Abstract
The total dependence of amphibian metamorphosis on thyroid hormone (T3) provides a unique vertebrate model for studying the molecular mechanism of T3 receptor (TR) function in vivo. In vitro transcription and developmental expression studies have led to a dual function model for TR in amphibian development, i.e., TRs act as transcriptional repressors in premetamorphic tadpoles and as activators during metamorphosis. We examined molecular mechanisms of TR action in T3-induced metamorphosis by using dominant-negative receptors (dnTR) ubiquitously expressed in transgenic Xenopus laevis. We showed that T3-induced activation of T3 target genes and morphological changes are blocked in dnTR transgenic animals. By using chromatin immunoprecipitation, we show that dnTR bound to target promoters, which led to retention of corepressors and continued histone deacetylation in the presence of T3. These results thus provide direct in vivo evidence for the first time for a molecular mechanism of altering gene expression by a dnTR. The correlation between dnTR-mediated gene repression and inhibition of metamorphosis also supports a key aspect of the dual function model for TR in development: during T3-induced metamorphosis, TR functions as an activator via release of corepressors and promotion of histone acetylation and gene activation.
Thyroid hormone (T3) affects a wide range of biological processes, from metabolism to development (72). The diverse effects of T3 are generally believed to be mediated through gene regulation by T3 receptors (TRs). In vitro and tissue culture studies have shown that T3 activates transcription by binding to TR, which most likely heterodimerizes with RXR (9-cis-retinoic acid receptor) and binds to thyroid hormone response elements (TREs) in T3 response genes. The binding of TREs by TR/RXR heterodimers is, however, independent of T3 (9, 35, 38, 43, 62, 69), implicating a role of unliganded TR in gene regulation. Indeed, various in vitro studies have revealed that unliganded TRs repress target transcription whereas, in the presence of T3, they enhance the transcription of these same genes (11, 23, 62, 69, 75). TRs exert these effects by recruiting TR-interacting cofactors. Many such cofactors have been isolated and characterized based on their ability to interact with TRs in the presence and/or absence of T3 (3, 4, 29, 39, 45, 70, 72, 75).
Compared to the enormous biochemical and molecular information on TR function from in vitro and tissue culture cell studies, much less is known about TR function and associated mechanisms in development. This has been largely due to the lack of proper animal models and/or suitable methodologies for in vivo studies. Recent genetic studies in mice have provided in vivo evidence to support the critical role of TRs in mediating developmental functions of T3. Interestingly, mice lacking TRα or TRβ or both have fewer developmental defects than mice lacking T3 (10, 12, 13, 16, 18, 67). In addition, a major cause of resistance to thyroid hormone syndrome in humans is due to mutations in the TRβ gene, leading to the formation of dominant-negative (dn) TRβs (1, 2, 46, 72). Mice with dnTRβ, which mimics resistance to thyroid hormone syndrome in humans, and dnTRα have been analyzed at the phenotypic level but, in most cases, very little information is available on the expression of genes known to be regulated by T3 (20, 32, 33, 61, 76).
The underlying molecular basis of the different phenotypes and differences in gene expression found in the various TR knockout and mutant animals remains unclear. Potential explanations for these differences may be found in different expression profiles and levels of different receptors, distinct isoform-dependent in vivo signaling pathways, or indirect effects through altered circulating T3 titer. In mice with a mutation introduced into either the TRα or TRβ locus, resulting in the expression of a dnTRα or dnTRβ, a number of known T3 response genes in different tissues were found to have different expression levels compared to wild-type mice (32, 33). However, the results from dnTRα and dnTRβ mice were quite different and could not be explained simply based on the regulation mechanisms obtained from in vitro or cell culture studies. Furthermore, it is unclear whether these genes are directly or indirectly affected by the TR knockout or transgene since no direct evidence is available to show whether TR binds to these genes and/or recruit cofactors to their promoters in the animals. In addition, other mechanisms of the regulation of T3 target genes in these animals, such as through nongenomic mechanisms acting via cytosolic proteins (6), cannot be ruled out. Thus, to understand the developmental and pathological roles of TR, it is important to study the molecular basis by which TR mediates the effects of T3 in various developmental processes in vertebrates.
We have been using Xenopus laevis metamorphosis as a model to investigate the developmental function and mechanism of gene regulation by TRs. Frog metamorphosis involves transformation of every organ and tissue of the tadpole and different organs and tissues undergo vastly different changes (7, 17, 52, 58, 73). Despite drastic differences across tissues, all are controlled by T3. This dependence on T3 makes frog metamorphosis a unique model for studying T3 function in vertebrate development.
Here, we have generated transgenic X. laevis tadpoles expressing a dn form of X. laevis TRα (dnTR). We show that the dnTR blocks T3-induced metamorphosis at the beginning of prometamorphosis (stage 54) (41) and that dnTR inhibits the expression of known T3 response genes. More importantly, we used chromatin immunoprecipitation (ChIP) to show that the dnTR binds to the TREs of endogenous-T3-response genes and retains corepressors at the target genes even when tadpoles are treated with T3. The ChIP assay also revealed reduced histone acetylation in dnTR transgenic tadpoles treated with T3, a finding consistent with the lack of gene activation in the presence of T3. Thus, our results provide, for the first time in vivo, direct evidence that T3-induced development requires TRE binding by TR, release of corepressors, and consequent chromatin modification.
MATERIALS AND METHODS
Transgenesis and tadpole treatment.
Transgenesis was performed as described previously (5, 14) by using the South African clawed frog (Xenopus laevis) (Nasco, Fort Atkinson, Wis.). The dnTR transgenesis vector pCS2G was a gift from A. Schreiber and D. Brown (51). The dn receptor has a 12-amino-acid deletion from the C terminus, preventing it from binding ligand. We also cloned the NotI fragment containing the crystallin promoter controlling green fluorescent protein (GFP) from CRY1/GFP3 construct (a gift from B. Grainger, University of Virginia) into the NotI site of pCS2G to make CGCGΔTR. This double promoter transgenesis vector regulates the expression of two genes from the same construct, GFP by the crystallin promoter and dnTR by the cytomegalovirus (CMV) promoter (14). Transgenic animals were identified by GFP expression in the eye and/or in gill and nasal regions. At stage 54, tadpoles were treated with 0, 5, or 50 nM T3 (3,5,3′-triiodothyronine; Sigma) for 1 to 3 days at room temperature with 1 to 3 tadpoles in 500 ml. Tadpoles in treatments were not fed, and water and hormone were changed daily.
