Abstract
cDNAs encoding the Drosophila 70-kDa S6 kinase (S6K) were isolated by low-stringency hybridization with mammalian p70S6k probes. Conceptual translation of S6k cDNA sequences yields a product containing all of the canonical features typical of serine/threonine kinases and has 78% amino acid identity in the catalytic domain with the human p70S6k homologue. The S6k gene, located at polytene chromosome site 65D, gives rise to two predominant transcripts of 3.0 and 5.0 kb and at least two smaller transcripts (<3.0 kb) that are found in whole-animal RNAs at all stages of development. Blood cells derived from the hematopoietic organs of ribosomal protein S6 (RpS6air8) mutant animals express higher levels of the smaller S6k transcripts, suggesting tissue- or genotype-specific differences in the regulation of the S6k gene. Drosophila S6K expressed in COS or NIH 3T3 cells phosphorylates mammalian RPS6 in a mitogen-dependent wortmannin- and rapamycin-sensitive manner, suggesting that its regulation is similiar to mammalian p70S6k.
The phosphorylation of ribosomal protein S6 (RPS6) is a rapid and highly conserved cellular growth response that is observed during development and/or in response to a variety of extracellular stimuli (1). This phosphorylation is correlated with regulation of mRNA translation which, in turn, may influence cell proliferation or differentiation (2, 3). The kinase responsible for the phosphorylation of RPS6 in mammalian cells is the serine/threonine kinase p85S6k/p70S6k (4, 5, 6, 7). Like RPS6 phosphorylation, p70S6k activation is a highly conserved mitogenic response. Although a direct activating kinase of p70S6k has not been identified, biochemical studies have revealed some of the upstream regulators, which indicate at least two distinct signaling pathways influence p70S6k. One pathway is regulated by phosphatidylinositol 3-kinase [P(I)3K], as revealed by a variety of genetic, mutational, and pharmacological analyses. The latter demonstrate that the P(I)3K inhibitor wortmannin abrogates the mitogen-stimulated activation of both p70S6k and Akt (also called protein kinase B, RacPK) (8, 9, 10, 11). Recent studies have demonstrated that Rho family members, Cdc42 and Rac1, are involved in the activation of p70S6k (11). A separate pathway contributing to p70S6k activation involves the FKBP12-rapamycin (RAP)-associated protein (FRAP, also known as mTOR, RAFT, and RAPT) and was demonstrated using the immunosuppressant RAP. RAP, through complex formation with FKBP12 and FRAP causes G1-phase arrest in T lymphocytes and other hematopoietic tissues (12, 13, 14). Many of the effects of RAP correlate with the inactivation of p70S6k and inhibition of protein synthetic activities.
Inhibition of p70S6k activation results in the reduction of RPS6 phosphorylation that apparently leads to the selective inhibition of protein synthesis and a delay or arrest at the G1/S phase of the cell cycle in certain cell types (15, 16, 17). The requirement for p70S6k activity during G1 phase was shown in experiments using neutralizing p70S6k antibodies that prevented both the activation of protein synthesis and the serum-induced entry of cells into S phase (18). The molecular events underlying the complex signaling leading to RPS6 phosphorylation are not fully resolved and it is unclear whether RPS6 phosphorylation alone or signaling to other downstream targets is responsible for regulating cell proliferation and/or differentiation. To more extensively analyze these pathways, we reasoned that a Drosophila p70S6k would be of value and have identified a cDNA encoding a homologue of the mammalian enzyme. In the present study, we report the sequence of Drosophila S6k and study its regulation during transient expression in mammalian cells.
MATERIALS AND METHODS
Cloning of S6k.
Drosophila S6k cDNAs were obtained by screening Drosophila embryonic and third-instar cDNA libraries (provided by C. Thummel, University of Utah, Salt Lake City, UT), constructed in λZAPII vectors using RNA isolated from a wild-type strain (Canton-S). Plaques containing recombinant clones from the cDNA libraries were blotted onto Hybond-N membranes (Amersham) using standard methods (19). The membranes were hybridized under low-stringency conditions at 37°C for 3 days with rat p70S6k cDNA sequences (a gift from J. Avruch, Massachusetts General Hospital, Charlestown, MA) that were random-prime labeled (GIBCO/BRL, Life Technologies, Gaithersburg, MD). Plaques DNAs that gave positive signals on filters were isolated and counter-screened using the same conditions as the primary screen. Replicate filters were probed with Drosophila S6k cDNA fragments that were PCR-amplified using p70S6k-specific primers and phage DNAs from the primary screen. These amplicons were sequenced and used to probe the filters at high stringency (65°C, 16 hr). Clones that remained positive through each round of screening were subjected to plasmid rescue using ExAssist (Stratagene) and were sequenced.
