Abstract
NF-κB is an ubiquitous transcription factor that is a key in the regulation of the immune response and inflammation. T-cell receptor (TCR) cross-linking leads to NF-κB activation, an IκB kinase (IKK)-dependent process. However, the upstream kinases that regulate IKK activity following TCR activation remain to be fully characterized. Herein, we demonstrate using genetic analysis, pharmacological inhibition, and RNA interference (RNAi) that the conventional protein kinase C (PKC) isoform PKCα, but not PKCβ1, is required for the activation of the IKK complex following T-cell activation triggered by CD3/CD28 cross-linking. We find that in the presence of Ca2+ influx, the catalytically active PKCαA25E induces IKK activity and NF-κB-dependent transcription; which is abrogated following the mutations of two aspartates at positions 246 and 248, which are required for Ca2+ binding to PKCα and cell membrane recruitment. Kinetic studies reveal that an early phase (1 to 5 min) of IKK activation following TCR/CD28 cross-linking is PKCα dependent and that a later phase (5 to 25 min) of IKK activation is PKCθ dependent. Activation of IKK- and NF-κB-dependent transcription by PKCαA25E is abrogated by the PKCθ inhibitor rottlerin or the expression of the kinase-inactive form of PKCθ. Taken together, our results suggest that PKCα acts upstream of PKCθ to activate the IKK complex and NF-κB in T lymphocytes following TCR activation.
Identification of the molecular events regulating T-cell activation is paramount to understanding the regulation of the immune response. The signal transduction pathways triggered by antigen presentation lead to the immediate activation of multiple transcription factors that further amplify the process of lymphocyte activation, ultimately leading to cellular proliferation and division. T-cell receptor (TCR) engagement, together with the second costimulatory signal derived from engagement of the CD28 receptor, results in NF-κB activation (13, 31, 36). When occurring together with NF-AT, AP-1, and octomer, NF-κB activation leads to interleukin-2 (IL-2) expression (17, 27, 30).
NF-κB is a heterodimer of transcription factors that belong to the Rel family of proteins. The canonical NF-κB is a heterodimer of p65 (RelA) with p50 or p52 (35, 50, 54). This heterodimer is anchored by a group of proteins named IκB, which function to retain NF-κB in the cytosol by masking its nuclear localization signal (1, 4, 45, 60). IκBα is the prototype IκB molecule known to control the subcellular localization of NF-κB (p50/p65). Following activation of certain signal transduction pathways, a site-specific hyperphosphorylation of IκBα at S32 and S36 renders the inhibitor molecule susceptible to site-specific ubiquitination and subsequent degradation by the proteasome complex (8, 9, 18, 62, 68). This releases NF-κB, allowing it to undergo nuclear translocation. Two IκBα kinases, IKKα (19, 52, 70) and IKKβ (46), which are contained within a high-molecular-weight complex, target the phosphorylation of S32 and S36 of IκBα following stimulation by various stimuli. While engagement of TCR/CD3 and CD28 activates the IKK complex (27), the molecular mechanisms and second messengers mediating it are poorly understood when compared to what is currently known about tumor necrosis factor (TNF)- and IL-1 receptor-initiated signaling (46, 52, 69). TCR/CD3- and CD28-generated signals converge on the mitogen-activated protein 3 (MAP3) type kinase, Cot, which in turn has been suggested to lead to the activation of the IKK complex via induction of the NF-κB-inducible kinase (NIK) (39). The relevance of NIK in this process, however, remains controversial, because NIK preferentially activates IKKα but not IKKβ (40, 52), and both IKKα and IKKβ are activated following CD3/CD28 ligation (34). Moreover, the activation of IKKβ, but not of IKKα, is essential for IL-2 expression (34). Other second messengers downstream of the TCR/CD3 and CD28 activation leading to activation of the IKK complex remain to be characterized. Engagement of TCR/CD3 by the complex formed between its cognate peptide and the major histocompatibility complex induces phospholipase C (PLC) activation, which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) (28) to inositol 1,4,5-triphosphate (IP3), which ultimately releases Ca2+ from intracellular stores and diacylglycerol (DAG) (6, 14). While free intracellular Ca2+ targets the Ca2+/calmodulin-activated phosphatase calcineurin, both Ca2+ and DAG activate protein kinase C (PKC) isoforms that mediate a critical positive signal necessary for IL-2 induction through their synergy with calcineurin (22). The specific PKC isoform that critically mediates TCR-initiated signaling is unclear, and this is an area of intense investigation (3, 25, 64).
To date, 11 closely related PKC isoenzymes have been described and classified into three subfamilies based on their domain structure and their ability to respond to Ca2+ and DAG (48, 49). The “conventional” PKC isoforms (α, β1, βII, and γ) are regulated by DAG, which binds the C1 domain, and by Ca2+, which binds the C2 domain. In contrast, the “novel” PKC isoforms (δ, ɛ, η, and θ) are not regulated by Ca2+ but respond to DAG. The molecular structure of the novel PKC isoforms is similar to that of the classical isoforms except for differences in the Ca2+ binding domain. The third group of PKC isoforms includes the “atypical” PKC isoforms (ζ, λ/ι, and μ), which are regulated neither by DAG nor Ca2+. Elevated concentrations of intracellular Ca2+ and DAG following TCR stimulation can potentially activate either conventional or novel PKC isoforms. Among the PKC isoforms in T cells, PKCα and PKCθ are recruited to the inner leaflet of the plasma membrane within minutes following TCR ligation (59), an event that temporally correlates with IKK and NF-κB activation (26, 34). This suggests that these two PKC isoforms may be involved in mediating the TCR-induced IKK and NF-κB activation in T lymphocytes. In fact, PKCθ has been recently shown to mediate CD3/CD28-induced NF-κB activation (16, 40, 58) by specifically activating the IKK complex (40). As for PKCα, it has been shown in nonlymphoid cells to potently activate IKKβ (37) and NF-κB (16).
Our group has previously demonstrated that the activation of IKK, and hence NF-κB by phorbol esters and ionomycin in primary T cells and transformed T-cell lines is dependent on conventional PKC isoforms (63). Because these two stimuli mimic the effects of DAG and increased intracellular Ca2+ that ensue following TCR/CD3 and CD28 activation, we have sought to investigate the role PKCα plays in mediating the activation of IKK and NF-κB following TCR/CD3 and CD28 cross-linking in T lymphocytes, as well as its relationship to PKCθ. Using a combination of pharmacological, genetic, and RNA interference approaches, we demonstrate that PKCα mediates activation of the IKK complex and NF-κB following CD3/CD28 cross-linking. Moreover, we show that PKCα lies upstream of PKCθ in this relevant signaling cascade in T lymphocytes.
MATERIALS AND METHODS
Plasmids.
