Abstract
The adenoviral early region 1A (E1A) protein mediates sensitization to different stimulus-induced apoptosis, such as tumor necrosis factor alpha, UV and gamma irradiation, and different categories of anticancer drugs. However, the molecular mechanisms underlying E1A-mediated sensitization to apoptosis are still not completely defined. Here, we show that E1A-mediated sensitization to apoptosis by the inactivation of a key survival factor Akt and the activation of a pro-apoptotic factor p38. Also, inactivation of Akt by either a specific inhibitor or a genetic knockout of Akt1 results in p38 activation, possibly through the release of the activity of p38 upstream kinases, including ASK1 and MEKK3. In addition, we showed that p38 phosphorylation is downregulated and Akt phosphorylation is upregulated in multiple human tumor tissues, and this correlates with tumor stage in human breast cancer. A deletion mutation of a conserved domain of E1A, which is required for E1A-induced downregulation of Akt activity, disrupts E1A-mediated upregulation of p38 activity and also eliminates E1A-mediated chemosensitization. Thus, activation of p38 and inactivation of Akt may have general implications for tumor suppression and sensitization to apoptosis.
Many types of tumors are associated with activated oncogenic kinases, and two complementary roles of these oncogenic kinases are stimulating signaling pathways that enable cells to function independent of their environment and making tumor cells resistant to genotoxic therapies, such as chemo- and radiotherapy (22, 24, 48). Deregulated growth signaling pathways and acquired resistance toward apoptosis therefore constitute two hallmarks of most, if not all, human tumors (18). For example, it has been shown that the serine/threonine kinase Akt and its family members Akt 2 and 3 are either amplified or their activity is constitutively elevated in human carcinomas such as breast, pancreatic, ovarian, brain, prostate, and gastric adenocarcinomas (39, 50). As it is a direct downstream target of phosphatidylinositol 3-kinase (PI3K), Akt is also a key oncogenic survival factor and can phosphorylate and inactivate a panel of critical proapoptotic molecules, including Bad, caspase 9, the Forkhead transcription factor FKHRL1 (known to induce expression of proapoptotic factors such as Fas ligand), GSK3-β, cell cycle inhibitors p21 and p27, and tumor suppressor TSC2, etc. (4, 25, 39, 50, 58). Akt can also inactivate p53, a key tumor suppressor, through phosphorylation and nuclear localization of MDM2 (33, 50, 59). Activation of Akt has been shown to induce resistance to apoptosis induced by a range of drugs (41). Thus, molecules that can block Akt activity may have important significance in cancer therapy and drug sensitization.
The adenovirus early region 1A protein (E1A) induces chemosensitization among different categories of anticancer drugs, including cisplatin, adriamycin, etopside, staurosporine, 5-fluorouracil, and paclitaxel (Taxol) (5, 14, 16, 32, 45, 53), suggesting that a general cellular mechanism may exist to regulate E1A-mediated chemosensitization. However, the molecular mechanisms underlying E1A-mediated chemosensitization are still not completely defined. Earlier studies on normal fibroblast cells revealed that E1A-mediated sensitization to cytotoxic anticancer drugs depends on the expression of functional p53 and p19ARF, an alternative splicing form of p16INK4a (12, 31, 32). E1A was also shown to downregulate Her-2/neu overexpression and facilitate E1A-mediated sensitization to the cytotoxicity of anticancer drugs in human breast and ovarian cancer cells (53, 55, 56). In another study, E1A was reported to mediate sensitization to anticancer drugs in human osteosarcoma cells (16) in a p53- and Her-2/neu-independent manner. Similarly, there is no correlation between p53 protein level and sensitivity of DNA-damaging agents in keratinocytes carrying adenovirus E1A (45). A few other critical molecules were also proposed to be involved in E1A-induced chemosensitization, such as the proapoptotic protein Bax, caspase 9, or a yet unidentified inhibitor that ordinarily provides protection against cell death (14, 15, 34, 43, 49, 52). However, none of the above molecules or pathways can really serve as a general cellular mechanism for E1A-mediated sensitization to apoptosis in a diverse cellular context. Recently, transcriptional upregulation of procaspases (such as pro-caspase 3, 7, 8, and 9) through E1A-mediated disruption of pRB function and subsequent release of free E2F-1 was reported to contribute to both p53-dependent and p53-independent drug sensitization by E1A in diploid normal fibroblast cells (37). In the present study, we found that E1A can activate p38 and inactivate Akt and showed that this pathway may provide a general cellular mechanism for E1A to mediate sensitization to different categories of anticancer drugs.
MATERIALS AND METHODS
Cell culture, cell harvest, and Western blot.
Human breast, ovarian, prostate, pancreatic, and colon cancer cell lines were grown in Dulbecco's modified Eagle's medium-F-12 (Life Technologies, Inc., Rockville, Md.) supplemented with 10% fetal bovine serum. The stable E1A-expressing cell lines in breast cancer MDA-MB-231 and MCF-7 were established as described previously (36, 52). Similarly, domain deletion mutant constructs of E1A were transfected into MDA-MB-231 cells, and stable clones were screened and selected in the presence of G418. Akt1 knockout mouse embryonic fibroblasts (MEFs) and myristoylated, membrane-bound, constitutively active Akt1 (myr-Akt)-transfected stable Rat1 cells were provided by Nissim Hay (University of Illinois at Chicago, Chicago) (10).
For the analysis of basal Akt and p38 expression and activity, cells were serum starved overnight before harvesting. Cells were then washed twice with cold phosphate-buffered saline (PBS) and lysed in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate (NaVO3), and 1.5% aprotinin. The cell extracts were clarified by centrifugation, and protein concentrations were determined by using a Bio-Rad (Hercules, Calif.) protein assay reagent and analyzed in a spectrophotometer with bovine serum album (Sigma, St. Louis, Mo.) as the protein standard. Aliquots of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Millipore Corp., Bedford, Mass.) by using standard procedures. The membranes were then subjected to Western blotting, and the blots were developed with the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, N.J.).
Primary antibodies.
For Western blot analysis, rabbit polyclonal antibodies against phospho-Akt (Ser 473, catalog no. 9271, 1:1,000 dilution) and phospho-p38 (Thr 180/Tyr 182, catalog no. 9211, 1:1,000 dilution) were purchased from Cell Signaling Technology (Beverly, Mass.). Rabbit polyclonal antibodies against nonphosphorylated total Akt (catalog no. 9272, 1:500 dilution), p38 (catalog no. 9212, 1:500 dilution), and cleaved poly(ADP-ribose) polymerase (PARP) (Asp214, catalog no. 9541, 1:1,000 dilution) were also from Cell Signaling Technology. A rabbit polyclonal antibody against beta-actin was used as a loading control for Western blotting and was purchased from Sigma. For immunohistochemical (IHC) study, we used an IHC-specific rabbit polyclonal antibody against phospho-Akt (Ser 473, catalog no. 9277) or an IHC-specific monoclonal antibody against phospho-p38 (Thr180/Tyr 182, catalog no. 9216) at a 1:75 dilution for both antibodies. The monoclonal antibody used against the E1A proteins was M58 (PharMingen, San Diego, Calif.). Rabbit polyclonal anti-Bax antibodies, a hamster anti-human Bcl-2 monoclonal antibody, and rabbit polyclonal anti-ASK1 (H-300) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Mouse monoclonal antibodies against human caspase 3 and 7 were from Transduction Laboratories (1:1,000 dilution, C31720; Lexington, Ky.) and BD PharMingen (1:1,000 dilution, 66871A), respectively. Rabbit polyclonal antibodies against human caspase 8 and 9 were from Santa Cruz Biotechnology (1:500 dilution, SC-7890/H-134) and Cell Signaling Technology (1:500 dilution, no. 9502), respectively. To detect hemagglutinin (HA)-tagged proteins, a monoclonal anti-HA antibody was used (1:1,000 dilution, catalog no. 1,583,816; Boehringer Mannheim, Indianapolis, Ind.). A monoclonal anti-FLAG antibody (M2) was purchased from Sigma (1:1,000 dilution).