Southern and Northern Blot Analyses.
Genomic DNAs derived from the strains y w67c23; H{Lw2}55A, y w67c23; H{Lw2}D85.6b, y w67c23; H{Lw2}J9.102 and y w67c23; Gla/SM6a (20) were digested, electrophoresed on agarose gels, and blotted as described below. Total RNA was isolated from wild-type animals and cultured cells using either a modified LiCl/urea method (21) or a guanidine salt/urea extraction protocol (RNazol, Biotecx Laboratories, Houston). The poly(A)+ RNA fraction was isolated from total RNA using a PolyAtract mRNA system (Promega). The RNA samples were resolved on 1% agarose/formaldehyde gels, blotted onto Hybond-N membranes (Amersham), UV-crosslinked, hybridized in 1% bovine serum albumin/0.5 M sodium phosphate, pH 7.2/7% SDS buffer, and washed in 0.1% SDS/0.1× SSC (standard saline citrate) at 65°C. Probes were random-primed labeled (GIBCO/BRL, Life Technologies) with [α-32P]dCTP (NEN).
PCR.
DNA was amplified using the polymerase chain reaction under the following conditions: 100–200 ng of template DNA, 150 ng each of forward and reverse primers, all four dNTPs (each at 1.25 mM), 1× buffer (Promega), and 2.5 units of Taq DNA polymerase (Promega). The PCR regimen involved 35 cycles of 94°C for 1 min, 47–70°C for 2 min (or 1–5°C below the lowest primer melt temperature), and 72°C for 3.5 min, followed by 1 cycle of 47–70°C for 2 min and 72°C for 10 min in a Perkin–Elmer/Cetus DNA thermal cycler. The amplicons from these reactions were recovered from agarose gels and used as probes in hybridization experiments or as templates in sequencing reactions.
DNA Sequencing.
DNAs derived from phage and plasmid recombinant clones and from PCR reactions were sequenced using dideoxynucleotide chain-termination methods with Sequenase (United States Biochemical) or double-stranded cycle sequencing (GIBCO/BRL, Life Technologies). In either application, both DNA strands were sequenced using a series of 17- to 20-mer oligonucleotide primers.
In Situ Hybridization to Polytene Chromosomes.
Polytene chromosomes were prepared from Oregon R third-instar salivary gland cells. A modified version of the biotinylated DNA in situ hybridization protocol (22) was used with the omission of the acetylation steps and an increase in the length and number of washes. S6k cDNAs were random-primed using biotin-dATP substitution and hybridization was done at 37°C for 18 hr. Biotin-labeled chromosome sites were detected using streptavidin/peroxidase reactions (Enzo Biochem) and chromosomes were counter-stained using Giemsa.
Mammalian Cell Transfections and S6 Kinase Assays.
Epitope-tagged Drosophila S6 kinase (S6k) constructs were generated by cloning the S6k cDNA in-frame, downstream of the hemagglutinin (HA) or myc epitope sequences in the pJ3Ω series vectors. NIH 3T3 cells were transfected with HA-p70S6k or HA-S6k (11). Cells were starved for 24 hr and then stimulated with platelet-derived growth factor (PDGF) for various time periods in the presence or absence of wortmannin (100 nM) or RAP (20 ng/ml). Cytosolic extracts were prepared and the recombinant proteins were immunoprecipitated with anti-HA antibodies (23). S6 kinase activity was measured as previously described using RPS6 as substrate (6). COS cells were transfected with myc-S6k using the DEAE-dextran method (11). Myc-S6K was immunoprecipitated from growing cells treated with or without wortmannin or RAP. Kinase assays were performed using RPS6 as substrate (7).