The NF-κB-dependent firefly luciferase reporter expression vector (κB-luc) has been previously described (63). The IL-2, RE/AP, and NF-AT/AP-1 promoter-luciferase reporter plasmids were gifts from D. McKean (Mayo Clinic, Rochester, Minn.), and the AP-1 reporter plasmid was described previously (22). The pRL-TK expression vector, which provides constitutive expression of Renilla luciferase, is commercially available (Promega, Madison, Wis.). Both wild-type and kinase-dead (KD) cDNAs of IKKβ were obtained from M. Roth (Tularik, Inc., South San Francisco, Calif.). Wild-type IKKβ with an N-terminal three-hemagglutinin (HA) tag was provided by E. Zandi (University of California—San Diego). IKKα KD was a kind gift from A. Israel (Institute Pasteur, Paris, France). The expression vector pEF-BOS was a gift from L. Karnitz (Mayo Clinic). The mammalian expression vectors (pEF1/Myc-His) were purchased from Invitrogen (Carlsbad, Calif.), and pCI was purchased from Promega. The expression vector pSRα4ΔCaM-AI has been described previously (63). Wild-type and KD PKCα and PKCθcDNAs were kindly provided by A. Altman (La Jolla Institute, La Jolla, Calif.). Constitutively active PKCαA25E was generated by site-directed mutagenesis with mutagenic primers sense A25E (CGCAAAGGGGACCTGAGGCAGAAG); mutated codon in boldface) and antisense A25E (CTTCTGCCTCAGCTCCCCTTTGCG), along with wild-type primers (5′-gcggccgctATGGCTGACGTC and 3′-tctagatcaTACGCGGCTCTGCAG; appended enzyme site in lowercase), and cloned into pGEM-T Easy (Promega). PKCαA25E was then excised by NotI digestion and subcloned into HA-pCDNA3 at the NotI site. The insert coding HA-tagged PKCα was subcloned into pEF-BOS by using XbaI sites. Constitutively active PKCα with mutations D246N and D248N was generated by PCR with a sense mutagenic primer (5′-GAAATCTGGAACTGGAACCGAACCAC) and an antisense primer (5′-GTGGTTCGGTTCCAGTTCCAGATTTC) and with HA-PKCαA25E as a template. HA-PKCαA25E/D246, 248N was subcloned into pEF-BOS by using XbaI sites. Constitutively active PKCθA148E was generated by site-directed mutagenesis with mutagenic primers (sense, 5′-CGCCGGGGTGAAATCAAGCAG; antisense, 5′-CTGCTTGATTTCACCCCGGCG; sense PKCθ with BamHI site, cgggatccATGTCGCCATTTCTT; and antisense PKCθ with XbaI site, cgtctagaTCAGGATATCAGCCG) and cloned into pGEM-T Easy. The insert coding PKCθA148E was excised and subcloned into pCI at NotI sites or cloned into pEF1/Myc-His at BamHI and NotI sites. The sequences of all generated constructs were verified by sequencing.
Generation of pFRT-H1P and the PKC suppression vectors.
A 210-bp fragment containing the RNA polymerase III-dependent H1 RNA promoter was amplified from Jurkat T-cell DNA as previously described (10). The H1 promoter fragment was subcloned into a modified mammalian expression vector as an EcoRI-HindIII fragment in order to generate the pFRT-H1P parental vector (FRT = for RNA targeting). In order to produce PKC-specific targeting short hairpinned RNA (shRNA) molecules, complementary oligonucleotides were synthesized as previously described (10). In brief, each oligonucleotide pair contains a 5′ BglII and 3′ HindIII overhang, an RNA polymerase III start and termination sequence, and 19 to 21 nucleotides (N19) of PKC specific sequence separated by a 9-nucleotide loop. The invariant nucleotide sequences of both the upper and lower oligonucleotide strands are 5′-GATCCCC(N19)ttcaagaga(61N)TTTTTGGAAA-3′ and 3′-GGG(N19)aagttctct(61N)AAAAACCTTTTCGA-5′. The specific targeting sequence (N19/21) for each PKC isoform was designed to be specific for the desired isoform and was subsequently subjected to BLAST search algorithm against the human expressed sequence tag (EST) database to confirm targeting specificity. The sequences for the three PKC isoforms targeted in this paper are as follows: PKCα, 5′-GAACAACAAGGAATGACTT-3′; PKCβ1, 5′-GGAAGCTGTGGCCATCTGC-3′; and PKCθ, 5′-TTGGATGAGGTGGATAAAA-3′.
Cell culture and reagents.
Jurkat T cells were obtained from the American Type Culture Collection, Rockville, Md., and maintained in RPMI 1640 (Bio-Whittaker, Walkersville, Md.) supplemented with 5% heat-inactivated fetal bovine serum, 100 U of penicillin-streptomycin per ml, and 2 mM l-glutamine. Cells were grown to a density of 3 × 105 to 5 × 105 per ml at the time of the different experiments. HEK 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 50 U of penicillin-streptomycin per ml, and 2 mM l-glutamine. Sodium orthovanadate and p-nitrophenyl phosphate (PNPP) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Ionomycin, Gö6976, β-glycerophosphate, Gö6850, and rottlerin were purchased from CalBiochem, and TNF-α was purchased from R&D Systems (Minneapolis, Minn.). Leupeptin, aprotinin, and pepstatin A were obtained from Boehringer-Mannheim (Indianapolis, Ind.). Anti-HA high-affinity antibodies were purchased from Boehringer Mannheim. Anti-IKKα (H-744, M-280), anti-IKKβ (H-470), anti-PKCα (C-20), anti-p65 (C-20), and anti-IκBα (C-21) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-human CD3 (UCHT1) and OKT3 antibodies were obtained from Ancell (Bayport, Minn.) and Ortho Biotech (Raritan, N.J.), and anti-CD28 was purchased from BD Biosciences (San Jose, Calif.). Anti-PKCβ1 and anti-PKCθ were purchased from BD Transduction Laboratories (San Diego, Calif.). Protein A agarose beads were obtained from Life Technologies (Gaithersburg, Md.). Dimeric human TNF receptor p80/immunoglobulin G1 (IgG1) Fc fusion protein was a kind gift from D. Lynch (Immunex, Seattle, Wash.). The preparation of substrate GST-IκBα(1-53) for the in vitro IKK kinase assay has been previously described (63).
To isolate CD3+ T cells, peripheral blood mononuclear cells (PBMCs) from healthy volunteer blood donors were obtained from buffy coats by density gradient centrifugation (Ficoll-Hypaque; Pharmacia LKB Biotechnology, Inc., Piscataway, N.J.). PBMCs were then depleted of monocytes by two cycles of plastic adherence, and CD3+ T cells were purified by neuraminidase-treated sheep erythrocyte (SRBC) rosetting. The remaining cell population was repeatedly found to be 98% CD3+ T cells, as determined by flow cytometry. CD3+ T cells used in the various experiments were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (Invitrogen), 2 mM l-glutamine, and antibiotics (penicillin, 100 U/ml; streptomycin, 100 μg/ml) at 0.5 × 106 cells per ml. CD3+ T cells were stimulated and harvested on the second day after isolation.
Where indicated, cells were pretreated with 2 μM Gö6976 for 15 min. FK506 was used at 20 ng/ml. For Jurkat T cells, ionomycin was used at 3.5 μg/ml, and TNF-α was used at 10 ng/ml. Jurkat and CD3+ T cells were cross-linked with 3 μg of anti-CD3 and anti-CD28 antibodies or isotype control antibodies per ml (63).
Cell extract preparation, immunoblotting, and kinase assay.
To obtain total cellular proteins, cells were washed with cold phosphate-buffered saline (PBS), resuspended in a modified whole-cell extract (WCE) PD buffer (63) (40 mM Tris-HCl [pH 8], 0.3 M NaCl, 0.1% Nonidet P-40, 6 mM EDTA, 6 mM EGTA, 10 mM NaF, 10 mM PNPP, 10 mM b-glycerophosphate, 300 μM sodium orthovanadate, 1 mM dithiothreitol, 2 μM phenylmethylsylfonyl fluoride [PMSF], 10-μg/ml aprotinin, 1-μg/ml leupeptin, 1-μg/ml pepstatin) and centrifuged at 12,000 × g for 15 min at 4°C. The resultant supernatant contained total cellular protein. The amount of cellular protein present in the clarified supernatant was calculated by using the Bio-Rad (Hercules, Calif.) protein assay.