Transient transfection, MTT assay, luciferase assay, and FACS analysis.
The standard MTT assay was performed to measure the viable cells after treatment with anticancer drugs as described previously (52). Expression vectors for HA-p38, constitutively active Akt (CA-Akt), dominant-negative Akt (DN-Akt), and cytomegalovirus driving luciferase (pcDNA3-Luc) were used in this study. First, 105 cells in a 60-mm dish were transfected with 2.2 μg of total DNA by using the DC-Chol cationic liposome as described previously (52). After 48 h, the cells were split into three sets: one used for a luciferase assay after exposure with or without paclitaxel for 24 h, one used to analyze Akt and p38 protein expression, and one fixed in 75% ethanol, stained with propidium iodide (25 μg/ml), and sent for fluorescence-activated cell sorter (FACS) analysis. The percentage of paclitaxel-treated cells that exhibited luciferase activity was normalized by using the luciferase activity of the untreated cells as the baseline (100%). Standard deviations from three independent experiments were calculated.
Establishment of IPTG-inducible DN-p38 stable cell lines.
One E1A-expressing MDA-MB-231 clone was cotransfected with an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible DN-p38α construct (a gift from Philipp E. Schere, Albert Einstein College of Medicine, Bronx, N.Y.) and the plasmid pCMVLacI (Stratagene, La Jolla, Calif.). Stable clones were selected in the presence of 200 μg of hygromycin/ml.
Immunoprecipitation.
After transient transfection with HA-tagged p38 or CA-Akt, cells were stimulated with 10 μM insulin for 15 min. Cells were then lysed, and cell lysates were centrifuged at 16,000 g for 30 min. The supernatants were then transferred to a fresh tube. Proteins were cleared via addition of a normal mouse or rabbit immunoglobulin G and immunoprecipitated with anti-p38, anti-Akt, or anti-HA antibodies. Immunoprecipitates were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Akt, p38, and ASK1 were detected by Western blotting.
Kinase assay.
Nonradioactive kinase assay kits for p38 and Akt were purchased from Cell Signaling (New England BioLabs, Beverly, Mass.). The p38 and Akt kinase activities were measured according to the manufacturer's protocol with glutathione S-transferase (GST)-ATF-2 as the substrate for p38 and GST-GSK-3-β as the substrate for Akt.
Tissue microarray and immunohistochemistry.
Tissue microarray slides (HistoArray no. IMH-343/BA2 and IMH-304/CB2) were purchased from IMGENEX (San Diego, Calif.). Detailed information about each slide is available online. Slide processing and immunohistochemical staining were performed according to the manufacturer's protocol. Briefly, tissue slides were heated at 60°C, deparaffinized in xylene, hydrated in graded ethanol, and then immersed in tap water. Antigen retrieval was performed with 0.01 M citrate buffer at pH 6.0 for 20 min in a 95°C water bath. Endogenous peroxidase activity was quenched in 3% hydrogen peroxide solution followed by three sequential PBS washes (5 min each). Slides were then blocked by the respective normal serum for each primary antibody, incubated with primary antibody diluted in TBS-T (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.1% Tween 20) containing 1% ovalbumin and 1 mg of sodium azide/ml, incubated with biotinylated secondary antibody for 30 min at room temperature, washed with PBS again, and incubated with avidin-biotin complex (Vestastain Elite ABC kit; Vector Laboratories, Inc., Burlingame, Calif.). Slides were washed with PBS again, incubated with the AEC (3-amino-9-ethylcarbazole) substrate kit (catalog no. Sk-4200; Vector Laboratories, Inc.), and then counterstained in Meyer's xylene.
One representative slide per case was evaluated with the antibodies mentioned above. The intensities of staining seen in different areas of the same slide were analyzed according to criteria described previously in the literature (1). The intensity was designated 0 when no cells stained, 1+ when 10 to 20% of cells stained (weak), 2+ when 20 to 50% of cells stained (moderate), and 3+ when more than 50% of cells stained (strong).
Statistics.
For statistical analysis, groups scored as 0 and 1+ were combined as weak staining while groups scored as 2+ and 3+ were combined as strong staining. Similarly, to simplify the statistical analysis, breast tumors with stages 1 and 2 were combined as early stages of tumors (n = 25 cases) while tumors with stages 3 and 4 were combined as late stages of tumors (n = 25 cases). Statistical analysis was performed by using χ2 analysis.
RESULTS
E1A upregulates p38 activity and downregulates Akt activity.
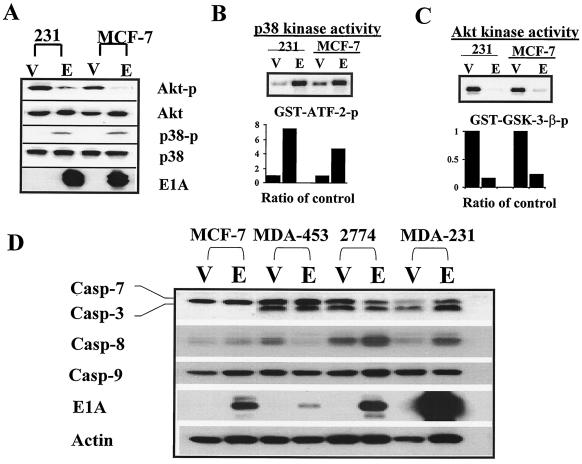
To determine whether apoptosis-related kinases are involved in E1A-mediated sensitization to apoptosis, we examined the phosphorylation status of three well-known kinases involved in regulation of apoptosis, p38, Akt, and JNK, in E1A-expressing MDA-MB-231 and MCF-7 cells (231-E1A and MCF-7-E1A) versus vector-transfected cells (231-Vect and MCF-7-Vect). We detected phosphorylated p38 in cells stably expressing E1A but not in vector-transfected cells. However, the level of phosphorylated Akt was much higher in vector-transfected cells than in E1A-expressing cells. The levels of total Akt and p38 were similar in both types of cells (Fig. 1A). Kinase assays showed that p38 activity was higher and Akt activity was lower in 231-E1A and MCF-7-E1A cells than in 231-Vect and MCF-7-Vect cells (Fig. 1B and C). We did not detect any difference in the level of phosphorylated JNK between E1A-expressing and vector-transfected cells (data not shown). These results indicated that E1A enhanced the activity of the proapoptotic kinase p38 and repressed the activity of the antiapoptotic kinase Akt but did not affect JNK phosphorylation.
FIG. 1.
Upregulation of p38 activity and downregulation of Akt activity by E1A correlated with E1A-mediated sensitization to paclitaxel-induced apoptosis. (A) Phospho-p38 (p38-p) and phospho-Akt (Akt-p) levels in E1A-expressing cells versus those in vector-transfected MDA-MB-231 and MCF-7 cells are shown. Total p38 (p38) and Akt (Akt) were used as loading controls. Results of kinase assays of p38 (B) and Akt (C) and densitometric analysis of relative p38 activity with GST-ATF-2 as a substrate and Akt activity with GST-GSK-3-β as a substrate in E1A-expressing cells versus vector-transfected control cells. E, E1A-expressing cells; V, vector transfected control cells. (D) Expression of caspase 3, 7, 8, and 9 proenzymes in E1A stable cells established in human breast cancer cell lines MCF-7, MDA-MB-231, and MDA-MB-453 and ovarian cancer cell line 2774. V, vector control; E, E1A stable cells.