RESULTS AND DISCUSSION
The gene encoding Drosophila p85S6k/p70S6k, called S6k, was isolated by low-stringency hybridization with mammalian p70S6k probes. Three S6k cDNA classes were isolated from embryonic and third-instar larval libraries. Two classes of cDNA clones contained sequences predicting identical open reading frames (ORFs) encoding a polypeptide of 637 residues with a calculated molecular mass of 76 kDa (Fig. 1A). The first cDNA class (class 1) had a 530- nt 5′ untranslated region (UTR) whereas the second class (class 2) had an overlapping but shorter 5′ UTR of approximately 90 nt. The third cDNA class had divergent 5′-end coding sequences but contained an identical ORF starting from residue 52 through to the C terminus suggesting a second isoform (Fig. 1A). The significance of the two putative isoforms is unknown. Two forms, p85 and p70, are found in mammalian cells, with the former containing an N-terminal extension with a nuclear localization signal (25). The mammalian p85S6k isoform is primarily nuclear whereas the p70S6k isoform is both nuclear and cytoplasmic (26, 27).
Figure 1.

Predicted amino acid sequence of Drosophila melanogaster S6K and its alignment with human p85S6k/p70S6k. (A) Residue identities (vertical line) and conservative substitutions (:.) between S6K (Dm S6K) and p70S6k (Hs p85S6k/p70S6k) (5) are shown, with gaps introduced to maximize the alignment. Numeration of the protein sequences starts with the first in-frame methionine of S6K and the p70S6k isoform. The catalytic domain is outlined and subdomains are indicated with Roman numerals. Residues known to be phosphorylated in the mammalian protein (24) are numbered below the line and boxed with the consensus kinase recognition motif underlined adjacent to each phosphoresidue. Comparable residues conserved in Drosophila S6K are boxed and/or underlined. S6K contains the sequence motif FXGFT389YVAP that is found in members of the second messenger AGC family of related kinases (24). The pseudosubstrate autoinhibitory (SKAIPS) domain includes residues 400–424 of p70S6k (5) and the SKAIPS region in Drosophila S6K is represented by residues 409–431. The point of divergence of the putative S6K isoform is indicated above the sequence with an asterisk. Dm S6K accession no., U67304U67304; Hs p85S6k/p70S6k accession no., M60724M60724. (B) Comparisons between S6K and p70S6k showing the overall identities and similarities in the N-terminal, catalytic, and C-terminal domains. Clear box shows the location of the SKAIPS domain, and the p85S6k/p70S6k isoforms are indicated.
It is possible that the two Drosophila isoforms may perform similar roles as the mammalian isoforms. The conceptually translated S6K has all of the characteristic motifs of a serine/threonine protein kinase including the 12 canonical subdomains of the catalytic domain (28). The catalytic domain shows the highest degree of amino acid identity (≈78%) with mammalian p70S6k (25) (Fig. 1B). The N-terminal domain has 23% identity and 80% overall similarity with the human protein sequence suggesting that its general structure is important in the regulation or function of these enzymes. The C-terminal region contains sequences known as the SKAIPS (S6 kinase autoinhibitory pseudosubstrate) domain, which may regulate activity through interactions with the catalytic domain and the acidic N-terminal domain (5). The Drosophila S6K SKAIPS region has 26% sequence identity with Drosophila RPS6 (21), its proposed substrate. This is similar to the sequence relationship between mammalian p70S6k SKAIPS and the region of RPS6 that contains all of the known phosphorylation sites (5, 29).
Genomic blots probed with different portions of the cDNA sequence suggests the presence of a single S6k gene (Fig. 2). In support of this, in situ hybridization to polytene chromosomes using S6k cDNA probes detected only a single hybridization site on the third chromosome in polytene region 65D (data not shown).
Figure 2.
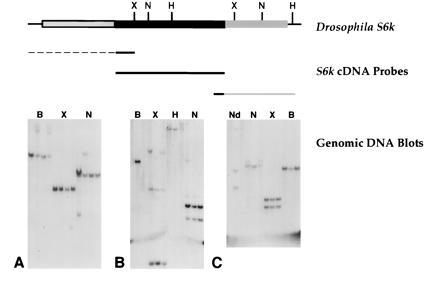
Hybridization of S6k cDNA sequences to Drosophila genomic DNAs. DNA samples derived from fly strains y w67c23; H{Lw2}55A (blot A only), y w67c23; H{Lw2}D85.6b, y w67c23; H{Lw2}J9.102 and y w67c23; Gla/SM6a(20) were digested with the restriction enzymes indicated above the lanes and probed with the following sequences. (A) N-terminal S6k cDNA (spanning subdomain II) and 5′-UTR sequences (common to class 1 and 2 S6k cDNAs). (B) Catalytic domain sequences. (C) C-terminal (from subdomain XI) and 3′-UTR sequences. B, BamHI; X, XhoI; N, NsiI; H, HindIII; Nd, NdeI.