For Western immunoblots, equal amounts of WCE were loaded and separated by sodium dodecyl sulfate-polyacrylamide (10%) gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P membranes (Millipore, Bedford, Mass.). Immunoblotting was performed with specific antibodies and visualized by using the ECL enhanced chemiluminescence Western blotting detection kit (Amersham, Buckinghamshire, England).
The isolation of membrane-bound PKCα and PKCθ were performed as previously described (11). The immunocomplex kinase assay from WCE Jurkat and CD3+ T cells using IκBα as a substrate has been described previously (63).
Gene transfection and reporter assays.
FuGENE6 (Roche Molecular Biochemicals, Indianapolis, Ind.) was used to transfect DNA plasmids into Jurkat T cells. In brief, 8 μl of FuGENE6 was mixed with 92 μl of RPMI 1640 medium and incubated for 5 min. FuGENE6/RPMI-1640 solution was added to a sterile tube containing 0.19 μg of κB-luc reporter plasmid, 0.01 μg of Tk-Renilla and 0 to 1.8 μg of a plasmid of interest up to a total of 2 μg of DNA and incubated for 15 min. The DNA/FuGENE6 solution was added to 106 log-phase Jurkat T cells. Lipofectamine Plus (Invitrogen) was used to transfect 293T cells according to the manufacturer's protocol.
Where indicated, Jurkat T cells were electroporated with a BTX Electro Square Porator T820 (BTX Corporation, San Diego, Calif.) at 325 V for 10 ms. Primary CD3+ T cells were electroporated at 360 V for 10 ms as previously described (5).
Jurkat T cells were transfected with the indicated plasmids and grown for 18 to 24 h. Cells were stimulated for 4 h with ionomycin (3.5 μg/ml) or TNF-α (10 ng/ml) or cross-linked with anti-CD3 and anti-CD28 antibodies as previously described (63). Thereafter, cells were washed twice in cold PBS and lysed with 100 μl of lysis buffer (Promega dual-luciferase reporter assay system). Firefly and Renilla luciferase activities from 20 μl of extract were assayed with the Promega dual-luciferase reporter assay system reagents and a Berthold Lumat following the manufacturer's recommendation. κB-luc activity was normalized to Renilla expression. All transfection experiments were performed in duplicate.
For in vitro kinase assays (IVK), Jurkat T cells were electroporated with 20 μg of the plasmid of interest or vector control.
RESULTS
Conventional PKC isoforms participate in the activation of the IKK complex following CD3/CD28 cross-linking in T lymphocytes.
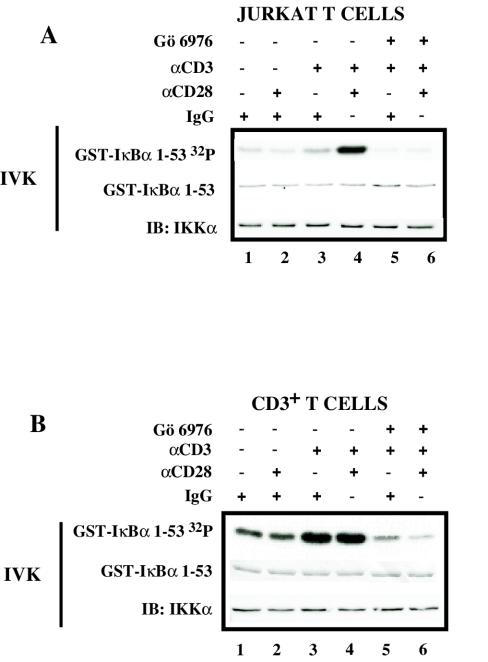
Our group has previously shown that phorbol myristate acetate (PMA) and ionomycin, two drugs that mimic events triggered by TCR ligation, can activate the IKK complex and induce NF-κB translocation to the nucleus, effects that were partially inhibited with pharmacological inhibition of conventional PKC isoforms (63). To initially address the involvement of conventional PKC isoforms in the TCR/CD3- and CD28-initiated signaling under more physiological conditions, the kinase activity of the IKK complex immunoprecipitated from Jurkat T cells and primary CD3+ T cells following the cross-linking of CD3 and CD28 and their pretreatment (or not) with the conventional PKC inhibitor Gö6976 was first analyzed. It has previously been shown that 2 μM Gö6976 does not inhibit either IKK activation by the PKC-independent stimulus TNF-α (63) or the kinase activity of PKCθ (16). Cross-linking of Jurkat T cells with isotype control IgG or CD28 alone did not induce IKK activity (Fig. 1A, lanes 1 and 2), whereas ligation of CD3 led to moderate IKK activation (Fig. 1A, lane 3). Cross-linking of both CD3 and CD28 resulted in a strong induction of the IKK complex activity (Fig. 1A, lane 4), which was inhibited by Gö6976 (Fig. 1A, lane 6).
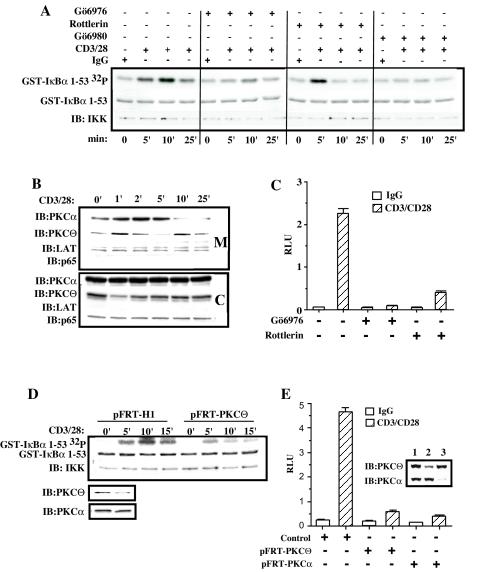
FIG. 1.
Conventional PKC isoforms participate in the activation of the IKK complex following CD3/CD28 cross-linking. (A) Jurkat T cells (5 × 106 per sample) were incubated with 6-μg/ml isotype control IgG (lane 1), 3-μg/ml (each) anti-CD3 and IgG (lanes3 and 5), 3-μg/ml (each) anti-CD28 and IgG (lane 2), or 3 μg (each) of anti-CD3 and anti-CD28 antibodies (lanes 4 and 6) and then plated onto wells precoated with goat anti-mouse IgG. Jurkat T cells were pretreated (+) or not (−) with 2 μM Gö6976 for 15 min before cross-linking with antibodies. IKK activity was measured in an IVK assay as described in Materials and Methods, using glutathione S-transferase (GST)-IκBα(1-53) as a substrate (GST-IκBα32P). Coomassie staining of the polyvinylidene difluoride membrane containing the IVK to detect the amount of IκBα substrate (GST-IκBα) and immunoblotting (IB) for IKKα were performed. Panel B is the same as panel A, except that primary human purified CD3+ T cells (10 × 106 cells per sample) were used.
In contrast to Jurkat T cells, primary resting purified CD3+ T cells demonstrated less of a requirement for CD3 and CD28 costimulation, since CD3 stimulation alone was sufficient to induce a strong IKK activity (Fig. 1B, lane3) which was not further increased following CD28 coligation (Fig. 1B, lane 4). Once again, pretreatment of primary CD3+ T cells with Gö6976 abolished IKK activation following either CD3 or CD3/CD28 cross-linking (Fig. 1B, lanes 5 and 6).