A recent report showed that transient transfection of E1A resulted in the accumulation of caspase proenzymes in human normal diploid fibroblasts (37). We therefore compared the expression levels of caspase proenzymes caspase 3, 7, 8, and 9 between E1A transfectants and vector-transfected carcinoma cells, including breast cancer MDA-MB-231, MDA-MB-453, and MCF-7 cells and ovarian cancer 2774 cells. Unlike what was demonstrated with normal fibroblast cells, we did not observe a unanimous increase of these caspase proenzymes in the E1A stable cells established in human cancer cells with epithelial origins (Fig. 1D). This suggests that transcriptional upregulation of the caspase proenzymes in these human cancer cells may not be as critical as it is in the normal fibroblast cells and that other cellular mechanisms may exist for the E1A-mediated sensitization to apoptosis in human cancer cells.
Upregulation of p38 activity and downregulation of Akt activity correlate with E1A-mediated sensitization to paclitaxel-induced apoptosis.
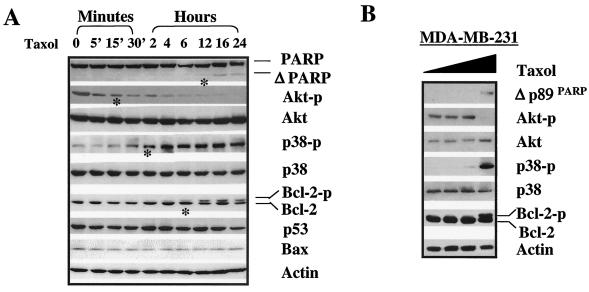
To test whether alteration of the kinase activity of Akt or p38 played a role in E1A-mediated sensitization to apoptosis, we compared the kinetics of phosphorylation of Akt or p38 with paclitaxel-induced apoptosis in 231-E1A cells by using PARP cleavage and Bcl-2 phosphorylation as apoptotic cell death markers. PARP cleavage and Bcl-2 phosphorylation occurred after decreased Akt phosphorylation and increased p38 phosphorylation in 231-E1A cells after exposure to 0.01 μM paclitaxel (Fig. 2A). However, no significant change was detected in the protein levels of p53 and Bax (Fig. 2A). The same concentration of paclitaxel did not trigger PARP cleavage, induce Bcl-2 phosphorylation, or modulate the levels of phosphorylated p38 and Akt in the parental MDA-MB-231 cells (data not shown). To trigger a similar response in parental MDA-MB-231 cells, a much higher dosage was required (Fig. 2B). The results suggest that downregulation of Akt and upregulation of p38 activities may be involved in the E1A-mediated sensitization to paclitaxel-induced apoptosis.
FIG. 2.
Upregulated p38 activity and downregulated Akt activity correlate with E1A-mediated sensitization to paclitaxel-induced apoptosis. (A) Kinetics of PARP, Akt, p38, Bcl-2, p53, and Bax protein expression in 231-E1A cells before and after exposure to 0.01 μM paclitaxel. Bcl-2 phosphorylation and PARP cleavage were detected at 6 and 12 h after exposure to paclitaxel and became more obvious thereafter. Phosphorylation of p38 was first detected after 30 min to 2 h and then become more obvious after 4 h of treatment. Dephosphorylation of Akt could be detected even earlier, at 5 min posttreatment. Actin was used as a loading control. A dramatic alteration of each molecule was marked with an asterisk. (B) Dose-dependent effect of PARP cleavage and Akt and p38 phosphorylation in MDA-MB-231 cells after exposure to paclitaxel for 24 h. The concentrations of paclitaxel used ranged from 0 to 0.001, 0.01, and 0.1 μM.
Activation of p38 and inactivation of Akt are required for E1A-mediated sensitization to drug-induced apoptosis.
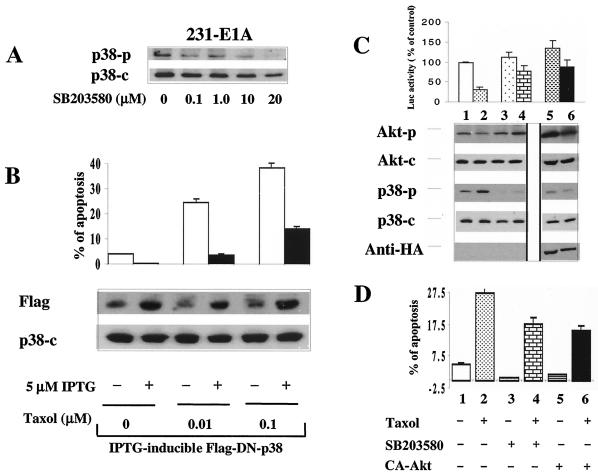
To evaluate whether activation of p38 is required for E1A-mediated sensitization to paclitaxel, we tested whether blocking p38 activity could inhibit E1A-mediated sensitization in 231-E1A cells. We used the specific p38 inhibitor SB203580 (Fig. 3A) and a DN-p38 mutant to block p38 activation (Fig. 3B). A pcDNA3-Luciferase (pcDNA-Luc) construct was transfected into 231-E1A cells, and luciferase activity was used as a measurement for cell survival. Pretreatment with SB203580 inhibited the phosphorylation of p38 in cells with or without exposure to paclitaxel (Fig. 3C, lanes 3 to 4) and protected cells from a paclitaxel-induced decrease of luciferase activity (Fig. 3C, lane 2 versus lane 4). In addition, FACS analysis showed that pretreatment with SB203580 protected 231-E1A cells from paclitaxel-induced apoptosis (27.5% versus 18.0%) (Fig. 3D, lanes 2 and 4, bottom). These data suggest that p38 activation is required for E1A-mediated sensitization to paclitaxel-induced apoptosis. Using a DN-p38 to block p38 activation further supported the above results (Fig. 3B). When the cells were switched to medium containing 5 μM IPTG for 24 h, expression of IPTG-inducible DN-p38 was induced in the presence or absence of paclitaxel (Fig. 3B, lower panel). FACS analysis showed that induction of DN-p38 by IPTG significantly inhibited paclitaxel-induced apoptosis (Fig. 3B, upper panel). Identical results were obtained when two additional stable clones were studied (data not shown). However, IPTG could not induce this effect in the 231-E1A cells without IPTG-induced DN-p38 (data not shown). Taken together, these data suggest that p38 activation is required for E1A-mediated sensitization to paclitaxel-induced apoptosis.
FIG. 3.
Activation of p38 and inactivation of Akt are required for E1A-mediated sensitization to paclitaxel-induced apoptosis. (A) Dose-dependent effect of SB203580 on p38 phosphorylation in 231-E1A cells. (B) An IPTG-inducible, Flag-tagged DN-p38 stable cell clone was established in 231-E1A cells. Repression of p38 activity by IPTG-inducible DN-p38 enhances Akt phosphorylation and eliminates E1A-mediated sensitization to paclitaxel in E1A-expressing cells in the presence of 5 μM IPTG for 24 h. (C) Western blot analysis of p38 and Akt in E1A-expressing MDA-MB-231 cells and luciferase assay. The viability of cells with (lanes 2, 4, and 6) or without (lanes 1, 3, and 5) exposure to paclitaxel was measured by luciferase assay. The pcDNA3-Luc vector was cotransfected into 231-E1A cells with (lanes 5 and 6) or without a HA-tagged, myristoylated, membrane-targeted CA-Akt (lanes 1 to 4) before treatment with paclitaxel. After exposure to paclitaxel for 4 h, a portion of cells was harvested for protein extraction while the rest were grown for 24 h. In the absence of 20 μM SB203580, the level of phosphorylated p38 increased after exposure to paclitaxel (lanes 1 and 2). Expression of CA-Akt was detected with an anti-HA monoclonal antibody (lanes 5 and 6). (D) A portion of the above-described cells was also subjected to FACS analysis to measure apoptosis. +, present; −, absent.