Histology and reverse transcriptase PCR (RT-PCR).
For histology, tadpole intestines were fixed for 1 h at room temperature or overnight at 4°C in 4% paraformaldehyde-60% phosphate-buffered saline, cryoprotected for 2 h in 0.5 M sucrose in 60% phosphate-buffered saline, embedded in OCT medium (TissueTek), and cryosectioned at 10 μm. Sections were stained with methyl green pyronin Y (Muto, Tokyo, Japan) (25).
For RT-PCR, total RNA from tadpole organs was isolated by using Trizol reagent (Invitrogen). RT-PCRs were performed by using Superscript One-Step RT-PCR (Invitrogen) and included 0.5 μg of total RNA and two primer sets per reaction tube: one for the control rpl8 (ribosomal protein L8) and one for the gene of interest. The RT-PCR primers were designed to bind in different exons to avoid unintentional amplification of potential genomic DNA contamination. The primers (5′-3′) used were CGTGGTGCTCCTCTTGCCAAG and GACGACCAGTACGACGAGCAG for rpl8 (57), CATCATGATTCCTGGTAACCGA and AAATTTCCATTTTCTGCTGTGC for BMP-4 (40), GGAACTTGGAAGGTTGACAGA and GCCTCTCTTGAAAATCCTTTTTG for IFABP (55), CCTGATGCATGCAAAACT and GTTCATCCTGGAAAGCAG for ST3 (42), GGGCAGTGGACATCACCAC and GTTGACCTTGGTCTGGGCC for xhh (59), CACTTAGCAACAGGGATCAGC and CTTGTCCCAGTAGCAATCATC for TH/bZip (15), and ATAGTTAATGCGCCCGAGGGTGGA and CTTTTCTATTCTCTCCACGCTAGC for TRβ (71). The reverse transcription reaction was carried out at 50°C for 30 min, followed by 28 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. These reaction conditions and the cycle number were based on those used previously by us and others for these genes (26, 47). Between 2 and 15 transgenic tadpoles were used for each gene-tissue combination.
ChIP assay.
ChIP assay was done as described previously (50). The following antibodies were used in the ChIP assay: anti-Xenopus TR (68), anti-GFP (Torrey Pines Biolabs, Inc., Houston, Tex.), anti-acetylated H4 (Upstate Biotechnology, Inc., Lake Placid, N.Y.), anti-Xenopus N-CoR (49), and anti-Xenopus SMRT, which was generated by immunizing a rabbit with the polypeptide KSKKQEMIKKLSTTNRSEQE, located in a 2-kb cDNA fragment corresponding to C-terminal part, encompassing the TR-binding domain, of the Xenopus laevis SMRT (T. Amano and Y.-B. Shi, unpublished results).
RESULTS
Transgenic expression of a dnTR blocks T3-induced gene regulation and metamorphosis in X. laevis.
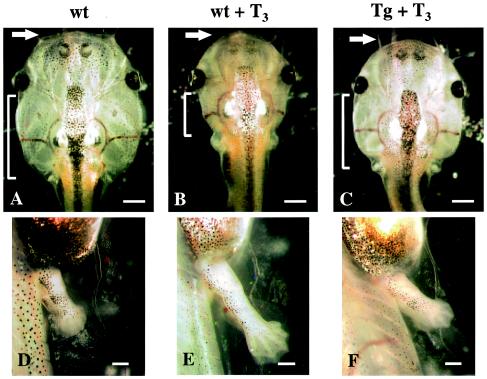
Using the ChIP assay, we have shown previously that TRs bind constitutively to T3 target genes in premetamorphic tadpoles (50), supporting a causative role of the activation of T3 target genes by T3-bound TR in initiating amphibian metamorphosis. To investigate directly the function and the underlying molecular mechanism of gene regulation by TR in development, we used a CMV promoter in transgenesis (34) to overexpress a dnTR with GFP fused at the N terminus, which is known to inhibit T3-induced morphological changes in very early premetamorphic tadpoles (51). When premetamorphic (stage 54) wild-type tadpoles were given a 3-day treatment of 5 nM T3, a concentration close to the peak level of plasma T3 during metamorphosis (36), metamorphosis was induced as expected (Fig. 1). The most noticeable external changes included the regression of gills, proliferation of Meckel's cartilage, and hind limb growth and morphogenesis (Fig. 1B and E). All of these changes were prevented in about 50% of the dnTR transgenic tadpoles treated similarly with T3 (Fig. 1C and F). The remaining transgenic tadpoles responded to T3 to some degree presumably due to lower transgene expression levels, as suggested by the fluorescence of the GFP fused to the dnTR in gill and nasal regions (not shown). Histological analysis of the intestine showed that T3 treatment of wild-type tadpoles but not transgenic tadpoles expressing dnTR led to the precocious intestinal remodeling, including proliferation of the cells of the adult epithelium, connective tissue, and muscles, as well as the degeneration of the larval epithelium (Fig. 2).
FIG. 1.
Transgenic expression of dnTR inhibits T3-induced morphological changes. Wild-type tadpoles (wt) or transgenic tadpoles expressing the dnTR under control of the CMV promoter (Tg) were treated at stage 54 with 0 or 5 nM T3 for 3 days and then examined for changes in external morphology. Transgene expression was confirmed by observation of fluorescence in the nares from the GFP moiety at the N terminus of the dnTR fusion protein. Note that regression of gills (brackets) and proliferation of Meckel's cartilage (arrows) were induced after 3 days in wild-type tadpoles (compare panels A and B), whereas such changes did not occur in the T3-treated transgenic animals (C). Similarly, hind limb growth and morphogenesis were induced by T3 treatment in the wild type (compare panels D and E) but not in transgenic animals (F). In the absence of T3, wild-type and transgenic animals are expected to be the same because endogenous TR (TRα is expressed during premetamorphosis) and dnTR function similarly without ligand, and they were indeed found to be the same during premetamorphosis (not shown). Thus, transgenic animals without T3 treatment were not used in this and other experiments. All experiments in Fig. 1 to 6 were repeated at least three times with similar results. Bars: 2 mm (A to C) and 1 mm (D to F).