These data suggest that conventional PKC isoforms participate in the TCR/CD3-initiated signal transduction pathway that leads to IKK activation in transformed and primary T lymphocytes.
PKCα is required for both IKK and NF-κB activation following T-cell stimulation by CD3 and CD28.
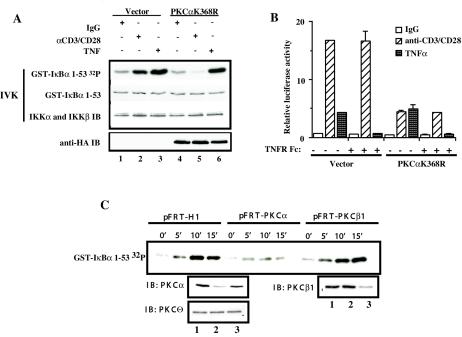
T lymphocytes express two conventional PKC isoforms, PKCα and PKCβ1, both of which are activated with different kinetics following TCR cross-linking (59). PKCα translocates within a few minutes, whereas PKCβ1 is recruited to the cell membrane 90 min after TCR cross-linking (59). To test whether PKCα mediates CD3/CD28-induced IKK activation, we first measured the activity of the IKK complex from TNF-α- or CD3/CD28-stimulated Jurkat T cells that had been transiently transfected with a catalytically inactive form of PKCα (PKCαK368R). Expression of the catalytically inactive PKCα specifically abrogated the CD3/CD28-, but not the TNF-α-induced IKK activation (Fig. 2A, compare lanes 2 and 5 with 3 and 6).
FIG. 2.
PKCα is required for CD3/CD28-induced activation of IKK complex and NF-κB-dependent transcriptional activity. (A) Jurkat T cells (10 × 106 per sample) were electroporated with 20 μg of catalytically inactive HA-PKCαK368R (lanes 4 to 6) or control vector pEF-BOS (lanes 1to 3). Eighteen hours later, transfected Jurkat T cells were cross-linked with anti-CD3 and anti-CD-28 antibodies (lanes 2 and 5) or were stimulated with TNF-α as a control (lanes 3 and 6). IKK activity was measured in an IVK assay using glutathione S-transferase (GST)-IκBα(1-53) as a substrate (GST-IκBα 32P). Coomassie staining of the polyvinylidene difluoride membrane containing the IVK indicates equal amounts of IκBα substrate (GST-IκBα), and immunoblotting (IB) for IKKα and β indicates equal amounts of complex immunoprecipitation per lane. Expression of HA-PKCαK368R was confirmed by immunoblotting with anti-HA antibodies (anti-HA IB). (B) Jurkat T cells (2 × 106 per sample) were transfected in duplicate with 2.4 μg of catalytically inactive HA-PKCαK368R or 2.4 μg of control vector pEF-BOS together with reporter κB-luc (0.38 μg) and Tk-Renilla (0.02 μg) plasmids by the FuGENE6 method. Twenty hours later, transfected Jurkat T cells were cross-linked with anti-CD3 and anti-CD28 antibodies or were stimulated with TNF-α. TNFR Fc fusion protein was added at the moment of TNF-α stimulation at 1 μg/ml. Four hours later, cells were harvested and luciferase activities were measured. The κ[/beta]-luc activity was normalized to Renilla luciferase activity (relative luciferase activity). (C) Jurkat T cells (10 × 106 per sample) were electroporated with 40 μg of pFRT-PKCα (lane 2), pFRT-PKCβ1 (lane 3), or pFRT control vector (lane 1). Forty-eight hours later, the transfected cells were treated with anti-CD3 (10 μg/ml) and anti-CD28 (10 μg/ml) antibodies for 45 min on ice and cross-linked with goat anti-mouse antibodies in solution at 37°C for the indicated periods of time. IKK activity was measured in IVK assay as described above. Protein levels following the suppression of endogenous PKCα or PKCβ1 expression were confirmed by immunoblotting. Equal amounts of protein per lane were demonstrated by immunoblotting with anti-PKCθ antibodies.
The ability of the catalytically inactive PKCα to inhibit the IKK complex activity following CD3/CD28 cross-linking was further confirmed by measuring its effect on NF-κB-dependent transcriptional activity. Jurkat T cells were transfected with the PKCαK368R expression vector and an NF-κB-dependent reporter plasmid and left unstimulated or were stimulated by CD3/CD28 cross-linking or TNF-α. Expression of the catalytically inactive PKCα reduced the NF-κB-dependent transcriptional activity following CD3/CD28 cross-linking, but it had no effect on TNF-α-induced transcription (Fig. 2B).
Since T-cell activation by CD3/CD28 cross-linking may induce autocrine secretion of TNF-α, which may further contribute to NF-κB-dependent transcriptional activity, we evaluated the effect of the catalytically inactive PKCα in the presence of recombinant TNF receptor (TNFR) fusion protein (TNFR Fc). As shown in Fig. 2B, addition of TNFR Fc to the cell culture media did not result in a significant decrease in NF-κB-dependent transcriptional activity in Jurkat T cells transfected with catalytically inactive PKCα, while totally abrogating the TNF-induced NF-κB-dependent transcriptional activity.
Altogether, these data indicate that PKCα can mediate CD3/CD28-induced activation of IKK and NF-κB in Jurkat T cells. However, these results do not rule out a possible role for PKCβ1 in mediating CD3/CD28-induced IKK activation, since the broad specificity of dominant-negative PKC mutants has been described (24).
To identify which of the two conventional PKC isoforms mediates CD3/CD28-induced IKK activation, we generated isoform-specific PKC suppression vectors (10), which direct synthesis of shRNA molecules specific for targeting of either human PKCα or human PKCβ1. As shown in Fig. 2C, transfection of Jurkat T cells with either pFRT-PKCα or pFRT-PKCβ1 resulted in selective and significant reduction of PKCα and PKCβ1 expression. Neither of the shRNA targeting vectors affected the levels of PKCθ. The kinetic analysis of the IKK activation demonstrated that suppression of PKCα, but not PKCβ1, resulted in the severe defect of IKK activation following CD3/CD28 cross-linking. Taken together, these results indicate that PKCα, but not PKCβ1, mediates activation of the IKK complex triggered by CD3/CD28 cross-linking in Jurkat T cells.
Constitutively active PKCα activates the IKK complex and NF-κB-dependent transcriptional activity in T cells.
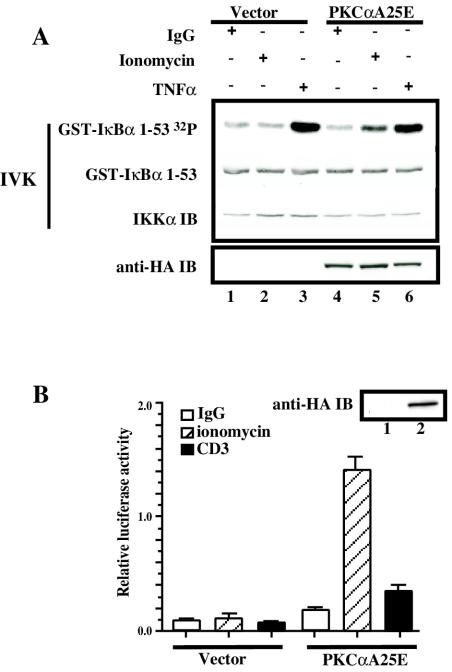
To further confirm the specific role of PKCα in the activation of the IKK complex and NF-κB-dependent transcriptional activity following CD3/CD28 stimulation in T cells, we evaluated the ability of a constitutively active PKCα isoform containing a substitution of Ala for Glu in the pseudosubstrate sequence (PKCαA25E) (3) in regulating the activity of IKK and of the NF-κB-dependent transcriptional activity. When PKCαA25E or control vector was expressed in Jurkat T cells, no detectable activation of the endogenous IKK complex was observed (Fig. 3A, lane 4). However, the addition of ionomycin to the PKCαA25E-transfected cells induced the activation of the IKK complex, while ionomycin treatment in the vector-transfected cells had no effect on IKK activity (Fig. 3A, lanes 2 and 5). These data indicate that the previously described constitutively active PKCα isoform can only activate the endogenous IKK complex in the presence of Ca2+ influx. How ionomycin and hence Ca2+ influx regulates PKCαA5E function is addressed below.