To determine whether downregulation of Akt activity is also required for E1A-mediated sensitization to paclitaxel, we examined whether activation of Akt by transfection of CA-Akt would inhibit paclitaxel-induced apoptosis in 231-E1A cells. The level of phosphorylated Akt and luciferase activity was increased in CA-Akt-transfected 231-E1A cells compared with that in the control 231-E1A cells (Fig. 3C, lanes 1 and 2 versus lanes 5 and 6). FACS analysis showed that fewer apoptotic cells were detected in CA-Akt-transfected cells (15.9%) than in control 231-E1A cells (27.5%) after exposure to paclitaxel (Fig. 3D, lane 2 versus lane 6, bottom). Thus, inhibition of Akt phosphorylation is also required for E1A-mediated sensitization to paclitaxel-induced apoptosis.
Activation of p38 and inactivation of Akt represent a general cellular mechanism in response to different apoptotic stimuli.
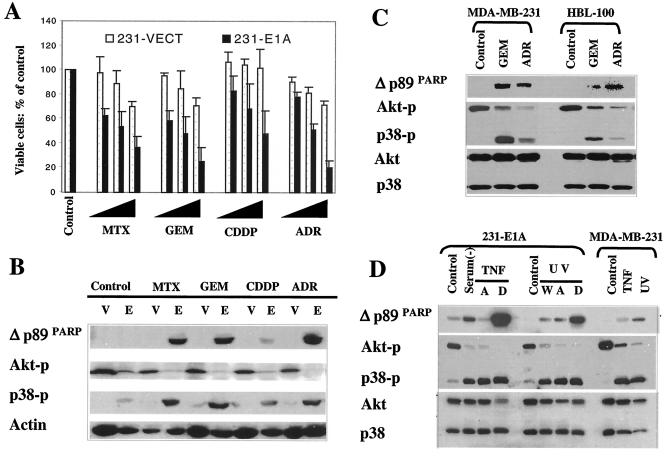
To determine whether the same mechanism of E1A-mediated sensitization to paclitaxel applies to other anticancer drugs, we tested the effects of four additional drugs used in the clinic for treatment of human cancer. These four anticancer drugs induce antitumor activities through different modes of action: doxorubicin-adriamycin (topoisomerase II inhibitor), cisplatin (DNA-damaging agent), methotrexate (antimetabolite drug), and gemcitabine (antimetabolite drug). The expression of E1A significantly enhanced each drug's cytotoxicity in MDA-MB-231 cells, determined by using the MTT assay (Fig. 4A). In addition, downregulation of Akt phosphorylation and upregulation of p38 phosphorylation and PARP cleavage were observed in 231-E1A cells but not in 231-Vect cells treated with each of the drugs at the same dosage (Fig. 4B). These results suggest that activation of p38 and inactivation of Akt may contribute to E1A-mediated sensitization to apoptosis induced by these different drugs.
FIG. 4.
Activation of p38 and inactivation of Akt represent a general cellular mechanism in response to different apoptotic stimuli. (A) Percentage of viable cells in vector-transfected (231-Vect) and E1A-expressing MDA-MB-231 (231-E1A) cells after exposure to different doses of adriamycin (ADR) (0.1, 1.0, and 10 μM), cisplatin (CDDP) (0.2, 2, and 10 μg/ml), gemcitabine (GEM) (0.2, 2, and 10 μg/ml), and methotrexate (MTX) (0.2, 2, and 10 μM) for 24 h. Cell viability was measured by using the MTT assay. (B) Downregulation of Akt activation and upregulation of p38 activation correlated with drug-induced PARP cleavage in 231-Vect (V) and 231-E1A (E) cells. The concentrations used were 1 μM ADR, 2-μg/ml CDDP, 2-μg/ml GEM, and 2 μM MTX. (C) The concentrations of GEM and ADR used for MDA-MB-231 and MCF-7 cells were 20 μg/ml and 20 μM, respectively. (D) Downregulation of Akt and upregulation of p38 phosphorylation correlated with PARP cleavage induced by serum starvation, TNF-α, and UV irradiation. 231-E1A cells were serum starved [serum(−)], exposed to TNF-α (5 ng/ml), or UV irradiated (6 J/cm2) while parental MDA-MB-231 cells were exposed to 10-times-higher doses of TNF-α (50 ng/ml) and UV radiation (60 J/cm2). Both the attached cells and cells in suspension were collected, if not specified. W, whole-cell lysate with both attached and suspended cells; A, attached cells only; D, detached or floated apoptotic cells.
To address whether activation of p38 and inactivation of Akt also applied to drug-induced apoptosis in the absence of E1A, we tested whether increasing the dosage of gemcitabine or adriamycin could also enhance p38 activation and inhibit Akt activation, which would then contribute to drug-induced apoptosis. When MDA-MB-231 and HBL-100 cells were exposed to a dose of gemcitabine or adriamycin 10 times higher than that used in experiment whose results are shown in Fig. 4B, we observed a similar pattern of downregulation of Akt and upregulation of p38 activation, which was correlated with PARP cleavage (Fig. 4C). Similar results were also observed in MDA-MB-231 cells when exposed to a higher dose of paclitaxel (Fig. 2B). These results suggest that p38 activation and Akt inactivation may not be limited to E1A-mediated sensitization to apoptosis but may also contribute to drug-induced apoptosis in the absence of E1A. Thus, downregulating Akt activity and upregulating p38 activity may represent a general cellular mechanism of response to apoptotic stimuli, and E1A may turn on this cellular mechanism and mediate sensitization to drug-induced apoptosis.
To determine the physiological relevance of inactivation of Akt and activation of p38 in the execution of apoptosis, we extended our investigation to apoptosis induced by serum starvation, tumor necrosis factor alpha (TNF-α), and UV irradiation. We observed that phosphorylation of p38 and dephosphorylation of Akt were correlated with serum starvation-, TNF-α-, and UV-induced PARP cleavage in 231-E1A cells, especially in detached apoptotic cells (Fig. 4D, lanes 1 to 8). However, a dose of 10 times higher is required for inducing a response in parental MDA-MB-231 cells similar to that in 231-E1A cells (Fig. 4D, lanes 9 to 11). Taken together, downregulation of Akt activation and upregulation of p38 activation may also represent a general cellular mechanism in response to different apoptotic stimuli.
The physiological regulation of p38 activity by Akt is through ASK1 and MEKK3, the upstream kinases of p38.
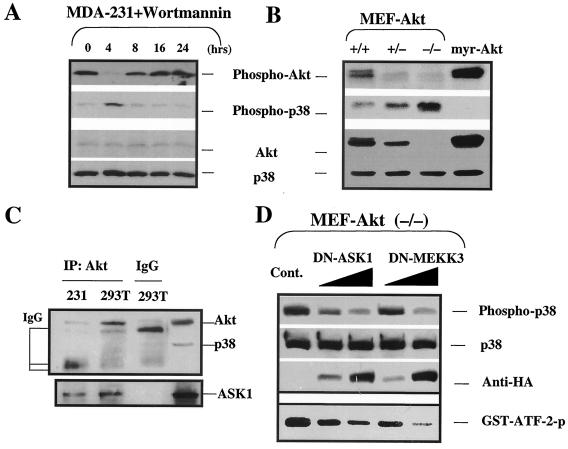
The above results suggest that both downregulation of Akt and upregulation p38 activities are involved in E1A-mediated sensitization to apoptosis. We noticed that reduced Akt phosphorylation occurs before enhanced p38 phosphorylation in the kinetic study of E1A-mediated sensitization to paclitaxel-induced PARP cleavage (Fig. 2A). We therefore asked whether Akt may act upstream of p38. To this end, Akt activity was blocked by either a specific PI3K inhibitor, wortmannin, or a genetic method to knock out Akt expression. Blocking Akt activation with wortmannin in MDA-MB-231 cells resulted in decreased Akt phosphorylation and increased p38 phosphorylation (Fig. 5A). And when Akt phosphorylation was recovered, the p38 phosphorylation was reduced again (8- to 24-h time points). These results indicate that Akt phosphorylation was required for repressing p38 activation, suggesting that the former is upstream from the latter. This conclusion was further supported by the study with Akt1-knockout MEFs and myr-Akt1-transfected stable cells. We observed that the level of phosphorylated p38 was increased in Akt1−/− MEFs compared with that in Akt+/+ and Akt+/− MEFs. Furthermore, the phospho-p38 protein was undetectable in the Akt constitutively activated myr-Akt1 stable cells (Fig. 5B). These results indicate that Akt is able to inhibit p38 activity.