The S6k gene gives rise to two major transcripts that are expressed throughout development, suggesting a continuous requirement for this enzyme (Fig. 3). Two major transcripts of 3.0 and 5.0 kb are found at abundant steady-state levels in whole animal poly(A)+ or total RNA (Fig. 3A). Two smaller transcripts (<3.0 kb) are expressed at lower levels at all tested developmental stages. All of these transcripts hybridized with probes that include the catalytic domain, C-terminal domain and the 3′ UTR (Fig. 3). Only the two major transcripts were detected with 5′-UTR probes common to class 1 and class 2 S6k cDNAs that also share a complete ORF (data not shown). These 5′-UTR probes did not detect the smaller transcripts (data not shown), suggesting that they have different N-terminal sequences. The smaller S6k transcripts are the predominant forms found in cultured air8 blood cells (Fig. 3B), which are derived from RpS6air8 (21) mutant larvae (K.L.W., D. J. Peel, W.M.G., R.L.E., and P. J. Bryant, unpublished results) suggesting that the S6k gene gives rise to tissue-specific expression patterns. An alternative explanation is that the S6k transcripts expressed in air8 cultured cells are influenced by the RpS6air8 mutation.
Figure 3.
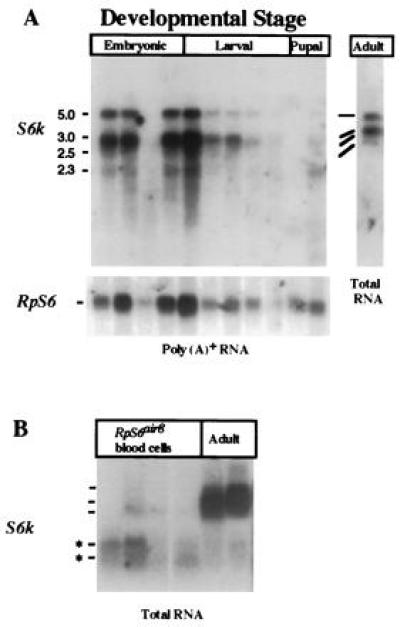
Expression of S6k during development and in Drosophila air8 cultured blood cells. (A) The 3.0-kb and 5.0-kb S6k transcripts are expressed at all stages of development and share a common open reading frame. A minimum of two smaller transcripts that differ in their 5′ ends from the major transcripts (data not shown) are found at all stages but in lower steady-state levels. (B) The smaller S6k transcripts are more abundant in total RNA derived from cultured Drosophila air8 blood cells [established from the neoplastic blood cells of RpS6air8 mutant larvae (21)] when compared with adult female RNAs. Poly(A)+ RNA was isolated from wild-type animals at the following developmental stages: embryonic, 4–8 hr, 8–12 hr, 12–16 hr, and 16–20 hr; larval, including first-instar, second-instar, early third-instar, and late (wandering) third-instar; pupal, including mid-pupae (degraded) and late pupae; adult total RNA from mixed-aged females. The S6k probe used in all of the experiments included sequences derived from the catalytic domain, C-terminal domain, and the 3′ UTR. Variations in sample loading on the gels were assessed by probing the filters with RpS6 sequences, which were shown to express consistent levels of 0.9- to 1.1-kb mRNA transcripts throughout development (21).
Mitogen stimulation results in the phosphorylation and activation of p70S6k (30, 31). In cultured cells, p70S6k shows a basal level of phosphorylation but additional phosphorylation is observed following mitogenic stimulation (30). Phosphorylation at multiple sites, including T229 and T389, appears to be critical for p70S6k activation as demonstrated through the use of kinase mutants and two inhibitors (wortmannin and RAP), which act by eliminating the phosphorylation of these residues (24, 29, 32). The high degree of sequence similarity between S6K and p70S6k, including the conservation of residues T229 and T389, suggests that they may have conserved biochemical functions and upstream regulators (Fig. 1A).