FIG. 3.
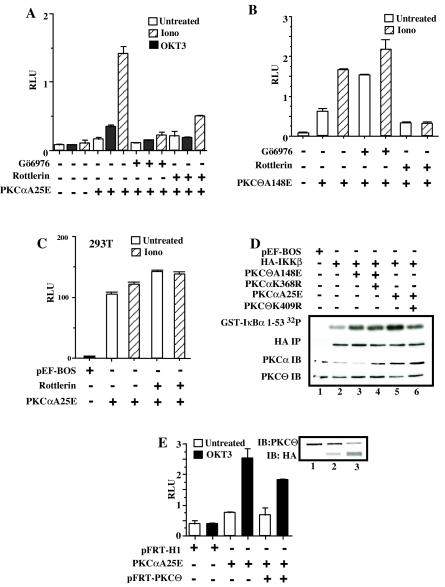
Catalytically active PKCα (PKCαA25E) activates the IKK complex and NF-κB-dependent transcriptional activity. (A) Jurkat T cells (10 × 106 per sample) were electroporated with 20 μg of catalytically active PKCα (pEF-BOS/HA-PKCαA25E) (lanes 4 to 6) or control vector pEF-BOS (lanes 1 to 3). Twenty hours later, transfected Jurkat T cells were treated with 3.5-μg/ml ionomycin (lanes 2 and 5) or were stimulated with TNF (lanes 3 and 6). IKK activity was measured in an IVK assay as described above. Expression of HA-PKCαA25E was analyzed by immunoblotting with anti-HA antibodies (anti-HA IB). (B) Jurkat T cells (10 × 106 per sample) were electroporated with 20 μg of catalytically active PKCα (pEF-BOS/HA-PKCαA25E) (lane 2) or control vector pEF-BOS (lane1) together with the reporter κB-luc (3.8 μg) and Tk-Renilla (0.2 μg) plasmids. Twenty hours later, transfected Jurkat T cells were treated with 3.5-μg/ml ionomycin or were stimulated with OKT3 monoclonal antibody (5 μg/ml). Four hours later, luciferase activity was measured and normalized as described in the legend to Fig. 2B. Expression of HA-PKCαA25E was analyzed by immunoblotting with anti-HA antibodies (anti-HA IB). All experiments were performed in duplicate.
The functional relevance of PKCα activation of the IKK complex was further studied by measuring NF-κB-dependent transcriptional activity. Transfection of PKCαA25E resulted in a two- to threefold increase in NF-κB-dependent transcriptional activity in unstimulated Jurkat T cells (Fig. 3B). Again, ionomycin treatment induced NF-κB-dependent transcriptional activity only in PKCαA25E-transfected cells but not in vector-transfected cells (Fig. 3B). Moreover, ligation of CD3, a receptor shown previously to trigger increases in intracellular Ca2+ (28), induced NF-κB activation in PKCαA25E- but not in vector-transfected cells (Fig. 3B). Therefore, we conclude that Ca2+ influx is required for NF-κB activation via a constitutively active PKCα.
Ca2+ influx is required for NF-κB activation by PKCαA25E.
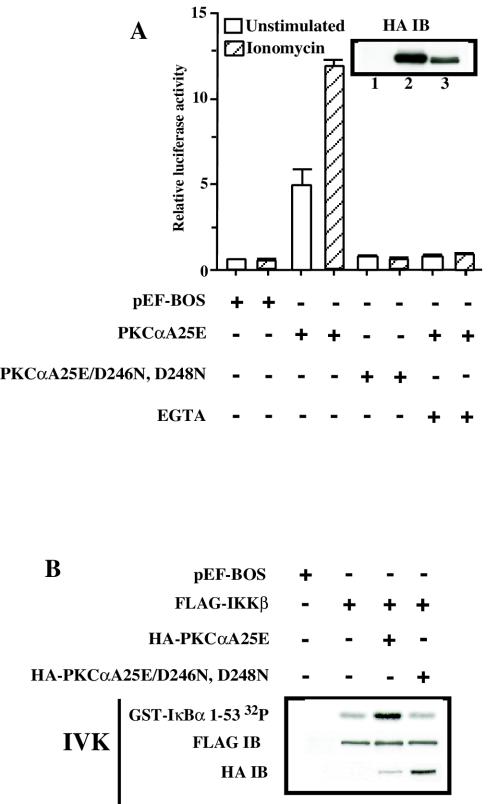
PKCα binds two Ca2+ ions when recruited to the cell membrane (44, 55, 65) where it becomes activated and undergoes autophosphorylation to achieve full catalytic competency (48). Ca2+ ions bind to five aspartic acid residues in the C2 Ca2+ binding domain of PKCα (15, 44, 55, 65). Based on the observation that PKCαA25E requires increases in intracellular Ca2+ to activate IKK and NF-κB, we investigated whether the effects of Ca2+ influx were secondary to its direct binding to PKCαA25E. To test this, the major Ca2+ binding sites, D246 and D248, were mutated to asparagine (44, 65), and the ability of this mutant (PKCαA25E/D246N-D248N) to activate NF-κB downstream of CD3/CD28 cross-linking was assessed. As shown in Fig. 4A, expression of this PKCα mutant failed to induce NF-κB-dependent transcriptional activity following ionomycin treatment, while not effecting TNF-α induced NF-κB activation (data not shown). Furthermore, EGTA depletion of extracellular Ca2+ in the culture media of Jurkat T cells transfected with PKCαA25E abrogated the ionomycin-induced NF-κB activity (Fig. 4A). Therefore, these observations suggest that PKCαA25E can efficiently induce NF-κB transcription only in the presence of Ca2+ influx.
FIG. 4.
Ca2+ binding to PKCαA25E is required to activate IKKβ and NF-κB. (A) Jurkat T cells (10 × 106 per sample) were electroporated with 20 μg of catalytically active PKCα (pEF-BOS/HA-PKCαA25E) (lane 2), 20 μg of mutant construct HA-PKCα25E/D246N, D248N (lane 3), or 20 μg of control vector pEF-BOS (lane 1) together with reporter κB-luc (3.6 μg) and Tk-Renilla (0.4 μg) plasmids. One group of cells transfected with HA-PKCα25E was pretreated with 1.5 mM EGTA before the addition of ionomycin to all samples. Expression of PKCαA25E (lane 2) or HA-PKCα25E/D246N, D248N (lane 3) was demonstrated by immunoblotting with anti-HA antibodies. (B) Jurkat T cells (10 × 106) were electroporated with 5 μg of FLAG-tagged IKKβ wild type with or without 20 μg of HA-PKCαA25E or HA-PKCαA25E/D246N, D248N or 20 μg of pEF-BOS. IKKβ was immunoprecipitated with anti-FLAG antibodies, and the kinase activity of IKKβ was measured as described above. The expression of FLAG-IKKβ was confirmed by immunoblotting with anti-FLAG antibodies (FLAG-IB).