FIG. 5.
Physiological regulation of Akt and p38 pathways. (A) Stable E1A-expressing or parental MDA-MB-231 cells were serum starved for 24 h before exposure to 20.0 μM 0.1 μM wortmannin. (B) Expression of phospho-p38 and phospho-Akt in Akt1 knockout MEFs and myr-Akt-transfected Rat1cells. (C) CA-Akt was transiently transfected into both MDA-MB-231 and 293T cells. The cells were lysed after transfection, and Akt was immunoprecipitated (IP). Western blot analyses of Akt, ASK1, and p38 interaction in 293T cells and MDA-MB-231 cells were performed. (D) Akt−/− MEFs were grown in six-well plates for 24 h and then transiently transfected by FuGENE 6 liposome (catalog no. 1 814 443; Roche Molecular Biochemicals, Indianapolis, Ind.) at a 3:1 ratio with either HA-tagged DN-ASK1 or HA-tagged DN-MEKK3 cDNA in the amounts of 1 and 10 μg, respectively. Cells were grown for another 36 h and were then harvested and analyzed for p38 kinase activity with ATF-2 as the substrate. The expressions of phospho-p38, total p38, and the HA tag were also detected by the respective antibodies. IgG, immunoglobulin G; Cont., control cells transfected with pcDNA3 plasmid DNA.
In an attempt to determine how Akt regulates p38, we sought to determine whether Akt is physically associated with p38 by using coimmunoprecipitation experiments. We did not detect p38 in immunoprecipitated Akt samples (Fig. 5C) or Akt in immunoprecipitated p38 samples (data not shown), suggesting that Akt and p38 were not directly associated under the conditions we used. A recent report demonstrated that ASK1 is a substrate of Akt (28), and ASK1 has been shown to be an upstream kinase of p38 (23, 51), suggesting that Akt may indirectly regulate p38 activity through ASK1. Indeed, we also detected that ASK1 was coimmunoprecipitated with Akt in our experimental system (Fig. 5C). To test whether Akt can downregulate p38 activation through the repression of p38 upstream kinases, such as ASK1, we blocked the activity of either ASK1 or MEKK3, both of which are p38 upstream kinases that can be inactivated by Akt (17, 28), by using a kinase-dead, DN mutant of ASK1 (DN-ASK1) or MEKK3 (DN-MEKK3). As expected, blockade of either ASK1 or MEKK3 activity by DN-ASK1 or DN-MEKK3 repressed p38 phosphorylation and its kinase activity, as measured by phosphorylation of ATF2 in a dose-dependent manner in Akt1−/− MEFs (Fig. 5D), suggesting that Akt inhibits p38 activation through repression of ASK1 and/or MEKK3 activation.
p38 inactivation is associated with Akt activation in human cancer.
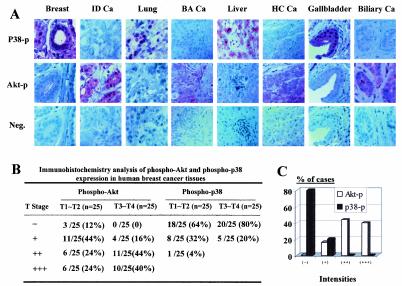
The above results suggest that Akt acts upstream of p38 and blocks p38 activation. Because activation of Akt is a common phenomenon in different types of human cancers, we asked whether p38 inactivation is also a common phenomenon in human cancer cells and correlates with Akt activation. To test whether p38 inactivation was accompanied by Akt activation in human tumor tissues in vivo, we utilized tissue array slides to screen phospho-p38 and phospho-Akt expression in tumor tissues of different origins and normal or parallel normal organ tissues. We found that the phospho-Akt level was dramatically higher while phospho-p38 was undetectable in most of the cancer tissues obtained from different types of solid tumors, such as breast, lung, liver, bile duct, gastric, colorectal, renal cell, ovarian, and uterine cancers; malignant lymphoma; and Schwannoma. In contrast, the intensity of phospho-p38 protein staining was relatively strong while that of phospho-Akt staining was very weak in normal organs and parallel healthy tissues. Representative data on the expression of phospho-p38 and phospho-Akt in healthy versus tumor tissues obtained from the breast, lung, liver, and biliary duct are shown in Fig. 6A. By screening a panel of breast, ovarian, prostate, pancreatic, and colorectal cancer cell lines with phosphospecific antibody against p38 or Akt, we also observed a correlation between enhanced Akt phosphorylation and reduced p38 phosphorylation in these human cancer cell lines (data not shown). These data support the hypothesis that p38 activity is repressed in different types of human cancer, which is associated with enhanced Akt activation.
FIG. 6.
Inactivation of p38 associated with Akt activation in human cancer. (A) Immunohistochemical staining of phospho-p38 and phospho-Akt in different types of human cancer tissues in the Histo-Array slides. Ca, carcinoma; ID Ca, infiltrating ductal carcinoma; BA Ca, bronchoalveolar carcinoma; HC Ca, hepatocellular carcinoma. (B) Intensity of phospho-Akt and phospho-p38 stained in the early and late stages of breast cancer. The staining intensity of phospho-Akt is significantly greater in late stage (stage III and IV) breast cancer while positive phospho-p38 staining is predominant in healthy breast epithelial cells and early stage (stage I and II) breast cancer (P < 0.001 for both). (C) Inverse correlation between phospho-Akt and phospho-p38 staining in late stage breast cancer tissue samples.
To test whether repression of p38 and activation of Akt also correlate with tumor stage in human cancer, we analyzed 10 healthy breast tissue samples (including 2 healthy nipple and 8 healthy breast tissues) and 50 cases of breast cancer at different stages on Histo-Array slides. These include 4 cases at stage I (T1), 21 cases at stage II (T2), 20 cases at stage III (T3), and 5 cases at stage IV (T4). Among the 25 cases of early stage tumors (T1 and T2), 12% of them are negative and half of them are weakly positive for phospho-Akt staining. In the advanced late-stage tumor samples, all of them are positive and more than 80% of them are moderately to strongly positive for phospho-Akt staining (Fig. 6B), whereas the relative intensity of phospho-p38 staining was inversely correlated with the tumor stage (Fig. 6B). In the early stage tumor samples, only one-third of them (36%) are positive for phospho-p38 staining and only 1 of 25 cases is moderately positive for phospho-p38. In the late-stage tumor samples (T3 and T4), 80% of them are negative for phospho-p38 staining and the rest are weakly positive for phospho-p38 staining (Fig. 6B). Comparing the phospho-p38 staining and phospho-Akt staining in the advanced late-stage tumor samples, we observed an inverse correlation between the intensity of strong phospho-Akt staining versus weak phospho-p38 staining (P < 0.0001) (Fig. 6C). Phosphorylated p38 could be detected in most of the healthy organ tissues but not in most of the cancer tissues or cell lines, indicating that p38 inactivation is also a common event in human cancer cells with Akt activation.