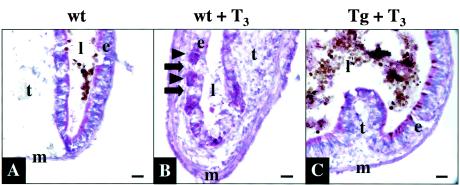
FIG. 2.
Transgenic expression of dnTR inhibits T3-induced intestinal remodeling. Wild-type (wt) or transgenic (Tg) tadpoles were treated with T3 as in Fig. 1. Cryosections of isolated intestines were stained with methyl green pyronine Y. (A) Note that the untreated control intestine had thin muscle layers (m) and sparse connective tissue except in the typhlosole (t), which is the single epithelial fold occupying much of the lumen (l) in the larval intestine (only part of the typhlosole is shown in the image). The majority of staining was seen in the larval epithelium (e). (B) In wild-type tadpoles after 3 days of T3 treatment, the muscle and connective-tissue layers increased in thickness largely due to active cell proliferation (56). The labeling in the larval epithelial cells decreased dramatically as these cells underwent apoptosis (arrows). Adult epithelial cells (arrowheads), whose origin remains unknown, were induced to proliferate and show intense staining. (C) All of these changes were prevented in the transgenic tadpoles treated with T3 for 3 days. Note that the typlosoles differ in size because of intestinal remodeling and/or the sections were from different locations within the anterior intestine. The brown material visible in panels A and C is gut contents in the lumen. The results are representative of three transgenic tadpoles examined. Bar, 20 μm.
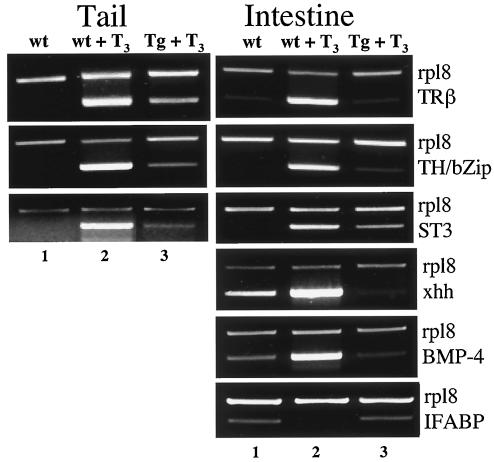
To investigate whether inhibition of T3-induced metamorphosis by transgenic dnTR expression was due to specific effects on T3-induced gene regulation cascade, we analyzed the expression of genes known to be T3-regulated during metamorphosis. We focused on the intestine and tail, where the dramatic gene regulation by T3 correlates with T3-induced morphological and cellular changes (53, 54, 65). First, we analyzed the expression of two ubiquitously regulated, direct-T3-response genes, TRβ and TH-induced bZip-containing transcription factor (TH/bZip) (28). Because direct-T3-response genes are known to be induced within 1 day of T3 treatment whereas indirect-response genes require longer T3 treatment, we investigated their regulation in wild-type and transgenic tadpoles after 1 day of T3 treatment with either 5 nM (not shown) or 50 nM T3 (Fig. 3). As expected, both TRβ and TH/bZip were induced by T3 in the intestine and tail of wild-type animals (Fig. 3, compare lane 2 to 1). In transgenic tadpoles expressing dnTR, their regulation was inhibited (Fig. 3, lane 3), suggesting that dnTR directly blocks T3 induction of these genes.
FIG. 3.
The induction of direct-T3-response genes is inhibited in transgenic animals expressing dnTR. Wild-type (lanes 1 and 2) or transgenic (lane 3) tadpoles at stage 54 were treated with 0 or 50 nM T3 for 1 day. RT-PCR analysis was carried out for the expression of the indicated T3-regulated genes on RNA isolated from the tail and intestine. Included in each reaction tube were a primer set for a T3-regulated gene and one for the T3-independent control gene rpl8 (57). The amplified DNA was analyzed by agarose gel electrophoresis. Note that TRβ and TH-induced bZip-containing transcription factor (TH/bZip) were induced both in the tails and intestines of wild-type tadpoles (compare lanes 1 and 2), as expected. Their induction was dramatically inhibited in the transgenic tadpoles expressing dnTR (lane 3).
We next examined whether transgenic expression of dnTR affects gene expression associated with T3-induced morphological changes after 3 days of treatment. We first analyzed three direct-T3-response genes that are regulated in different organs: TRβ, TH/bZip, and a fibroblast-specific stromelysin 3 (ST3) (42). All three were induced in both the tail and intestine by the treatment of premetamorphic tadpoles at stage 54 with 5 nM T3 for 3 days (Fig. 4, compare lanes 1 and 2), a treatment that induced observable morphological changes (Fig. 1 and 2). Their induction was prevented or drastically reduced in transgenic tadpoles expressing dnTR (Fig. 4, compare lane 3 to 2). In addition, several genes which are known to be regulated by T3 mainly or exclusively in the intestine failed to respond to the T3 treatment in the dnTR transgenic but not wild-type tadpoles (Fig. 4, compare lane 3 and 2 to 1 for the intestine samples). These included the epithelium-specific direct-T3-response gene Sonic hedgehog (xhh) (27, 59), the connective tissue-specific late upregulated gene bone morphogenic protein 4 (BMP-4) (26), and the epithelium-specific late downregulated gene intestinal fatty acid-binding protein (IFABP) (24, 55). In particular, the regulation of the late genes BMP-4 and IFABP is associated with morphological changes in the intestine. The failure of these genes to be regulated by T3 in the dnTR transgenic tadpoles throughout the treatment indicates that dnTR inhibits metamorphosis by blocking the regulation of both the direct and late, indirect-T3-response genes in different organs or tissues.