We further confirmed this by comparing the ability of PKCαA25E and its mutant form, PKCαA25E/D246N-D248N, to activate exogenously expressed IKKβ. As shown in Fig. 4B, only PKCαA25E increased the kinase activity of IKKβ in the presence of Ca2+ influx, in contrast to its mutant form. Since the mutations D246N and D248N of PKCα strongly impair its interaction with the plasma membrane (15), we infer that the lack of PKCαA25E/D246N-D248N binding to the plasma membrane may be responsible for the inability of the mutant form of PKCα to activate IKKβ.
PKCα and PKCθ mediate IKK activation following CD3/CD28 stimulation with different kinetics.
Having demonstrated a role for PKCα in the CD3/CD28 activation of NF-κB, we questioned its relationship to PKCθ in the CD3/CD28-initiated pathway leading to IKK and NF-κB activation.
To evaluate the relative contribution of each PKC isoform in the CD3/CD28 pathway, we analyzed the kinetics of the IKK complex activation following CD3/CD28 cross-linking in the presence of a variety of PKC inhibitors: the conventional PKC inhibitor Gö6976 (43), the PKCθ inhibitor rottlerin (66), and an inhibitor of both conventional and novel PKC isoforms, Gö6890 (61). We find that rottlerin did not inhibit the early (3 to 5 min) activation of the IKK complex, while Gö6976 did inhibit the early activation (Fig. 5). Interestingly, Gö6976 significantly impaired IKK activation at 10 min. Finally, both rottlerin and Gö6976 totally inhibited IKK activation 25 min after CD3/CD28 cross-linking. Moreover, we find that inhibition of both conventional and novel PKC isoforms by Gö6890 abrogates IKK activation at all times following CD3/CD28 stimulation. These observations suggest that at an early phase (up to 5 min) following CD3/CD28 stimulation, PKCα activates the IKK complex independently of PKCθ, while at later periods following CD3/CD28 stimulation, it is PKCθ-dependent signaling that leads to IKK activation.
FIG. 5.
PKCα and PKCθ mediate IKK activation following CD3/CD28 stimulation with different kinetics. (A) Jurkat T cells were pretreated with different PKC inhibitors for 20 min prior to CD3 and CD28 cross-linking: Gö6976 and Gö6890 were each used at 2 μM, and rottlerin was used at 30 μM. Cells were treated with anti-CD3 (10 μg/ml) and anti-CD28 (10 μg/ml) antibodies for 45 min on ice and cross-linked with goat anti-mouse antibodies in solution at 37°C for the indicated periods of time. Cells were harvested, and the IKK complex kinase activity was measured in an IVK assay, as described in the legends to Fig. 1 to 4. (B) Membrane (M) and cytoplasmic (C) fractions from CD3/CD28-stimulated Jurkat T cells were isolated and resolved by SDS-PAGE. The purity of the digitonin-extractable fraction (C) and NP-40-soluble fraction (M) was tested with antibodies specific for the raft-associated protein, LAT, and the cytoplasmic NF-κ B protein, p65. The quantity of PKCα and PKCθ was analyzed by immunoblotting. (C) Jurkat T cells were transfected with the reporter plasmids κB-luc (0.19 μg) and Tk-Renilla (0.01 μg) by the FuGENE6 method. Eighteen hours later, transfected Jurkat T cells were pretreated with Gö6976 at 2 μM and rottlerin at 30 μM for 20 min andwere then cross-linked with anti-CD3 (10 μg/ml) and anti-CD28 (10 μg/ml) antibodies. Four hours later, luciferase activity was measured, normalized as described above, and expressed as relative luciferase units (RLU). (D) Jurkat T cells (10 × 106 per sample) were electroporated with 40 μg of pFRT-PKCθ or control vector pFRT-H1. Forty-eight hours later, transfected Jurkat T cells were treated with anti-CD3 (10 μg/ml) and anti-CD28 (10 μg/ml) antibodies for 45 min on ice and cross-linked with goat anti-mouse antibodies in solution at 37°C for the indicated periods of time. IKK activity was measured in an IVK assay as described above. Suppression of endogenous PKCθ expression, but not PKCα, was confirmed by immunoblotting. (E) Jurkat T cells were electroporated with 40 μg of pFRT-PKCθ (lane 2), pFRT-PKCα (lane 3), or control vector pFRT-H1 (lane 1) together with reporter κB-luc (3.6 μg) and Tk-Renilla (0.4 μg) plasmids. Forty-eight hours later, transfected Jurkat T cells were treated with anti-CD3 (3 μg/ml) and anti-CD28 (3 μg/ml) antibodies for 45 min on ice and cross-linked on plated goat anti-mouse antibodies at 37°C. Four hours later, luciferase activity was measured, normalized as described above, and expressed as relative luciferase units (RLU). Suppression of endogenous PKCθ and PKCα protein levels was confirmed by immunoblotting.
To test whether the variation in the kinetics of IKK activation results from differences in the translocation rate of the PKCs to the cell membrane, we isolated membrane and cytoplasmic fractions from Jurkat T cells stimulated with cross-linking anti-CD3/CD28 antibodies for various times (Fig. 5B). The purity of membrane and cytoplasmic fractions was established by blotting for the membrane-associated protein, LAT, and cytoplasmic NF-κB protein, p65. Consistent with the IKK activation observed above, we demonstrated a rapid accumulation of PKCα into the membrane fraction (1 to 5 min). However, the kinetics of PKCθ membrane recruitment was distinct, demonstrating a biphasic recruitment with an initial rapid translocation of PKCθ at 1 to 2 min with a subsequent decrease at 5 min, followed by a second, more prolonged PKCθ accumulation at 10 to 15 min. These results indicate that the rate of PKCα translocation to the membrane fraction correlated with the early phase of IKK activation, while later IKK activation may be ascribed to the second phase of PKCθ accumulation on the cell membrane.
Further analysis of NF-κB-dependent transcriptional activity in Jurkat T cells pretreated with either Gö6976 or rottlerin demonstrated that Gö6976 totally inhibited CD3/CD28-induced NF-κB-dependent transcription, while rottlerin incompletely diminished NF-κB-dependent transcription (Fig. 5C). Altogether, these results suggest that the early phase of IKK activation (Fig. 5A) mediated by PKCα can result in NF-κB-dependent transcription, albeit impaired in the absence of PKCθ signaling (Fig. 5C).
To confirm this conclusion, we analyzed the kinetics of IKK activation following CD3/CD28 stimulation in Jurkat T cells transfected with a PKCθ RNA suppression vector. Forty-eight hours after transfection, we observed a strong reduction in the PKCθ expression and a preferential inhibition of IKK activation at 10 and 15 min following CD3/CD28 stimulation (Fig. 5D). This is in stark contrast to the effects of PKCα suppression (Fig. 2C) and Gö6976 treatment (Fig. 5A), which resulted in a significant reduction of IKK activation at all times following CD3/CD28 stimulation.
Finally, we measured the effect of suppression of either PKCα or PKCθ on CD3/CD28-induced NF-κB dependent transcriptional activity. As shown in Fig. 5E, decreasing either PKCα or PKCθ protein levels results in a profound defect in NF-κB activation following CD3/CD28 stimulation. Therefore, these data suggest that while PKCα and PKCθ may mediate different phases in IKK activation, the action of both PKCs is required for a prolonged IKK activation and consequently, for efficient NF-κB-mediated gene transcription.