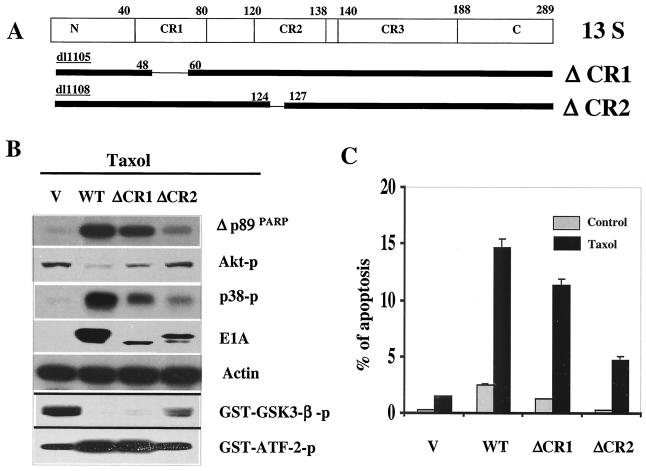
The CR2 domain of E1A is required for downregulation of Akt phosphorylation and chemosensitization.
The above results suggested that Akt regulated p38 activation in both physiological and pathological conditions, which indicates that E1A-mediated downregulation of Akt activity and upregulation of p38 activity are accompanied events, i.e., by repression of Akt activation, E1A enhanced p38 activity and sensitized cells to drug-induced apoptosis. To lay further genetic support for these conclusions, we proposed to map the domain(s) of E1A that is responsible for downregulation of Akt activity and demonstrate that the same domain is also critical for E1A-mediated upregulation of p38 and sensitization to drug-induced apoptosis. It is known that among the three conserved domains (CR) of E1A, CR1 and CR2 are associated with E1A-mediated sensitization to apoptosis (46). Therefore, we established E1A functional domain deletion mutation stable cells in MDA-MB-231 cells, including wild-type E1A, and deletion mutations of CR1 (ΔCR1) and CR2 (ΔCR2) (Fig. 7A). We found that deletion mutations of the CR2 domain dramatically disrupted E1A's ability to downregulate Akt kinase activity, eliminated E1A-mediated upregulation of p38 kinase activity, and remarkably repressed E1A-mediated sensitization to paclitaxel-induced apoptosis (Fig. 7B and C) while the CR1 domain mutant only slightly affected E1A-mediated chemosensitization and downregulation of Akt and upregulation of p38 activities (Fig. 7B and C). These results indicate that the same CR2 domain required for downregulation of Akt is also required for upregulation of p38 and sensitization to drug-induced apoptosis and thus supports the conclusions that Akt represses p38 activity and that E1A, by downregulation of Akt activity, enhances p38 activation and sensitizes cells to anticancer drug-induced apoptosis.
FIG. 7.
The CR2 domain of E1A is required for downregulation of Akt and sensitization to drug-induced apoptosis. (A) Domain structure and map for deletion mutation of CR1 and CR2. (B) Akt and p38 kinase activity were measured, and Western blot analysis of PARP cleavage, phospho-Akt, phospho-p38, or E1A expression was performed in different domain deletion mutant stable cells. Actin was used as a loading control. (C) FACS analysis of apoptosis of wild-type (WT) E1A and different domain deletion mutant E1A stable cells with or without treatment with 0.01 μM paclitaxel for 21 h. V, pSV-neo vector-transfected stable cells.
DISCUSSION
The present study shows that the activity of p38 is regulated by Akt and is deregulated partly due to Akt activation in human cancer. Activation of Akt antagonizes p38 activation while inactivation of Akt results in p38 activation. The adenoviral protein E1A, by downregulation of Akt activity, enhanced p38 activation and sensitized cells to apoptosis induced by different apoptotic stimuli. It is known that p38 participates in the regulation of apoptotic cell death through transcriptional upregulation of proapoptotic gene expression, such as Fas ligand (11, 13, 20, 38). p38 is also involved in negative regulation of cell growth, as it represses cyclin D1 expression and regulates the G2-M transition through the regulation of cdc25 protein phosphatase and p53 protein (6, 42). Recently, inactivation of p38 has been shown to contribute to the development of human cancers by suppressing p53 activation (7), suggesting a tumor-suppressive function of p38. In contrast, Akt is known to upregulate the cyclin D1 expression while repressing Fas ligand expression and p53 stabilization (39, 50). Akt is also involved in regulation of the G2-M transition (26, 40, 47). Thus, Akt may functionally antagonize the p38 effect on cellular processes ranging from cell cycle progression to cell death, though some cell types may respond differently (3, 8, 17, 19, 44). However, the regulation between the Akt and p38 pathways is still unclear in the literature, and the present study provides a link between activation of Akt and inactivation of p38. As discussed above, Akt positively regulates cell growth but negatively regulates cell death while p38 positively regulates cell death but negatively regulates cell growth. Given the results we obtained in Akt−/− MEFs (Fig. 5B and D) and the fact that Akt directly phosphorylates and negatively regulates the activation of ASK1 and MEKK3, the upstream kinase of p38 (17, 28), we propose that Akt may repress p38 activation through the phosphorylation and inactivation of ASK1 or MEKK3 and that inactivation of Akt may result in p38 activation through the release of ASK1 and/or MEKK3 activity. Because either DN-ASK1 or DN-MEKK3 sufficiently repressed enhanced p38 phosphorylation in Akt1−/− MEFs (Fig. 5D), it also suggests that both ASK1 and MEKK3 are involved in Akt-mediated inactivation of p38. Regulation of p38 activity by Akt2 through ASK1 was also demonstrated recently by Yuan et al. in their report on cisplatin-induced apoptosis (57), suggesting that both Akt1 and Akt2 may use a similar mechanism to inactivate p38.
We have shown that activation of p38 follows inactivation of Akt (Fig. 2 and 4B to D) when cells underwent apoptosis, suggesting that the pro- and antiapoptotic signals may integrate each other to prepare cells to commit suicide. The relative Akt and p38 activity may determine a cell's response to apoptotic stimuli, as they can be observed in E1A-mediated sensitization to apoptosis induced by serum starvation, TNF-α, UV irradiation, and different categories of chemotherapeutic drugs (Fig. 4B to D). Expression of E1A may shift the balance of the pro- and antiapoptotic signals by repressing Akt activity and enhancing p38 activity, thereby favoring the proapoptotic signal. Additional approaches could also be used by E1A to shift the intracellular signal integration to favor a proapoptotic signal, such as activation of p53 and caspase proenzymes, but these pathways may not contribute to the present study. For example, p53 and p14ARF were deleted in MDA-MB-231 cells and we did not detect any change in the expression level of p53 or p14ARF in E1A versus parental control cells (data not shown), i.e., the p53-dependent mechanisms may not contribute to E1A-mediated sensitization to apoptosis in the present study. Although expression of E1A by infection of cells with either retroviral or adenoviral vector resulted in the accumulation of caspase proenzymes, such as caspase 3, 7, 8, and 9, by a direct transcriptional mechanism through enforced E2F-1 release in normal diploid human fibroblasts (IMR90) (37). We did not observe a consistent increase of these caspase proenzymes in the E1A stable cells established in human cancer cells with epithelial origin (Fig. 1D). In addition, sensitization to the DNA damage agent adriamycin-induced apoptosis by E1A is dependent on p53 status in normal fibroblasts (37) while E1A dramatically sensitized the adriamycin therapeutic effect in ovarian cancer SKOV3.ip1 (5) and breast cancer MDA-MB-231 cells (Fig. 4A), which do not express functional p53. The discrepancy between normal diploid fibroblasts and epithelial carcinoma cells in E1A-mediated sensitization to apoptosis may reflect the nature of the intrinsic difference between normal fibroblasts and carcinoma cells. However, downregulation of Akt activity by E1A was also observed in E1A-mediated sensitization to cisplatin in human normal IMR90 fibroblasts (54). Thus, targeting the key oncogenic survival factor Akt may represent a critical mechanism for E1A-mediated sensitization to anticancer drug-induced apoptosis in human cancer cells and normal fibroblasts as well.