FIG. 4.
Transgenic expression of dnTR inhibits T3-induced changes in the expression of both ubiquitous and tissue-specific direct or indirect-T3-response genes. Wild-type (lanes 1 and 2) or transgenic (lane 3) tadpoles at stage 54 were treated with 0 or 5 nM T3 for 3 days, a treatment known to be sufficient to induce changes in the expression of both direct- and late, indirect-T3-response genes (53). RT-PCR analysis was carried out as in Fig. 3. TRβ and TH/bZip are induced by T3 ubiquitously, whereas ST3 is a fibroblast-specific direct-response gene. As expected, all three were induced both in the tails and intestines of wild-type tadpoles (compare lanes 1 and 2) but not in the transgenic tadpoles expressing dnTR (lane 3). In addition, the intestine-specific T3-induced direct-response gene Sonic hedgehog (xhh), the late upregulated gene for BMP-4, and the late downregulated gene for IFABP were regulated as expected in the wild-type animals (compare lanes 1 and 2). Again, T3 regulation of all of these genes was prevented or greatly inhibited in the transgenic tadpoles (lane 3). There are some variations in the degrees of inhibition on T3-induced changes in gene expression among different genes and/or in different organs. This is likely due to differences between the tail and intestine and/or among the promoters of different genes.
The dnTR inhibits T3-induced metamorphosis through recruitment of histone deacetylase-containing corepressor complexes even in the presence of T3.
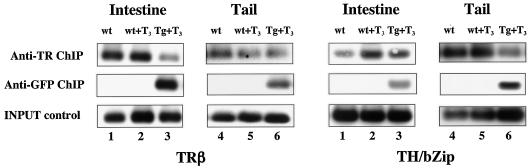
To investigate the molecular mechanism by which dnTR blocked gene activation by T3 in transgenic tadpoles, we first determined whether dnTR was capable of binding to endogenous-T3-response genes by competing for binding with endogenous wild-type TRs. We treated premetamorphic wild-type or transgenic tadpoles with 5 nM T3 for 1 day and isolated nuclei from the intestines, tails, or whole animals. The nuclei were subjected to ChIP assay with polyclonal antibodies against wild-type TRs (recognizing both TRα, TRβ, and dnTR) or GFP (recognizing GFP fused to the dnTR transgene) to detect TRs bound to the endogenous-T3-response genes TRβ and TH/bZip. As shown in Fig. 5, the TRE regions of both genes were bound by TRs constitutively in both wild-type and transgenic animals. The ChIP assay with the GFP antibody demonstrated that dnTR was also bound to endogenous TREs (Fig. 5). This is also supported by the weaker signals with the TR antibody in the transgenic animals, which was likely due to the competition for binding to the TREs by dnTR with wild-type TRs and the possible interference of the immunoprecipitation by the GFP moiety in the fusion protein dnTR.
FIG. 5.
The dnTR competes effectively with wild-type TRs for binding to endogenous-T3-response genes in transgenic tadpoles. Wild-type tadpoles (wt, lanes 1 to 2) or transgenic tadpoles carrying the GFP-TR fusion gene dnTR (Tg, lanes 3) were treated with (lanes 2 to 3) or without (lanes 1) 5 nM T3 for 1 day. Nuclei were isolated from intestine or tail and subjected to ChIP assay with the antibodies to indicated proteins for the binding of the TRs to the TRE regions of the endogenous TRβ and TH/bZip genes. Note that the ChIP assay with the GFP antibody clearly showed that the fusion protein is bound to the T3 response genes in different organs of the transgenic animals. The ChIP assay with the TR antibody, which recognizes both TRα and TRβ, showed that TR is constitutively bound to both genes in different organs or whole animals (not shown) as expected (the weaker signal in the transgenic animals is likely due to possible interference of the immunoprecipitation by the GFP moiety in the fusion protein. Regardless of the exact cause, it does not affect the main conclusion that dnTR can compete with endogenous TR for binding to TREs). It is unclear why the T3 treatment enhanced the binding of TR to the TH/bZip promoter in the intestines but not the tails. However, this has no impact on our conclusion regarding dnTR binding to endogenous promoters. The INPUT control shows the DNA level prior to immunoprecipitation with the antibodies.
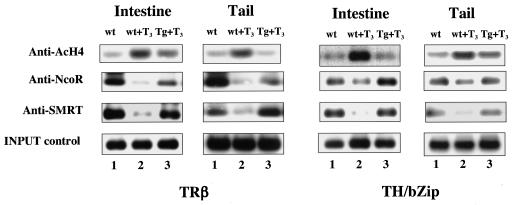
To further understand the molecular mechanism underlying altered transcriptional regulation in the dnTR transgenic tadpoles, we investigated whether the competitive binding of TREs by dnTR in vivo altered cofactor recruitment. For this purpose, we carried out a ChIP assay to address the recruitment of corepressors and changes in histone acetylation at the promoters of T3 response genes. Since the functional defect in dnTR is its inability to bird T3, no changes were expected in transgenic tadpoles compared to the wild type in the absence of T3. Thus, we focused on changes in T3-treated animals. When wild-type animals were treated with T3, the association of N-CoR to the TREs of the endogenous TRβ and TH/bZIP promoters was drastically reduced, and the acetylation levels of histone H4 was upregulated at the TRE regions in the tail and intestine, in agreement with previous observations (Fig. 6) (49, 50). In addition, by using an antibody against a peptide of the Xenopus corepressor SMRT, we found that it was also recruited to the TREs in the absence of T3 and was released upon T3 treatment in wild-type animals (Fig. 6). When transgenic animals expressing dnTR were treated with T3, both N-CoR and SMRT were retained at the TREs (Fig. 6). Since both N-CoR and SMRT are known to form complexes with histone deacetylases (19, 30, 31, 37, 64, 66, 74), this retention of corepressors suggests that the histones at the TRE regions would be underacetylated compared to wild-type animals in the presence of T3. Indeed, ChIP assay with anti-acetylated H4 antibody showed that the acetylation levels of H4 at the TRE regions were much lower compared to wild-type animals treated with T3. Thus, the retention of corepressor complexes by dnTR leads to histone deacetylation at the T3 response genes, thereby preventing their activation by T3 treatment.