PKCα acts upstream of PKCθ.
To further delineate the contribution of both PKCα and PKCθ in CD3/CD28-induced NF-κB regulation, we compared the effects of both Gö6976 and rottlerin on NF-κB-dependent transcription induced by expression of PKCαA25E or constitutively active PKCθ, PKCθA148E (Fig. 6A and B). As expected, Gö6976 did not inhibit PKCθA148E-induced NF-κB-dependent transcription (Fig. 6B), while it completely blocked the induction of NF-κB-dependent transcription by PKCαA25E (Fig. 6A).
FIG. 6.
PKCαA25E activates IKKβ and NF-κB in a PKCθ-dependent manner in T lymphocytes, but not in 293T cells. (A) Jurkat T cells were transfected with HA-PKCαA25E and reporter plasmids by electroporation. Eighteen hours later, transfected Jurkat T cells were pretreated with either Gö6976 at 2 μM or rottlerin at 30 μM for 20 min and stimulated with either ionomycin or OKT3 antibodies (5 μg/ml). Four hours later, luciferase activity was measured and normalized as described above. Panel B is the same as panel A, except that PKCθA148E was transfected. (C) 293T cells plated on 24-well plates were transfected with 200 ng of HA-PKCαA25E and reporter κB-luc (20 ng) and Tk-Renilla (1 ng) plasmids by using Lipofectamine Plus according to the manufacturer's protocol. Eighteen hours later, transfected 293T cells were pretreated with rottlerin at 30 μM for 20 min and stimulated or not with ionomycin. Four hours later, luciferase activity was measured and normalized as described above.(D) Jurkat T cells (10 × 106) were electroporated with 5 μg of HA-IKKβ wild type with or without 15 μg of PKCαA25E, PKCαK368R, PKCθA148E, or PKCθK409R. IKKβ was immunoprecipitated (IP) with anti-HA antibodies, and the kinase activity of IKKβ was measured as described above. (E) Jurkat T cells (10 × 106) were electroporated with 15 μg of PKCαA25E (lanes 2 and 3) with or without 40 μg of pFRT-PKCθ (lane 3) or control vector (lane 1) together with reporter κB-luc (3.6 μg) and Tk-Renilla (0.4 μg) plasmids. Forty-eight hours later, transfected Jurkat T cells were cross-linked with OKT3 antibodies (5 μg/ml) or isotype control mouse IgG2a (5 μg/ml). Four hours later, luciferase activity was measured, normalized as described above, and expressed as relative luciferase units (RLU). Suppression of endogenous PKCθ and HA-PKCαA25E protein levels was confirmed by immunoblotting.
Rottlerin, previously characterized as a potent PKCθ inhibitor (66), efficiently inhibited NF-κB-dependent transcription induced by PKCαA25E in the presence of Ca2+ influx (Fig. 6A), whereas it did not inhibit in vitro PKCα kinase activity (14, 66). It has been recently reported that rottlerin has a broader inhibitory activity (16) and can affect PKCδ activation through an indirect mechanism (56). To further explore this, we observed that 30 μM rottlerin efficiently inhibited PKCθΑ148Ε-induced NF-κB-dependent transcriptional activity (Fig. 6B), while it did not affect in vitro PKCα kinase activity, IKK activity, or NF-κB-mediated transcription induced by TNF-α (data not shown). More importantly, the effect of rottlerin on PKCαA25E-induced NF-κB-dependent transcription was only observed in T lymphocytes, since in nonhematopoetic cells, such as 293T cells, that lack PKCθ (2), PKCαA25E induced NF-κB-dependent transcription in a rottlerin-insensitive manner (Fig. 6C). These findings suggest that in T lymphocytes, PKCα signals upstream of PKCθ to activate IKK and NF-κB.
To further correlate this conclusion, the activity of IKKβ was measured in cells in which IKKβ was coexpressed with constitutively active PKCα A25E with or without catalytically inactive PKCθ (PKCθK409R) (Fig. 6D). Coexpression of PKCθK409R (Fig. 6D) or rottlerin treatment (data not shown) inhibited the PKCαA25E-induced IKKβ kinase activity.
To exclude possible competition or interference between PKCα and PKCθ, we studied the effect of the PKCαK409R on the PKCθΑ148Ε-induced IKKβ kinase activity. As shown in Fig. 6D, catalytically inactive PKCα had no effect on kinase activity of IKKβ induced by expression of PKCθA148E. Pretreatment of PKCθA148E-transfected cells with Gö6976 also did not affect the IKKβ kinase activity (data not shown). These data suggest that PKCα does not compete with PKCθ to activate IKKβ but rather acts upstream of this novel PKC isoform.
Finally, we analyzed PKCαA25E-induced NF-κB-dependent transcription in Jurkat T cells, cotransfected with a PKCθ RNA suppression vector, following T-cell activation with OKT3 antibody (Fig. 6E). Interestingly, we found that a reduction in PKCθ expression resulted in a greater accumulation of HA-PKCαA25E (Fig. 6E). Despite the severalfold increase in protein levels of HA-PKCαA25E in cells cotransfected with the PKCθ RNA suppression vector, PKCαA25E-induced NF-κB-dependent transcription was impaired.
Altogether, our data suggest that PKCα acts upstream of PKCθ in the TCR-initiated pathway that leads to NF-κB activation.
PKCα is required for IL-2 transcription.
While previously published data demonstrated that PKCθ positively regulates IL-2 transcription (16, 40, 58, 67) by targeting NF-AT (67), AP-1 (3, 67), NF-κB (16, 40), and CD28RE/AP (16), the role of PKCα in regulating IL-2 gene expression remains controversial (3, 25, 67). To confirm whether PKCα can induce IL-2 transcription in T cells, Jurkat T cells were transfected with the full-length IL-2 promoter-luciferase reporter gene construct and increasing doses of either PKCαA25E or PKCθA148E plasmid (Fig. 7A). As expected, expression of PKCθA148E induced IL-2-dependent transcription in a dose-dependent manner that is strongly amplified by ionomycin. In contrast, expression of PKCαA25E induced significant IL-2-dependent transcriptional activity in a dose-dependent manner only in the presence of ionomycin. The ability of PKCαA25E to induce IL-2 transcription was not restricted only to Jurkat T cells, since PKCαA25E together with ionomycin efficiently activated IL-2-dependent transcription in primary human CD3+ cells (Fig. 7B).
FIG.7.