E1A has been shown to facilitate cytochrome c release from the mitochondria, which also contributes to E1A-mediated sensitization to anticancer drugs. However, the mechanism by which E1A facilitates cytochrome c release is unclear (14). Although we did not test whether E1A expression affected Bax translocation, which may also facilitate cytochrome c release, E1A expression or treatment with paclitaxel did not affect the levels of Bax protein in our system (Fig. 2A). Akt is known to play an important role in maintaining mitochondrial integrity and inhibiting the release of cytochrome c (11, 27). Overexpression of Akt confers resistance to paclitaxel by inhibiting paclitaxel-induced cytochrome c release (41). However, p38 is also involved in regulation of cytochrome c release (2). Therefore, it is possible that E1A may alter mitochondrial potential by downregulating Akt and upregulating p38, thereby facilitating the release of cytochrome c upon treatment with chemotherapeutic drugs, such as paclitaxel. Thus, downregulation of key survival factor Akt activity and subsequent upregulation of a proapoptotic factor p38 activity by E1A may constitute a fundamental approach for E1A-mediated sensitization to apoptosis.
The mechanisms underlying E1A-mediated downregulation of Akt activity are not yet clear. Obviously, E1A-mediated downregulation of Her-2/neu and/or Axl may contribute to reduced Akt activation, as activation of either Her-2/neu or Axl leads to PI3K-Akt kinase activation and downregulation of Her-2/neu and/or Axl also contributes to E1A-mediated sensitization to apoptosis (21, 29, 30, 58). However, downregulation of Akt activity by E1A may not necessarily depend on E1A-mediated downregulation of Her-2/neu and/or Axl, because both MDA-MB-231 and MCF-7 cells are low Her-2/neu-expressing cells and MCF-7 cells have an undetectable expression level of Axl (35). In addition, deletion mutation of the CR2 domain affects E1A-mediated downregulation of Akt (Fig. 7B), but it has no effect on E1A-mediated transcriptional repression of Her-2/neu (9). Thus, in addition to the Her-2/neu-dependent pathway, a Her-2/neu-independent pathway must exist for E1A to mediate downregulation of Akt activity leading to sensitization to apoptosis. In addition, overexpression of Her-2/neu or activation of Akt also leads to p53 destabilization (50, 59), suggesting that downregulation of Akt activity by E1A may constitute an alternative pathway for stabilization of p53. Like Her-2/neu and p53, Akt also plays a critical role in the regulation of apoptotic cell death and the development of human cancer. Therefore, E1A-mediated downregulation of Akt and upregulation of p38 activities may have general implications for E1A-mediated tumor suppression and sensitization to apoptosis.
Acknowledgments
We thank Richard R. Vaillancourt (The University of Arizona College of Pharmacy, Tucson, Ariz.) for providing the HA-tagged kinase-dead DN mutant MEKK3 (MEKK3-KM) and Philipp E. Schere (Albert Einstein College of Medicine of Yeshiva University) for providing the IPTG-inducible DN-p38 constructs. We also thank N. Hay (University of Illinois at Chicago) for providing us with a panel of Akt-knockout MEFs and myr-Akt stable cells. We acknowledge Zheng Huang's technical support for staining and reading of Histo-Array slides. We thank Stephanie Miller for reading and editing the manuscript.
This work was supported by grant RO1-CA58880 and the SPORE grant for ovarian cancer research from the National Institutes of Health (to M.-C.H.) and by grant DAMD17-01-1-0300 from the U.S. Department of Defense Army Breast Cancer Research Program (to Y.L.).
REFERENCES
- 1.Allred, D. C., G. M. Clark, R. Elledge, S. A. Fuqua, R. W. Brown, G. C. Chamness, C. K. Osborne, and W. L. McGuire. 1993. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J. Natl. Cancer Inst. 85:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Assefa, Z., A. Vantieghem, M. Garmyn, W. Declercq, P. Vandenabeele, J. R. Vandenheede, R. Bouillon, W. Merlevede, and P. Agostinis. 2000. p38 mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochrome c release in ultraviolet B radiation-induced apoptosis. J. Biol. Chem. 275:21416-21421. [DOI] [PubMed] [Google Scholar]
- 3.Berra, E., M. T. Diaz-Meco, and J. Moscat. 1998. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J. Biol. Chem. 273:10792-10797. [DOI] [PubMed] [Google Scholar]
- 4.Blain, S. W., and J. Massague. 2002. Breast cancer banishes p27 from nucleus. Nat. Med. 8:1076-1078. [DOI] [PubMed] [Google Scholar]
- 5.Brader, K. R., J. K. Wolf, M. C. Hung, D. Yu, M. A. Crispens, K. L. van Golen, and J. E. Price. 1997. Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin. Cancer Res. 3:2017-2024. [PubMed] [Google Scholar]
- 6.Bulavin, D. V., S. A. Amundson, and A. J. Fornace. 2002. p38 and Chk1 kinases: different conductors for the G(2)/M checkpoint symphony. Curr. Opin. Genet. Dev. 12:92-97. [DOI] [PubMed] [Google Scholar]
- 7.Bulavin, D. V., O. N. Demidov, S. Saito, P. Kauraniemi, C. Phillips, S. A. Amundson, C. Ambrosino, G. Sauter, A. R. Nebreda, C. W. Anderson, A. Kallioniemi, A. J. J. Fornace, and E. Appella. 2002. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 31:210-215. [DOI] [PubMed] [Google Scholar]
- 8.Chen, D., R. V. Fucini, A. L. Olson, B. A. Hemmings, and J. E. Pessin. 1999. Osmotic shock inhibits insulin signaling by maintaining Akt/protein kinase B in an inactive dephosphorylated state. Mol. Cell. Biol. 19:4684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, H., D. Yu, G. Chinnadurai, D. Karunagaran, and M. C. Hung. 1997. Mapping of adenovirus 5 E1A domains responsible for suppression of neu-mediated transformation via transcriptional repression of neu. Oncogene 14:1965-1971. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross, T. G., D. S. Toellner, N. V. Henriquez, E. Deacon, M. Salmon, and J. Lod. 2000. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 256:34-41. [DOI] [PubMed] [Google Scholar]
- 12.de Stanchina, E., M. E. McCurrach, F. Zindy, S. Y. Shieh, G. Ferbeyre, A. V. Samuelson, C. Prives, M. F. Roussel, C. J. Sherr, and S. W. Lowe. 1998. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 12:2434-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Zutter, G. S., and R. J. Davis. 2001. Pro-apoptotic gene expression mediated by the p38 mitogen-activated protein kinase final transduction pathway. Proc. Natl. Acad. Sci. USA 98:6168-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duelli, D. M., and Y. A. Lazebnik. 2000. Primary cells suppress oncogene-dependent apoptosis. Nat. Cell Biol. 2:859-862. [DOI] [PubMed] [Google Scholar]
- 15.Fearnhead, H. O., J. Rodriguez, E. E. Govek, W. Gou, R. Kobayashi, G. Hannon, and Y. A. Lazebnik. 1998. Oncogene-dependent apoptosis is mediated by caspase-9. Proc. Natl. Acad. Sci. USA 95:13664-13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisch, S. M., and K. E. Dolter. 1995. Adenovirus E1a-mediated tumor suppression by a c-erbB-2/neu-independent mechanism. Cancer Res. 55:5551-5555. [PubMed] [Google Scholar]
