Abstract
We studied the effects of hypothermia on mortality rate, concentrations of tumor necrosis factor alpha and interleukin 6 in plasma, and the end products of nitric oxide (NO) in endotoxemia. It was found that moderate and mild hypothermia improved the mortality rate and attenuated cytokine responses and the elevation of the end products of NO after endotoxin injection and that these beneficial effects were similar for moderate and mild hypothermia.
Endotoxin shock, a common problem in patients with endotoxemia that often resists even intensive medical treatment, is characterized by profound hypotension, progressive metabolic acidosis, and dysfunction of multiple organs. Although its pathophysiology is not well defined, cytokines and nitric oxide (NO) are considered important in mediating the cardiovascular disturbance (10, 11, 17). Circulating endotoxin increases the production and release of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), which are dilators of resistance vessels in skeletal muscle (5). When macrophages are activated by endotoxin or cytokines, the inducible NO synthase gene is expressed. This, in turn, leads to the production of NO synthase, which, once activated, continuously releases large amounts of NO, with a vasodilating effect (13, 14). Enhanced production of NO thus contributes importantly to cardiovascular dysfunction.
Several investigators have documented that hypothermia has an organ-protective effect in controlled hemorrhagic shock and provides myocardial protection (6, 18). Moreover, several studies have shown that hypothermia delays the induction of proinflammatory cytokines in vitro (7). At the same time, however, several studies have reported that the mortality rate of hypothermic patients with sepsis is twice that of febrile septic patients (2, 4, 9). We studied the effects of hypothermia on mortality rate, concentrations of TNF-α and IL-6 in plasma, and the end products of NO in endotoxin-exposed rats.
MATERIALS AND METHODS
Animal preparation.
All experimental procedures were approved by the Animal Care Committee of Kanazawa University School of Medicine and were in accordance with the National Institutes of Health guidelines for animal use. Male Wistar rats (n = 36) weighing 375 ± 15 g (mean ± standard deviation [SD]) were anesthetized with an intraperitoneal injection of pentobarbital sodium (30 mg/kg of body weight). Ventilation was performed through a tracheotomy. The femoral artery was cannulated to monitor blood pressure and to draw blood samples. Lactated Ringer's solution containing a muscle relaxant (0.02 mg of pancuronium bromide/ml) and pentobarbital sodium (0.5 mg/ml) was infused continuously at a rate of 10 ml · kg−1 · h−1 through a femoral vein cannula because animals anesthetized with this dose of pentobarbital alone did not demonstrate escape behavior. The heart rate (HR) was recorded from lead II of the electrocardiogram. The rats were connected to a pressure-controlled ventilator (Servo 900B; Siemens-Elema, Solna, Sweden) delivering 100% oxygen at a frequency of 30 breaths/min with an inspiratory/expiratory ratio of 1:1. The animals were then rested for at least 15 min to allow hemodynamic parameters to be established, and baseline readings of HR and systolic arterial pressure (SAP) were taken.
Study protocols.
After baseline measurements were taken, the animals were allocated randomly to one of three groups, all of which received endotoxin (n = 12 per group).
Normothermia group.
Endotoxin shock was induced by a bolus injection of endotoxin (15 mg/kg of body weight). We used lipopolysaccharide prepared from Escherichia coli (O111:B4; Difco Laboratories, Detroit, Mich.) as the endotoxin. Rectal body temperatures of this normothermia group were maintained between 36 and 38°C with the aid of a heating pad.
Mild hypothermia group.
Endotoxin shock was induced as for the normothermia group. Immediately after the injection of endotoxin, the animals were cooled externally to maintain rectal body temperatures between 34 and 35°C.
Moderate hypothermia group.
Endotoxin shock was induced as for the normothermia group. Immediately after the injection of endotoxin, the animals were cooled externally to maintain rectal body temperatures between 30 and 31°C.
Arterial blood samples (1.0 ml) were drawn for the measurement of the baseline for cytokine concentrations in plasma 2 and 5 h after the endotoxin injection. Another set of samples (0.5 ml) was drawn for the measurement of the end products of NO 6 h after the endotoxin injection. The total amount of blood drawn from each animal was 4.0 ml over 6 h.
Sample analysis.
The blood used for determination of cytokine concentrations was centrifuged for 10 min at 3,000 × g at 4°C. Plasma was then decanted and stored at −70°C until analysis. Cytokine (TNF-α and IL-6) concentrations were measured by an enzyme-linked immunosorbent assay (BioSource, Camarillo, Calif.). The lower limits of detection were 4.5 pg of TNF-α and 7.0 pg of IL-6/ml. NO-related compounds were measured by a chemiluminescent assay using a nitric oxide analyzer (NOA 280; Sievers Instruments, Boulder, Colo.). Data are reported as nitrite plus nitrate concentrations (NOx) in μmol/liter of plasma.