FIG. 6.
The dnTR retains corepressors at the T3 response genes even in the presence of T3 and prevents T3-induced histone acetylation at the target genes. Wild-type tadpoles (wt, lanes 1 and 2) or transgenic tadpoles carrying the GFP-TR fusion gene dnTR (Tg, lanes 3) were treated with (lanes 2 and 3) or without (lanes 1) 50 nM (for acetylated H4 [AcH4]) or 5 nM (for all others) T3 for 1 day. ChIP assay was carried out as in Fig. 5 except that different antibodies were used as indicated. Note that T3 induced the release of corepressors in wild-type animals but, in transgenic animals expressing dnTR, this release was inhibited significantly if not completely (the extent of the inhibition appeared to vary slightly in different tissues for the two genes but this does not affect the conclusion), and as a result, histone acetylation levels at the target genes in the transgenic animals were lower compared to wild-type animals even though both were treated with T3. The INPUT control shows the DNA level prior to immunoprecipitation with the antibodies.
DISCUSSION
TRs are dual-function transcription factors. They bind to their target genes through TREs and repress T3-inducible genes in the absence of T3 and activate them when T3 is present. They are presumed to mediate most, if not all, of the biological function of T3 in development and organ function. Recent genetic studies in mice have provided some support for this model. However, the molecular basis for the differences in phenotypes among various TR-knockout mice, transgenic/mutant TR-knockin mice, and hypothyroid mice is unclear due to the lack of molecular studies on the regulation of T3 target genes in these animals (10, 12, 13, 16, 18, 20, 32, 33, 61, 67, 76). This raises the possibility that T3 may function through TR-independent pathways by acting through cytosolic proteins (6). In the present study we show, by using frog metamorphosis as a model, that a dnTR expressed in transgenic animals specifically inhibits the expression of endogenous-T3-response genes to block the biological effects of T3, i.e., the induction of metamorphosis. More importantly, we demonstrate here for the first time in developing animals that the dnTR inhibits T3 response gene regulation by binding to TREs in the target genes, thereby retaining corepressor complexes to deacetylate histones at the target gene promoter even in the presence of T3.
Functions of TRs during frog development.
Based on the expression studies on TRs and their heterodimerization partners RXRs in X. laevis, we have previously proposed a dual-function model for the role of TR in frog development (48). That is, TR/RXR heterodimers function as transcriptional repressors of T3-inducible genes in premetamorphic tadpoles to allow for animal growth and prevent premature metamorphosis and as transcriptional activators of these genes when T3 becomes available later to initiate metamorphic changes in different tissues. Earlier work has provided several independent lines of evidence in support of this model. First, by introducing TRs and/or RXRs into developing X. laevis embryos, we had shown that TR/RXR heterodimers are capable of repressing endogenous T3 resposne genes in the absence of T3 and activating them when T3 is present (44). Second, by transiently transfecting tail muscle cells in vivo, Tata and coworkers showed a dnTR was able to inhibit the T3 induction of a cotransfected T3-inducible promoter in premetamorphic tadpoles (63). Finally, we had demonstrated that TR and RXR are bound constitutively to the TREs of T3 response genes in premetamorphic tadpoles (50), implicating a role of unliganded TR/RXR in premetamorphic tadpole development. Our data presented here showing molecular evidence in vivo is the most direct support for this model to date.
The dual function of TRs in development may not be unique to amphibian metamorphosis because recent genetic studies support, by implication without direct evidence, this model in mammals. For example, mice lacking either TRα or TRβ or both have fewer abnormalities in development and/or organ functions than are associated with hypothyroidism (12, 13, 16, 18, 21, 67). Furthermore, as in frogs, during early embryogenesis, mammalian fetuses have little or no detectible T3 in plasma, although TRs are expressed (60). As in amphibian TR expression studies, these data imply a role for unliganded TR during early mammalian embryogenesis before zygotic synthesis of T3, which subsequently would activate T3-inducible genes through binding to TRs. Unfortunately, few mammalian T3-inducible genes have been identified and analyzed during development, and no study has examined the molecular mechanism of T3-induced transcription in vivo to determine the role of TRs in mediating the developmental effects of T3. We directly address these issues here in vivo by using ubiquitous transgenic overexpression of a dnTR that inhibits amphibian metamorphosis. We showed that the dnTR specifically inhibited the regulation of all known T3 response genes analyzed thus far by binding to TREs in different organs and tissues. Thus, our data provide direct in vivo molecular support for the dual-function model for TR function in development by revealing a critical role of transcriptional activation of T3-inducible genes by T3-bound TRs in activating morphogenic transformation in various tissues and organs.
Role of corepressors in the regulation of T3 response genes in development.
Since the cloning of TRs in 1986 (9), extensive effort has been directed toward understanding the molecular mechanisms governing transcriptional regulation by TRs, leading to the identification of many cofactors (3, 4, 29, 39, 45, 70, 72, 75). Studies in vitro and in tissue culture cells, as well as in frog oocytes, have shown that transcriptional activation by T3-bound TRs involves the recruitment of coactivator complexes with (e.g., SRC and p300) or without (e.g., TRAP or DRIP) histone acetyltransferase activity, in a process that also involves chromatin remodeling (3, 4, 22, 23, 29, 39, 45, 69, 70, 72, 75). In the absence of T3, the unliganded TR repressed transcription by recruiting corepressors. The best-studied corepressors for TRs are N-CoR and SMRT. Many of the cofactors are expressed in many different cell types and tissues and thus are likely to participate in gene regulation by TRs. However, there have been few studies addressing the function of these cofactors in gene regulation by TRs in developing animals due to the lack of proper systems for molecular analysis.