To determine whether S6K is regulated in a manner similar to the mammalian enzyme, HA-epitope-tagged S6K was transiently expressed in NIH 3T3 cells (Fig. 4 A and B). The addition of PDGF led to the decreased electrophoretic mobility of HA-S6K as determined by immunoblot analysis (Fig. 4B). The kinetics of the mobility shift were similar to that of p70S6k, which exhibits multiple size forms in response to PDGF that are attributable to phosphorylation. This mobility shift coincided with the activation of HA-S6K, as measured by its ability to phosphorylate RPS6 (Fig. 4B). Futhermore, pretreatment with wortmannin or RAP potently inhibited PDGF-dependent phosphorylation and activation of HA-S6K (Fig. 4B). Thus these results indicate that the upstream regulatory elements present in mammalian cells are capable of activating Drosophila S6K and that activated S6K can use mammalian RPS6 as a substrate. Expression of another epitope-tagged S6K (myc-S6K) in COS cells confirmed that the components required for the regulation of Drosophila S6K are present in other mammalian cell types (Fig. 4C).
Figure 4.
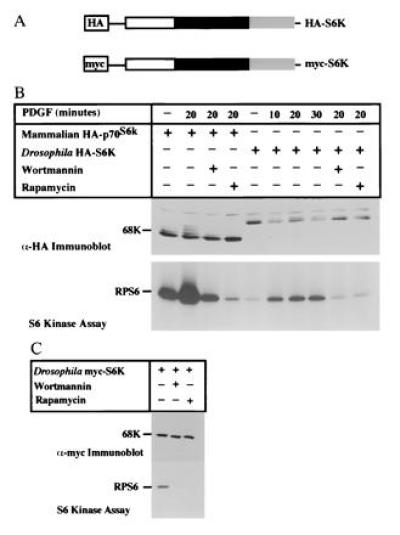
Characterization of epitope-tagged Drosophila S6K in transiently transfected mammalian cell lines. (A) Epitope-tagged Drosophila S6k constructs containing either HA or myc epitope sequences. (B) Growth factor stimulation of p70S6k. NIH 3T3 cells were transfected with HA-p70S6kor HA-S6k. Cells were starved for 24 hr and then stimulated with PDGF for the time periods indicated above the lanes in the presence or absence of wortmannin (100 nM) or RAP (20 ng/ml). The recombinant proteins were immunoprecipitated with anti-HA antibodies and S6 kinase activity was measured using RPS6 as substrate. (C) Regulation of S6K in monkey COS cells. COS cells were transfected with myc-S6k, and myc-S6K was immunoprecipitated from growing cells treated with or without wortmannin or RAP. Kinase assays were performed using RPS6 as substrate.
Although a variety of external stimuli can induce the activation of p70S6k, its regulation is complex and multiple signals are required to achieve its full activation. The cascades relaying these signals seem to have important roles in cell cycle progression through the translation of specific messages, the regulation of gene expression (30, 31, 33, 34), or the direct interaction with other downstream targets. Our results provide evidence that the structure of Drosophila S6K is conserved with mammalian p70S6k such that S6K responds to signal transduction pathways present in mammalian cells. The Drosophila system should facilitate the identification of genetic components modulating S6k expression and reveal the phenotypic consequences of altering their expression. This will elucidate the molecules with key roles in various biological processes that utilize these signaling pathways to affect cell growth, proliferation, and differentiation.
Acknowledgments
We thank and acknowledge J. Avruch, P. Bryant, R. Nicholls, G. Serbedzija, R. Siggins, C. Thummel, and D. Woods who contributed materials or suggestions that greatly facilitated this work. This investigation was supported by Grants CA42580 (R.L.E.), GM51405 (J.B.), and GM28669 (W.M.G.) from the National Institutes of Health. K.L.W. is supported by an American Cancer Society fellowship. M.M.C. is supported by a fellowship from the Cancer Research Institute/F. M. Kirby Foundation. J.B. is an established investigator of the American Heart Association. R.L.E. is an American Cancer Society Professor of Cellular and Developmental Biology.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: RPS6, ribosomal protein S6; S6K, S6 kinase; RAP, rapamycin; HA, hemagglutinin; UTR, untranslated region; SKAIPS, S6 kinase autoinhibitory pseudosubstrate; PDGF, platelet-derived growth factor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank data base (accession no. U67304U67304).