Statistical analysis.
Data are presented as the means ± SDs. Differences between the groups at baseline were analyzed with the unpaired Student's t test and Mann-Whitney U test. Hemodynamic and cytokine changes during the study were analyzed by means of two-way analysis of variance with repeated measures followed by a post hoc test (Bonferroni's correction). Comparisons of mortality rates among the groups were made by the Kaplan Meier and Mantel-Cox methods. Statistical analyses were performed using the StatView application (Macintosh version 5.0; Abacus Concepts, Berkeley, Calif.). Statistical significance was defined as a P of < 0.05.
RESULTS
Hemodynamics and mortality rate.
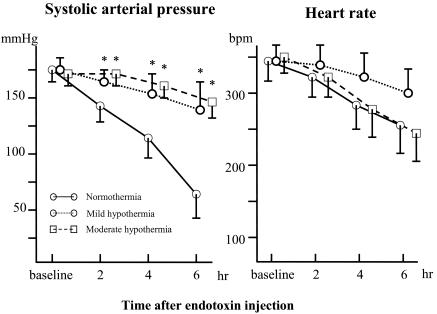
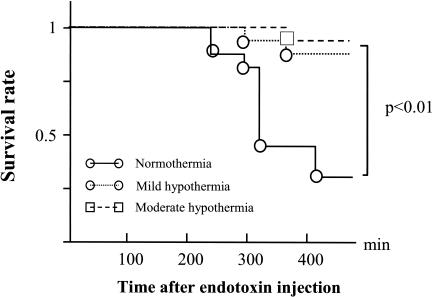
No significant differences were noted in baseline HR or SAP among the groups (Fig. 1). Injections of endotoxin reduced SAP in the normothermia group but not in the mild hypothermia and moderate hypothermia groups. Mortality rates 6 h after endotoxin injection were 75, 16, and 8% for the normothermia, mild hypothermia, and moderate hypothermia groups, respectively (Fig. 2). The mortality rate for the normothermia group was thus significantly higher than that for the other groups (P < 0.001).
FIG. 1.
HR and SAP at baseline and after injection of endotoxin (means ± SDs). An asterisk (*) indicates a P of < 0.05 that SAP will decrease versus that for the normothermia group. bpm, beats per min.
FIG. 2.
Survival curves for the normothermia, mild hypothermia, and moderate hypothermia groups.
Plasma cytokine concentrations.
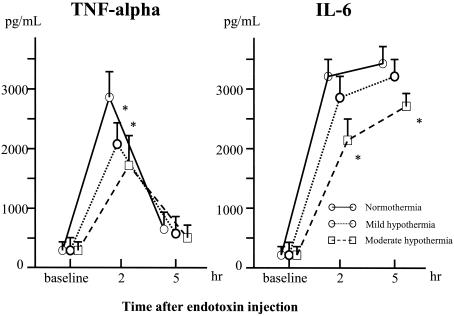
All baseline values for the three groups were similar (Fig. 3). Endotoxin injection increased the TNF-α concentrations in all the groups, but concentrations remained significantly lower in the mild hypothermia and moderate hypothermia groups than in the normothermia group 2 h after injection. Concentrations of IL-6 in plasma became elevated in all the groups. Significantly lower concentrations were seen in the moderate hypothermia group than in the normothermia group (P < 0.01).
FIG. 3.
Changes in concentrations of TNF-α and IL-6 in plasma from baseline after endotoxin injection (means ± SDs). An asterisk (*) indicates a P of < 0.05 that TNF-α and IL-6 concentrations will increase versus those for the normothermia group.
Concentrations of NOx in plasma.
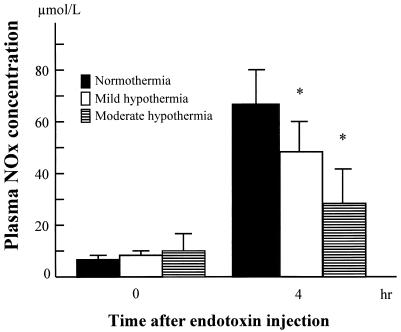
All baseline values were similar for the three groups (Fig. 4). Concentrations of NOx in plasma significantly increased in all the groups 6 h after endotoxin injection, but the concentrations were lower in the mild hypothermia and moderate hypothermia groups than in the normothermia group (P < 0.05).