Frog metamorphosis is an excellent system for analyzing TR regulation at the molecular level. Diverse changes in different organs, such as resorption of the larval gills and tail and de novo development of the limbs and adult intestine, await the cue from T3 to initiate the metamorphic changes (7, 17, 52, 58, 73). Thus, investigation of the role of cofactors in different developmental processes is simplified by the uniform state of TR function in premetamorphosis where there is no T3. By using the ChIP assay, we had previously shown that TR/RXR heterodimers bind TREs in premetamorphic tadpoles constitutively (50). More recently, we demonstrated recruitment of N-CoR by unliganded TRs to the target genes in premetamorphic tadpoles and its release by T3 treatment (49). Consistent with the association of N-CoR and SMRT with histone deacetylases (19, 30, 31, 37, 64, 66, 74), T3 treatment of premetamorphic tadpoles leads to an increase in local histone acetylation levels at the target genes upon the release of N-CoR (49). In the present study, we have extended these earlier findings by showing that Xenopus SMRT is also recruited by unliganded TRs in premetamorphic tadpole tissues and is released upon T3 treatment. Thus, both N-CoR and SMRT appear to have similar function in tadpole development.
More importantly, our results for the first time demonstrate that the activation of T3 response genes by TR is essential for amphibian metamorphosis. Transgenic expression of the dnTR specifically inhibits the regulation of both direct and indirect-T3-response genes, and this inhibition is tightly associated with the blockage of metamorphosis. Transgenic animals in which T3 response genes were not influenced by transgene expression (due to lower expression levels as reflected by the fluorescence of the GFP fused to the dnTR in gill and nasal regions) were able to undergo T3-induced morphological changes (data not shown) (It is worth pointing out that, due to low expression levels of endogenous TR, which is not detectable by standard Western blot analysis [8], and the lack of an antibody that recognizes equally both the endogenous TR and transgenic dnTR, it is difficult to directly compare the levels of transgenic TR with endogenous TR. On the other hand, our phenotype and molecular analyses clearly indicated that sufficient transgenic TR was expressed in the transgenic animals to affect their development.) By ChIP assay, we have shown that this inhibition involves the binding of dnTR to TREs in T3-inducible genes. This leads to the retention of the corepressors SMRT and N-CoR at the target genes even in the presence of T3, a finding consistent with the inability of dnTR to bind T3. This retention of the corepressors appears to lead in turn to histone deacetylation at the target genes, in agreement with the ability of SMRT and N-CoR to form complexes containing histone deacetylases. Thus, our data show that the release of corepressors by T3 and subsequent histone acetylation are critical for the activation of T3-inducible genes by TR, the first step in the gene regulation cascade responsible for T3-dependent tissue transformation during frog metamorphosis.
In conclusion, we have shown here that during development the ligand-binding and activation function of TR is essential for amphibian metamorphosis. The total dependence of this process on T3 and concurrent transformation of different tissues and organs have allowed us to show, for the first time in developing animals, that corepressor release and histone acetylation are important for gene activation by T3 and subsequent morphological changes. The development of reagents for analyzing the function of coactivators should allow us in the future to test whether coactivator recruitment after corepressor release participates in this process. Further analysis in different organs and tissues with similar approaches should help to determine whether different cofactors are utilized by TR in different developmental programs, such as cell proliferation, differentiation, and apoptosis.
Acknowledgments
We thank A. Shreiber and D. D. Brown for the gift of the dnTR plasmid.
D.B. and S.-C.V.H. contributed equally to this study.
REFERENCES
- 1.Adams, M., C. Matthews, T. N. Collingwood, Y. Tone, P. Beck-Peccoz, and K. Chatterjee. 1994. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. J. Clin. Investig. 94:506-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brucker-Davis, F., M. C. Skarulis, M. B. Grace, J. Benichou, P. Hauser, E. Wiggs, and B. D. Weintraub. 1995. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. National Institutes of Health Prospective Study. Ann. Intern. Med. 123:572-583. [DOI] [PubMed] [Google Scholar]
- 3.Burke, L. J., and A. Baniahmad. 2000. Co-repressors 2000. FASEB J. 14:1876-1888. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J. D., and H. Li. 1998. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit. Rev. Eukaryot. Gene Expr. 8:169-190. [DOI] [PubMed] [Google Scholar]
- 5.Damjanovski, S., L. M. Sachs, and Y.-B. Shi. 2002. Function of thyroid hormone receptors during amphibian development, p. 153-176. In A. Baniahmad (ed.), Methods in molecular biology: thyroid hormone receptors, vol. 202. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed]
- 6.Davis, P. J., and F. B. Davis. 1996. Nongenomic actions of thyroid hormone. Thyroid 6:497-504. [DOI] [PubMed] [Google Scholar]
- 7.Dodd, M. H. I., and J. M. Dodd. 1976. The biology of metamorphosis, p. 467-599. In B. Lofts (ed.), Physiology of the amphibia. Academic Press, Inc., San Diego, Calif.
- 8.Eliceiri, B. P., and D. D. Brown. 1994. Quantitation of endogenous thyroid hormone receptors alpha and beta during embryogenesis and metamorphosis in Xenopus laevis. J. Biol. Chem. 269:24459-24465. [PubMed] [Google Scholar]
- 9.Evans, R. M. 1988. The steroid and thyroid hormone receptor superfamily. Science 240:889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flamant, F., and J. Samarut. 2003. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol. Metabol. 14:85-90. [DOI] [PubMed] [Google Scholar]
- 11.Fondell, J. D., A. L. Roy, and R. G. Roeder. 1993. Unliganded thyroid hormone receptor inhibits formation of a functional preinitiation complex: implications for active repression. Genes Dev. 7:1400-1410. [DOI] [PubMed] [Google Scholar]
- 12.Forrest, D., E. Hanebuth, R. J. Smeyne, N. Everds, C. L. Stewart, J. M. Wehner, and T. Curran. 1996. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 15:3006-3015. [PMC free article] [PubMed] [Google Scholar]
- 13.Fraichard, A., O. Chassande, M. Plateroti, J. P. Roux, J. Trouillas, C. Dehay, C. Legrand, K. Gauthier, M. Kedinger, L. Malaval, B. Rousset, and J. Samarut. 1997. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 16:4412-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu, L., D. Buchholz, and Y.-B. Shi. 2002. A novel double promoter approach for identification of transgenic animals: a tool for in vivo analysis of gene function and development of gene-based therapies. Mol. Reprod. Dev. 62:470-476. [DOI] [PubMed] [Google Scholar]
- 15.Furlow, J. D., and D. D. Brown. 1999. In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol. Endocrinol. 13:2076-2089. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier, K., O. Chassande, M. Plateroti, J. P. Roux, C. Legrand, B. Pain, B. Rousset, R. Weiss, J. Trouillas, and J. Samarut. 1999. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J. 18:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert, L. I., J. R. Tata, and B. G. Atkinson. 1996. Metamorphosis: post-embryonic reprogramming of gene expression in amphibian and insect cells. Academic Press, Inc., San Diego, Calif.