- 17.Gratton, J. P., M. Morales-Ruiz, Y. Kureishi, D. Fulton, K. Walsh, and W. C. Sessa. 2001. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J. Biol. Chem. 276:30359-30365. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich, K. A., and J. L. Kummer. 1996. Inhibition of p38 mitogen-activated protein kinase by insulin in cultured fetal neurons. J. Biol. Chem. 271:9891-9894. [DOI] [PubMed] [Google Scholar]
- 20.Hsu, S. C., M. A. Gavrilin, M. H. Tsai, J. Han, and M. Z. Lai. 1999. p38 mitogen-activated protein kinase is involved in Fas ligand expression. J. Biol. Chem. 274:25769-25776. [DOI] [PubMed] [Google Scholar]
- 21.Hung, M. C., G. N. Hortobagyi, and N. T. Ueno. 2000. Development of clinical trial of E1A gene therapy targeting HER-2/neu-overexpressing breast and ovarian cancer. Adv. Exp. Med. Biol. 465:171-180. [DOI] [PubMed] [Google Scholar]
- 22.Hunter, T. 2000. Signalin—2000 and beyond. Cell 100:113-127. [DOI] [PubMed] [Google Scholar]
- 23.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 24.Igney, F. I., and P. H. Krammer. 2002. Death and anti-death: tumor resistance to apoptosis. Nat. Rev. Cancer 2:277-288. [DOI] [PubMed] [Google Scholar]
- 25.Inoki, K., Y. Li, T. Zhu, J. Wu, and K. L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648-657. [DOI] [PubMed] [Google Scholar]
- 26.Kandel, E. S., J. Skeen, N. Majewski, A. Di Cristofano, P. P. Pandolfi, C. S. Feliciano, A. Gartel, and N. Hay. 2002. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol. Cell. Biol. 22:7831-7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy, S. G., E. S. Kandel, T. K. Cross, and N. Hay. 1999. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, A. H., G. Khursigara, X. Sun, T. F. Franke, and M. V. Chao. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, W. P., Y. Liao, D. Robinson, H. J. Kung, E. T. Liu, and M. C. Hung. 1999. Axl-gas6 interaction counteracts E1A-mediated cell growth suppression and proapoptotic activity. Mol. Cell. Biol. 19:8075-8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, W. P., Y. Wen, B. Varnum, and M. C. Hung. 2002. Akt is required for Axl-Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene 21:329-336. [DOI] [PubMed] [Google Scholar]
- 31.Lowe, S. W. 1999. Activation of p53 by oncogenes. Endocr. Relat. Cancer 6:45-48. [DOI] [PubMed] [Google Scholar]
- 32.Lowe, S. W., H. E. Ruley, T. Jacks, and D. E. Housman. 1993. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957-967. [DOI] [PubMed] [Google Scholar]
- 33.Mayo, L. D., and D. B. Donner. 2001. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 98:11598-11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCurrach, M. E., T. M. F. Connor, C. M. Knudson, S. J. Korsmeyer, and S. W. Lowe. 1997. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meric, F., W. P. Lee, A. Sahin, H. Zhang, H. J. Kung, and M. Hung. 2002. Expression profile of tyrosine kinases in breast cancer. Clin. Cancer Res. 8:361-367. [PubMed] [Google Scholar]
- 36.Meric, F., Y. Liao, W. P. Lee, R. E. Pollock, and M. C. Hung. 2000. Adenovirus 5 early region 1A does not induce expression of the Ewing sarcoma fusion product EWS-FLI1 in breast and ovarian cancer cell lines. Clin. Cancer Res. 6:3832-3836. [PubMed] [Google Scholar]
- 37.Nahle, Z., J. Polakoff, R. V. Davuluri, M. E. McCurrach, M. D. Jacobson, M. Narita, M. Q. Zhang, Y. Lazebnik, D. Bar-Sagi, and S. W. Lowe. 2002. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 4:859-864. [DOI] [PubMed] [Google Scholar]
- 38.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 40.Okumura, E., T. Fukuhara, H. Yoshida, S. Hanada, R. Kozutsumi, M. Mori, K. Tachibana, and T. Kishimoto. 2002. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2-M-phase transition. Nat. Cell Biol. 4:111-116. [DOI] [PubMed] [Google Scholar]
- 41.Page, C., H. J. Lin, Y. Jin, V. P. Castle, G. Nunez, M. Huang, and J. Lin. 2000. Overexpression of Akt/AKT can modulate chemotherapy-induced apoptosis. Anticancer Res. 20:407-416. [PubMed] [Google Scholar]
- 42.Pearce, A. K., and T. C. Humphrey. 2001. Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol. 11:426-433. [DOI] [PubMed] [Google Scholar]
- 43.Putzer, B. M., T. Stiewe, K. Parssanedjad, S. Rega, and H. Esche. 2000. E1A is sufficient by itself to induce apoptosis independent of p53 and other adenoviral gene products. Cell Death Differ. 7:177-188. [DOI] [PubMed] [Google Scholar]
- 44.Rane, M. J., P. Y. Coxon, D. W. Powell, R. Webster, J. B. Klein, W. Pierce, P. Ping, and K. R. McLeish. 2001. p38 kinase-dependent MAPKAPK-2 activation functions as 3-phopshoinositide-dependent kinase-2 for AKt in human neutrophils. J. Biol. Chem. 276:3517-3523. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Prieto, R., M. Lleonart, and S. Ramon y Cajal 1995. Lack of correlation between p53 protein level and sensitivity of DNA-damaging agents in keratinocytes carrying adenovirus E1a mutants. Oncogene 11:675-682. [PubMed] [Google Scholar]
- 46.Shisler, J., P. Duerksen-Hughes, T. M. Hermiston, W. M. Wold, and L. R. Gooding. 1996. Induction of susceptibility to tumor necrosis factor by E1A is dependent on binding to either p300 or p105-Rb and induction of DNA synthesis. J. Virol. 70:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shtivelman, E., J. Sussman, and D. Stokoe. 2002. A role of PI 3-kinase and PKA activity in the G2/M phase of the cell cycle. Curr. Biol. 12:919-924. [DOI] [PubMed] [Google Scholar]
- 48.Skorski, T. 2002. Oncogenic tyrosine kinases and the DNA-damage repsonse. Nat. Rev. Cancer 2:351-360. [DOI] [PubMed] [Google Scholar]
- 49.Teodoro, J. G., G. C. Shore, and P. E. Branton. 1995. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene 11:467-474. [PubMed] [Google Scholar]
- 50.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueno, N. T., C. Bartholomeusz, J. L. Herrmann, Z. Estrov, R. Saho, M. Andreeff, J. Price, R. W. Paul, P. Anklesaria, D. Yu, and M. C. Hung. 2000. E1A-mediated paclitaxel sensitization in Her-2/neu-overexpressing ovarian cancer SKOV3. ip1 through apoptosis involving the caspase-3 pathway. Clin. Cancer Res. 6:250-259. [PubMed] [Google Scholar]
- 53.Ueno, N. T., D. Yu, and M. C. Hung. 1997. Chemosensitization of Her-2/neu-overexpressing human breast cancer cells to paclitaxel (Taxol) by adenovirus type 5 E1A. Oncogene 15:953-960. [DOI] [PubMed] [Google Scholar]
- 54.Viniegra, J. G., J. H. Losa, V. J. Sanchez-Arevalo, C. P. Cobo, V. M. Soria, S. Ramon y Cajal, and R. Sanchez-Prieto 2002. Modulation of PI3K/Akt pathway by E1a mediates sensitivity to cisplatin. Oncogene 21:7131-7136. [DOI] [PubMed] [Google Scholar]
- 55.Yu, D., and M. C. Hung. 2000. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 19:6115-6121. [DOI] [PubMed] [Google Scholar]
- 56.Yu, D., and M. C. Hung. 2000. Role of erbB2 in breast cancer chemosensitivity. Bioessays 22:673-680. [DOI] [PubMed] [Google Scholar]
- 57.Yuan, Z. Q., R. I. Feldman, G. E. Sussman, D. Coppola, S. V. Nicosia, and J. Q. Cheng. 15 April 2003, posting date. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J. Biol. Chem. 278:23432-23440. [Online.] http://www.jbc.org. [DOI] [PubMed]
- 58.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, B. P., Y. Liao, W. Xia, Y. Zou, B. Spohn, and M. C. Hung. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973-982. [DOI] [PubMed] [Google Scholar]