FIG. 4.
Changes in concentrations of NOx in plasma from baseline to 4 h after endotoxin injection. An asterisk (*) indicates a P of < 0.05 that NOx concentrations will decrease versus those for the normothermia group.
DISCUSSION
Endotoxin produced hypotension as well as increased cytokine and NOx concentrations in plasma in rats. Moreover, the mortality rate 6 h after endotoxin injection was very high. With moderate and mild hypothermia, however, these endotoxin-induced changes were attenuated. In addition, the mortality rates for moderate- and mild-hypothermic rats were significantly lower than those for normothermic rats. These inhibitory effects of moderate and mild hypothermia were the most important findings of our study.
Several investigators have shown that hypothermia has beneficial effects on endotoxemia, and their clinical studies have demonstrated that hypothermia improves outcomes for patients in comas after resuscitation (3) and that moderate hypothermia during cardiopulmonary bypass surgery reduces myocardial cell damage and myocardial cell death (18). On the other hand, several studies have found that hypothermia with endotoxemia has a deteriorating effect on hemodynamics and survival rate. Marik and Zaloga observed that hypothermic patients in septic shock had a higher mortality rate with a higher incidence of organ dysfunction than febrile septic shock patients (9), while other studies have reported that the mortality rate of hypothermic patients with sepsis was twice that of febrile septic patients (2, 4). Thus, it is inconclusive whether hypothermia is beneficial or not, although our study demonstrated that moderate and mild hypothermia improved the survival rate during endotoxemia in rats.
Of particular interest are the anti-inflammatory effects of hypothermia on endotoxemia observed in our study. Several investigators have examined the relationship between cytokine responses and hypothermia. Kimura et al. (7) showed that moderate hypothermia delayed the induction of proinflammatory cytokines in human peripheral blood mononuclear cells in vitro, while several animal and clinical studies reported that hypothermia attenuated the elevation of circulating concentrations of IL-6 (1). In contrast, Lee et al. (8) showed that hypothermia induced an anti-inflammatory-T-cell-cytokine profile in vitro, and several other reports showed that concentrations of TNF-α and IL-6 in plasma were not significantly different in hypothermic and normothermic septic patients (2, 9). The study presented here showed that moderate and mild hypothermia attenuated the elevation of TNF-α and IL-6 concentrations after endotoxin injection. NOx is known to increase in endotoxemia, causing vasodilation and myocardial toxicity (13, 14). However, there are few reports on the relationship between NO and hypothermia in endotoxemia. Our study showed that, during normothermia, concentrations of NOx in plasma became remarkably elevated but that the elevation of NOx concentrations in plasma is attenuated during moderate and mild hypothermia. These findings suggest that these anti-inflammatory effects of hypothermia may be the reason for the improvement in mortality rates.
We compared the effects of moderate and mild hypothermia on endotoxemia in rats. Qing et al. (12) showed that moderate hypothermia, in contrast to normothermia and deep hypothermia, produced anti-inflammatory effects, including reduction of leukocyte mobilization and inhibition of TNF-α production during cardiac surgery. However, there are few other reports on different degrees of hypothermia. We were able to show that both moderate and mild hypothermia improved mortality rates and attenuated endotoxin-induced changes, including attenuation of TNF-α, IL-6, and NOx production.
Two important and related questions remain unanswered. One is whether hypothermia has similar beneficial effects when induced at different times after endotoxin injection and whether the dose of endotoxin used in the present study is clinically feasible. With regard to endotoxin, previous studies found that the same endotoxin dose as used in the present study caused endotoxin-induced shock at 4 h and a high mortality rate (60 to 80%) at 6 h (15, 16). This dose was obviously high, but we used this dose in the present study in order to evaluate the mortality rate. Moreover, another question, that of species, remains. We used rats as the endotoxin shock model, but the responses to endotoxin in this model may be different from those in humans and other animals. Further studies need to focus on these questions.
In summary, our study showed that moderate and mild hypothermia improved mortality rates and attenuated cytokine responses and NOx elevation in rats receiving a bolus injection of endotoxin. Our findings also indicated that these beneficial effects were similar for moderate and mild hypothermia.