References
- 1.Erikson R L. J Biol Chem. 1991;266:6007–6010. [PubMed] [Google Scholar]
- 2.Frost V, Morley S J, Mercep L, Meyer T, Fabbro D, Ferrari S. J Biol Chem. 1995;270:26698–26706. doi: 10.1074/jbc.270.44.26698. [DOI] [PubMed] [Google Scholar]
- 3.Edelmann H M, Kuhne C, Petritsch C, Ballou L M. J Biol Chem. 1996;271:963–971. doi: 10.1074/jbc.271.2.963. [DOI] [PubMed] [Google Scholar]
- 4.Kozma S C, Ferrari S, Bassand P, Siegmann M, Totty N, Thomas G. Proc Natl Acad Sci USA. 1990;87:7365–7369. doi: 10.1073/pnas.87.19.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee P, Ahmad M F, Grove J R, Kozlosky C, Price D J, Avruch J. Proc Natl Acad Sci USA. 1990;87:8550–8554. doi: 10.1073/pnas.87.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blenis J, Chung J, Erikson E, Alcorta D A, Erikson R L. Cell Growth Differ. 1991;2:279–285. [PubMed] [Google Scholar]
- 7.Chung J, Kuo C J, Crabtree G R, Blenis J. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 8.Burgering B M Th, Coffer P J. Nature (London) 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 9.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 10.Weng Q-P, Andrabi K, Kliffep A, Kozlowski M T, Williams L T, Avruch J. Proc Natl Acad Sci USA. 1995;92:5744–5748. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou M M, Blenis J. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 12.Brown E J, Beal P A, Keith C T, Chen J, Shin T B, Schreiber S L. Nature (London) 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 13.Quesniaux V F, Wehrli S, Steiner C, Joergensen J, Schuurman H J, Herrman P, Schreier M H, Schuler W. Blood. 1994;84:1543–1552. [PubMed] [Google Scholar]
- 14.Dumont F J, Altmeyer A, Kastner C, Fischer P A, Lemon K P, Chung J, Blenis J, Staruch M J. J Immunol. 1994;152:992–1003. [PubMed] [Google Scholar]
- 15.Terada N, Patel H R, Takase K, Kohno K, Nairn A C, Gelfand E W. Proc Natl Acad Sci USA. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terada N, Takase K, Papst P, Nairn A C, Gelfand E W. J Immunol. 1995;155:3418–3426. [PubMed] [Google Scholar]
- 17.Reinhard C, Fernandez A, Lamb N J C, Thomas G. EMBO J. 1994;13:1557–1565. doi: 10.1002/j.1460-2075.1994.tb06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane H A, Fernandez A, Lamb N J C, Thomas G. Nature (London) 1993;363:170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 20.Smith D, Wohlgemuth J, Calvi B R, Franklin I, Gelbart W M. Genetics. 1993;135:1063–1076. doi: 10.1093/genetics/135.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson K L, Konrad K D, Woods D F, Bryant P J. Proc Natl Acad Sci USA. 1992;89:11302–11306. doi: 10.1073/pnas.89.23.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashburner M. Drosophila: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 23.Cheatham L, Monfar M, Chou M M, Blenis J. Proc Natl Acad Sci USA. 1995;92:11696–11700. doi: 10.1073/pnas.92.25.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson R B, Dennis P B, Han J W, Williamson N A, Kozma S C, Wettenhall R E, Thomas G. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grove J R, Banerjee P, Balasubrajmanyam A, Coffer P J, Price D J, Avruch J, Woodgett J R. Mol Cell Biol. 1991;11:5541–5550. doi: 10.1128/mcb.11.11.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhard C, Fernandez A, Lamb N J C, Thomas G. EMBO J. 1994;13:1557–1565. doi: 10.1002/j.1460-2075.1994.tb06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffer P J, Woodgett J R. Biochem Biophys Res Commun. 1994;198:780–786. doi: 10.1006/bbrc.1994.1112. [DOI] [PubMed] [Google Scholar]
- 28.Hanks S K, Quinn A M, Hunter T. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 29.Weng Q-P, Andrabi K, Kozlowski M T, Grove J R, Avruch J. Mol Cell Biol. 1995;15:2333–2340. doi: 10.1128/mcb.15.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou M M, Blenis J. Curr Opin Cell Biol. 1995;7:806–814. doi: 10.1016/0955-0674(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 31.Kozma S C, Thomas G. Semin Cancer Biol. 1994;5:255–260. [PubMed] [Google Scholar]
- 32.Han J W, Pearson R B, Dennis P B, Thomas G. J Biol Chem. 1995;270:21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- 33.Jefferies H B, Reinhard C, Kozma S C, Thomas G. Proc Natl Acad Sci USA. 1994;91:4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Groot R P, Ballou L M, Sassone-Corsi P. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]


