T lymphocytes from patients with endemic pemphigus foliaceus (fogo selvagem). J. Clin. Investig. 105:207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin, M. S., S. J. Swartz, A. Lopez, X. Ding, M. A. Fernandez-Vina, P. Stastny, J. A. Fairley, and L. A. Diaz. 1997. Development and characterization of desmoglein-3 specific T cells from patients with pemphigus vulgaris. J. Clin. Investig. 99:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moll, R., and I. Moll. 1998. Epidermal adhesion molecules and basement membrane components as target structures of autoimmunity. Virchows Arch. 432:487-504. [DOI] [PubMed] [Google Scholar]
- 20.Moraes, J. R., M. E. Moraes, M. Fernandez-Vina, L. A. Diaz, H. Friedman, I. T. Campbell, R. R. Alvarez, S. A. Sampaio, E. A. Rivitti, and P. Stastny. 1991. HLA antigens and risk for development of pemphigus foliaceus (fogo selvagem) in endemic areas of Brazil. Immunogenetics 33:388-391. [DOI] [PubMed] [Google Scholar]
- 21.Niizeki, H., N. Inamoto, K. Nakamura, K. Tsuchimoto, T. Hashimoto, and T. Nishikawa. 1994. A case of pemphigus foliaceus after interferon alpha-2a therapy. Dermatology 189:129-130. [DOI] [PubMed] [Google Scholar]
- 22.Parodi, A., M. Semino, R. Gallo, and A. Rebora. 1993. Bullous eruption with circulating pemphigus-like antibodies following interferon-alpha therapy. Dermatology 186:155-157. [DOI] [PubMed] [Google Scholar]
- 23.Stanley, J. R., L. Koulu, and C. Thivolet. 1984. Distinction between epidermal antigens binding pemphigus vulgaris and pemphigus foliaceus autoantibodies. J. Clin. Investig. 74:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren, S. J., M. S. Lin, G. J. Giudice, R. G. Hoffmann, G. Hans-Filho, V. Aoki, E. A. Rivitti, V. Santos, L. A. Diaz, et al. 2000. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. N. Engl. J. Med. 343:23-30. [DOI] [PubMed] [Google Scholar]