REFERENCES
- 1.Aibiki, M., S. Maekawa, S. Ogura, Y. Kinoshita, N. Kawai, and S. Yokono. 1999. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J. Neurotrauma 16:225-232. [DOI] [PubMed] [Google Scholar]
- 2.Arons, M. M., A. P. Wheeler, G. R. Bernard, B. W. Christman, J. A. Russell, R. Schein, W. R. Summer, K. P. Steinberg, W. Fulkerson, P. Wright, W. D. Dupont, B. B. Swindell, and Ibuprofen in Sepsis Study Group. 1999. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit. Care Med. 27:699-707. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, S. A., T. W. Gray, M. D. Buist, B. M. Jones, W. Silvester, G. Gutteridge, and K. Smith. 2002. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 346:557-563. [DOI] [PubMed] [Google Scholar]
- 4.Clemmer, T. P., C. J. Fisher, R. C. Bone, G. J. Slotman, C. A. Metz, and F. O. Thomas. 1992. Hypothermia in the sepsis syndrome and clinical outcome. Crit. Care Med. 20:1395-1401. [DOI] [PubMed] [Google Scholar]
- 5.Forfia, P. R., X. Zhang, M. Ochoa, X. Xu, R. Bernstein, N. R. Ferreri, and T. H. Hintze. 1998. Relationship between plasma NOx and cardiac and vascular dysfunction after LPS injection in anesthetized dogs. Am. J. Physiol. 274:H193-H201. [DOI] [PubMed] [Google Scholar]
- 6.Gundersen. Y., P. Vaagenes, A. Pharo, E. T. Valo, and P. K. Opstad. 2001. Moderate hypothermia blunts the inflammatory response and reduces organ injury after acute haemorrhage. Acta. Anaesthesiol. Scand. 45:994-1001. [DOI] [PubMed] [Google Scholar]
- 7.Kimura, A., S. Sakurada, H. Ohkuni, Y. Todome, and K. Kurata. 2002. Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit. Care Med. 30:1499-1502. [DOI] [PubMed] [Google Scholar]
- 8.Lee, S. L., F. D. Battistella, and K. Go. 2001. Hypothermia induces T-cell production of immunosuppressive cytokines. J. Surg. Res. 100:150-153. [DOI] [PubMed] [Google Scholar]
- 9.Marik, P. E., and G. P. Zaloga. 2000. Hypothermia and cytokines in septic shock. Inten. Care Med. 26:716-721. [DOI] [PubMed] [Google Scholar]
- 10.Minghini, A., L. D. Britt, and M. A. Hill. 1998. Interleukin-1 and interleukin-6 mediated skeletal muscle arteriolar vasodilation: in vitro versus in vivo studies. Shock 9:210-215. [DOI] [PubMed] [Google Scholar]
- 11.Petros, A., G. Lamb, A. Leone, S. Moncada, D. Bennett, and P. Vallance. 1994. Effect of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc. Res. 28:34-39. [DOI] [PubMed] [Google Scholar]
- 12.Qing, M., J. F. Vazquez-Jimenez, B. Klosterhalfen, M. Sigler, K. Schumacher, J. Duchateau, B. J. Messmer, G. von Bernuth, and M. C. Seghaye. 2001. Influence of temperature during cardiopulmonary bypass on leukocyte activation, cytokine balance, and post-operative organ damage. Shock 15:372-377. [DOI] [PubMed] [Google Scholar]
- 13.Salvemini, D., R. Korbut, E. Anggard, and J. Vane. 1990. Immediate release of a nitric oxide-like factor from bovine aortic endothelial cells by Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA 87:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuehr, D. J., and M. A. Marletta. 1987. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J. Immunol. 139:518-525. [PubMed] [Google Scholar]
- 15.Taniguchi, T., K. Shibata, and K. Yamamoto. 2001. Ketamine inhibits endotoxin-induced shock in rats. Anesthesiology 94:928-932. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi, T., K. Yamamoto, N. Ohmoto, K. Ohta, and T. Kobayashi. 2000. Effects of propofol on hemodynamic and inflammatory responses to endotoxemia in rats. Crit. Care Med. 28:1101-1106. [DOI] [PubMed] [Google Scholar]
- 17.Tracey, K. J., B. Beutler, S. F. Lowry, J. Merryweather, S. Wolpe, I. W. Milsark, R. J. Hariri, T. J. Fahey III, A. Zentella, J. D. Albert, G. T. Shires, and A. Cerami. 1986. Shock and tissue injury induced by recombinant human cachectin. Science 234:470-474. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez-Jimenez, J. F., M. Qing, B. Hermanns, B. Klosterhalfen, M. Woltje, R. Chakupurakal, K. Schumacher, B. J. Messmer, G. Bernuth, and M. C. Seghaya. 2001. Moderate hypothermia during cardiopulmonary bypass reduces myocardial cell damage and myocardial cell death related to cardiac surgery. J. Am. Coll. Cardiol. 38:1216-1223. [DOI] [PubMed] [Google Scholar]