- 18.Gothe, S., Z. Wang, L. Ng, J. M. Kindblom, A. C. Barros, C. Ohlsson, B. Vennstrom, and D. Forrest. 1999. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. EMBO J. 13:1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar, and R. Shiekhattar. 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14:1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto, K., F. H. Curty, P. P. Borges, C. E. Lee, E. D. Abel, J. K. Elmquist, R. N. Cohen, and F. E. Wondisford. 2001. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl. Acad. Sci. USA 8:3998-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetzel, B. S. 1989. The story of iodine deficiency: an international challenge in nutrition. Oxford University Press, Oxford, England.
- 22.Hsia, V. S.-C., and Y.-B. Shi. 2002. Chromatin disruption and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Mol. Cell. Biol. 22:4043-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsia, V. S.-C., H. Wang, and Y.-B. Shi. 2001. Involvement of chromatin and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Cell Res. 11:8-16. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuya-Oka, A., A. Shimozawa, H. Takeda, and Y.-B. Shi. 1994. Cell-specific and spatio-temporal expression of intestinal fatty acid-binding protein gene during amphibian metamorphosis. Roux's Arch. Dev. Biol. 204:150-155. [DOI] [PubMed] [Google Scholar]
- 25.Ishizuya-Oka, A., and S. Ueda. 1996. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 286:467-476. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuya-Oka, A., S. Ueda, T. Amano, K. Shimizu, K. Suzuki, N. Ueno, and K. Yoshizato. 2001. Thyroid-hormone-dependent and fibroblast-specific expression of BMP-4 correlates with adult epithelial development during amphibian intestinal remodeling. Cell Tissue Res. 303:187-195. [DOI] [PubMed] [Google Scholar]
- 27.Ishizuya-Oka, A., S. Ueda, T. Inokuchi, T. Amano, S. Damjanovski, M. Stolow, and Y.-B. Shi. 2001. Thyroid hormone-induced expression of Sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation 69:27-37. [DOI] [PubMed] [Google Scholar]
- 28.Ishizuya-Oka, A., S. Ueda, and Y.-B. Shi. 1997. Temporal and spatial regulation of a putative transcriptional repressor implicates it as playing a role in thyroid hormone-dependent organ transformation. Dev. Genet. 20:329-337. [DOI] [PubMed] [Google Scholar]
- 29.Ito, M., and R. G. Roeder. 2001. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12:127-134. [DOI] [PubMed] [Google Scholar]
- 30.Jepsen, K., and M. G. Rosenfeld. 2002. Biological roles and mechanistic actions of co-repressor complexes J. Cell Sci. 115:689-698. [DOI] [PubMed] [Google Scholar]
- 31.Jones, P. L., L. M. Sachs, N. Rouse, P. A. Wade, and Y. B. Shi. 2001. Multiple N-CoR complexes contain distinct histone deacetylases. J. Biol. Chem. 276:8807-8811. [DOI] [PubMed] [Google Scholar]
- 32.Kaneshige, M., K. Kaneshige, X.-G. Zhu, A. Dace, L. Garrett, T. A. Carter, R. Kazlauskaite, D. G. Pankratz, A. Wynshaw-Boris, S. Refetoff, B. D. Weintraub, M. C. Willingham, C. Barlow, and S.-Y. Cheng. 2000. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc. Natl. Acad. Sci. USA 97:13209-13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneshige, M., H. Suzuki, K. Kaneshige, J. Cheng, H. Wimbrow, C. Barlow, M. C. Willingham, and S.-Y. Cheng. 2001. A targeted dominant negative mutation of the thyroid hormone alpha1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc. Natl. Acad. Sci. USA 98:15095-15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroll, K. L., and E. Amaya. 1996. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122:3173-3183. [DOI] [PubMed] [Google Scholar]
- 35.Lazar, M. A. 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocrinol. Rev. 14:184-193. [DOI] [PubMed] [Google Scholar]
- 36.Leloup, J., and M. Buscaglia. 1977. La triiodothyronine: hormone de la métamorphose des amphibiens. C. R. Acad. Sci. 284:2261-2263. [Google Scholar]
- 37.Li, J., J. Wang, J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and C-CoR exist in large protein complexes containing HDAC3. EMBO J. 19:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocrinol. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 40.Metz, A., S. Knoechel, P. Buechler, M. Koester, and W. Knoechel. 1998. Structural and functional analysis of the BMP-4 promoter in early embryos of Xenopus laevis. Mech. Dev. 74:29-39. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwkoop, P. D., and J. Faber. 1956. Normal table of Xenopus laevis, 1st ed. North Holland Publishing Co., Amsterdam, The Netherlands.
- 42.Patterton, D., W. P. Hayes, and Y. B. Shi. 1995. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev. Biol. 167:252-262. [DOI] [PubMed] [Google Scholar]
- 43.Perlman, A. J., F. Stanley, and H. H. Samuels. 1982. Thyroid hormone nuclear receptor. Evidence for multimeric organization in chromatin. J. Biol. Chem. 257:930-938. [PubMed] [Google Scholar]
- 44.Puzianowsak-Kuznicka, M., S. Damjanovski, and Y.-B. Shi. 1997. Both thyroid hormone and 9-cis retinoic acid receptors are required to efficiently mediate the effects of thyroid hormone on embryonic development and specific gene regulation in Xenopus laevis. Mol. Cell. Biol. 17:4738-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rachez, C., and L. P. Freedman. 2000. Mechanisms of gene regulation by vitamin D3 receptor: a network of coactivator interactions. Gene 246:9-21. [DOI] [PubMed] [Google Scholar]
- 46.Refetoff, S., R. E. Weiss, and S. J. Usala. 1993. The syndromes of resistance to thyroid hormone. Endocrinol. Rev. 14:348-399. [DOI] [PubMed] [Google Scholar]
- 47.Sachs, L. M., T. Amano, N. Rouse, and Y. B. Shi. 2001. Involvement of histone deacetylase at two distinct steps in gene regulation during intestinal development in Xenopus laevis. Dev. Dyn. 222:280-291. [DOI] [PubMed] [Google Scholar]
- 48.Sachs, L. M., S. Damjanovski, P. L. Jones, Q. Li, T. Amano, S. Ueda, Y. B. Shi, and A. Ishizuya-Oka. 2000. Dual functions of thyroid hormone receptors during Xenopus development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 126:199-211. [DOI] [PubMed] [Google Scholar]
- 49.Sachs, L. M., P. L. Jones, E. Havis, N. Rouse, B. A. Demeneix, and Y.-B. Shi. 2002. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol. Cell. Biol. 22:8527-8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachs, L. M., and Y.-B. Shi. 2000. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 97:13138-13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreiber, A. M., B. Das, H. Huang, N. Marsh-Armstrong, and D. D. Brown. 2001. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc. Natl. Acad. Sci. USA 98:10739-10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi, Y.-B. 1999. Amphibian metamorphosis: from morphology to molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 53.Shi, Y.-B. 1996. Thyroid hormone-regulated early and late genes during amphibian metamorphosis, p. 505-538. In L. I. Gilbert, J. R. Tata, and B. G. Atkinson (ed.), Metamorphosis: post-embryonic reprogramming of gene expression in amphibian and insect cells. Academic Press, Inc., San Diego, Calif.
- 54.Shi, Y.-B., and D. D. Brown. 1993. The earliest changes in gene expression in tadpole intestine induced by thyroid hormone. J. Biol. Chem. 268:20312-20317. [PubMed] [Google Scholar]
- 55.Shi, Y.-B., and W. P. Hayes. 1994. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev. Biol. 161:48-58. [DOI] [PubMed] [Google Scholar]
- 56.Shi, Y.-B., and A. Ishizuya-Oka. 1996. Biphasic intestinal development in amphibians: embryogensis and remodeling during metamorphosis. Curr. Top. Dev. Biol. 32:205-235. [DOI] [PubMed] [Google Scholar]
- 57.Shi, Y.-B., and V. C.-T. Liang. 1994. Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim. Biophys. Acta 1217:227-228. [DOI] [PubMed] [Google Scholar]
- 58.Shi, Y. B., L. Fu, S. C. Hsia, A. Tomita, and D. Buchholz. 2001. Thyroid hormone regulation of apoptotic tissue remodeling during anuran metamorphosis. Cell Res. 11:245-252. [DOI] [PubMed] [Google Scholar]
- 59.Stolow, M. A., and Y. B. Shi. 1995. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 23:2555-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tata, J. R. 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239-248. [DOI] [PubMed] [Google Scholar]
- 61.Tinnikov, A., K. Nordstrom, P. Thoren, J. M. Kindblom, S. Malin, B. Rozell, M. Adams, O. Rajanayagam, S. Pettersson, C. Ohlsson, K. Chatterjee, and B. Vennstrom. 2002. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J. 21:5079-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai, M. J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 63.Ulisse, S., G. Esslemont, B. S. Baker, K. Chatterjee, and J. R. Tata. 1996. Dominant-negative mutant thyroid hormone receptors prevent transcription from Xenopus thyroid hormone receptor beta gene promoter in response to thyroid hormone in Xenopus tadpoles in vivo. Proc. Natl. Acad. Sci. USA 93:1205-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Underhill, C., M. S. Qutob, S. P. Yee, and J. Torchia. 2000. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1 J. Biol. Chem. 275:40463-40470. [DOI] [PubMed] [Google Scholar]
- 65.Wang, Z., and D. D. Brown. 1993. Thyroid hormone-induced gene expression program for amphibian tail resorption. J. Biol. Chem. 268:16270-16278. [PubMed] [Google Scholar]
- 66.Wen, Y. D., V. Perissi, L. M. Staszewski, W. M. Yang, A. Krones, C. K. Glass, M. G. Rosenfeld, and E. Seto. 2000. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc. Natl. Acad. Sci. USA 97:7202-7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wikstrom, L., C. Johansson, C. Salto, C. Barlow, A. C. Barros, F. Baas, D. Forrest, P. Thoren, and B. Veenstrom. 1998. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor. EMBO J. 17:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong, J., and Y.-B. Shi. 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J. Biol. Chem. 270:18479-18483. [DOI] [PubMed] [Google Scholar]
- 69.Wong, J., Y. B. Shi, and A. P. Wolffe. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TRβ A gene by the thyroid hormone receptor. Genes Dev. 9:2696-2711. [DOI] [PubMed]
- 70.Xu, L., C. K. Glass, and M. G. Rosenfeld. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9:140-147. [DOI] [PubMed] [Google Scholar]
- 71.Yaoita, Y., Y. B. Shi, and D. D. Brown. 1990. Xenopus laevis alpha and beta thyroid hormone receptors. Proc. Natl. Acad. Sci. USA 87:7090-7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yen, P. M. 2001. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 81:1097-1142. [DOI] [PubMed]
- 73.Yoshizato, K. 1989. Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int. Rev. Cytol. 119:97-149. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, J., M. Kalkum, B. T. Chait, and R. G. Roeder. 2002. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol. Cell 9:611-623. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, J., and M. A. Lazar. 2000. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 62:439-466. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, X.-Y., M. Kaneshige, Y. Kamiya, K. Kaneshige, P. McPhie, and S.-Y. Cheng. 2002. Differential expression of thyroid hormone receptor isoforms dictates the dominant negative activity of mutant β receptor. Mol. Endocrinol. 16:2077-2092. [DOI] [PubMed] [Google Scholar]