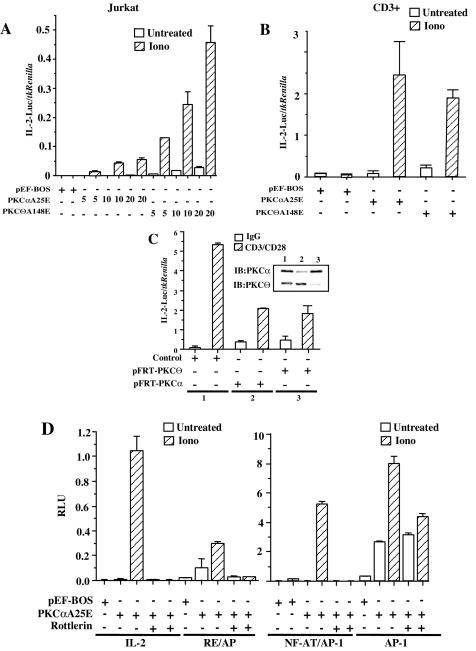
PKCαA25E induces IL-2 transcription in Jurkat and primary CD3+ T cells in a PKCθ-dependent manner. (A) Jurkat T cells (10 × 106 per sample) were electroporated with increasing doses of either HA-PKCαA25E or PKCθA148E or pEF-BOS together with reporter IL-2-luciferase (3.8 μg) and Tk-Renilla (0.2 μg) plasmids. Eighteen hours later, Jurkat T cells were stimulated or not with ionomycin. Luciferase activity was measured as described in the legend to Fig. 2B. (B) Primary CD3+ T cells from healthy donors were electroporated (BTX, 360 V, 10 ms) with 60 μg of HA-PKCαA25E, PKCθA148E, or pEF-1 together with IL-2-luciferase (34 μg) and Tk-Renilla (6 μg) plasmids. Eighteen hours later, CD3+ T cells were stimulated or not with ionomycin, and luciferase activity was measured. (C) Jurkat T cells were electroporated with 40 μg of pFRT-PKCθ (lane 3), pFRT-PKCα (lane 2), or control vector (lane 1) together with reporter IL-2-luciferase (3.6 μg) and Tk-Renilla (0.4 μg) plasmids. Forty-eight hours later, transfected Jurkat T cells were stimulated with anti-CD3 and anti-CD28 antibodies as described in the legend to Fig. 6D. Suppression of endogenous PKCθ and PKCα protein expression was confirmed by immunoblotting. (D) Jurkat T cells (106 per sample) were transfected with 0.19 μg of IL-2- or RE/AP-, NF-AT/AP-1- or AP-1-luciferase reporter plasmids with 1.8 μg of HA-PKCαA25E or pEF-BOS. Eighteen hours later, transfected Jurkat T cells were pretreated with rottlerin and stimulated with ionomycin. Four hours later, luciferase activity was measured and normalized to Tk-Renilla expression.
To confirm these observations, we compared the CD3/CD28-induced IL-2 transcription in Jurkat T cells transfected with RNA suppression vectors for either PKCα or PKCθ. As shown in Fig. 7C, suppression of either PKCθ or PKCα results in a reduction of IL-2 transcription following CD3/CD28 cross-linking. This indicates that PKCα and PKCθ are each required for efficient IL-2 transcription in T cells.
Having demonstrated that PKCαA25E, in the presence of Ca2+ influx, can activate NF-κB-dependent transcription in a PKCθ-dependent manner, we asked whether PKCαA25E induced the activity of the IL-2 promoter in a similar fashion. Jurkat T cells transfected with PKCαA25E and IL-2 promoter-luciferase reporter genes were pretreated with rottlerin before ionomycin stimulation. As shown in Fig. 7D, PKCαA25E induced IL-2-dependent transcriptional activity exclusively in a rottlerin-sensitive manner. To further characterize which of the transcription factors that regulate the IL-2 promoter are targeted by PKCαA25E, Jurkat T cells were transfected with PKCαA25E and CD28RE/AP, NF-AT, and AP-1 reporter genes accordingly. Similar to NF-κB- and IL-2-dependent transcription, PKCαA25E induced RE/AP- and NF-AT-dependent transcription following ionomycin treatment in a rottlerin-dependent manner (Fig. 7D). However, PKCαA25E strongly activated AP-1-dependent transcription in the absence of ionomycin and was not inhibited by rottlerin treatment (Fig. 7D). Interestingly, PKCαA25E-mediated AP-1-dependent transcription was further enhanced by the addition of ionomycin, and this increase was only partially sensitive to rottlerin inhibition. Therefore, these results suggest that PKCαA25E, in the presence of Ca2+ influx, can efficiently induce IL-2 transcription in both Jurkat and primary CD3+ T cells. While IL-2, NF-κB, RE/AP, and NF-AT reporter genes were induced by PKCαA25E in a PKCθ-dependent manner, AP-1 induction by PKCαA25E was PKCθ independent, pointing to a potential divergence in PKCα-initiated pathways in T lymphocytes.
DISCUSSION
In this study, we have demonstrated a novel role for PKCα in T lymphocytes downstream of the CD3/CD28 engagement leading to the activation of the IKK complex and NF-κB. We conclude this based on observations that pharmacological inhibition of PKCα, expression of catalytically inactive PKCα or suppression of PKCα, but not PKCβ1, expression, results in specific abrogation of the CD3/CD28-induced activity of the endogenous heterodimeric IKK complex without any effect on basal or TNF-α-induced IKK activity. This conclusion is further supported by a recent report that inhibition of PKCα expression, but not PKCβ1, resulted in a profound decrease in IL-2R, IL-2, and TNF-α induction in Jurkat T cells (42). In addition, while the absence of PKCβ abolished B-cell receptor-mediated IKK and NF-κB activation (53, 57), it did not affect the TCR-mediated signal transduction (38). Altogether these data indicate that PKCα mediates TCR-induced IKK and NF-κB activation, in contrast to PKCβ, which plays a unique role in NF-κB regulation in B cells. Moreover, although PKCθ has been recently shown to be a critical molecule involved in the activation of NF-κB downstream of the TCR (16, 40, 58), our data place PKCα upstream of PKCθ in this process.
Another group of evidence that establishes the role of PKCα in NF-κB activation triggered by TCR/CD28 ligation was provided by using a constitutively active form of PKCα. Previous studies utilizing the constitutively active form of PKCα demonstrated that it had no significant effect on NF-κB activation in T cells (3, 16, 25, 40). However, we observed that the ability of such constitutively active form of PKCα to induce IKK and NF-κB in T cells depends on Ca2+ binding to its C2 regulatory domain. This contrasts with the Ca2+ independence of PKCθ, a kinase lacking the C2 regulatory domain, and explains the ability of a constitutively active PKCθ to activate NF-κB in the absence of Ca2+ influx. An increase in Ca2+ influx is known to promote PKCα translocation to the plasma cell membrane and subsequent binding to negatively charged phospholipids, steps that are required for PKCα autophosphorylation (12, 15, 44, 55, 65). Substitution of a key alanine for a charged residue within the PKCα pseudosubstrate (A25E) yields pseudosubstrate release and catalytic activity in vitro (3). However, this constitutively active PKCα fails to activate NF-κB until a secondary Ca2+ signal is given. We interpret the inability of PKCαA25E to induce NF-κB in the absence of Ca2+ as likely due to the lack of catalytic competency of PKCαA25E and/or the lack of required interaction with downstream targets on the cell membrane. It has been previously shown that mutations in the Ca2+ acceptor sites in the C2 domain block PKCα translocation to the plasma cell membrane (15). This would explain why the PKCαA25E/D246N-D248N mutant, which retains the A25E mutation that provides pseudosubstrate sequence release and catalytic activity (3), is unable to induce NF-κB activity compared to PKCαA25E, even when both are in the presence of ionomycin. From these observations, it is inferred that only the plasma membrane-bound PKCα can activate the IKK complex and hence, NF-κB. This conclusion is also supported by a previous report (29) indicating that expression of a PKCα transgene results in an accumulation of overexpressed PKCα on the cell membrane of T cells and subsequent hyperresponsiveness of these T cells to such weak stimuli as soluble antibodies to TCR. Therefore, it seems that targeting PKCα to the membrane significantly reduces the threshold of TCR signaling. It also appears that membrane localization is an absolute requirement for PKCs to activate NF-κB, since PKCθ can activate NF-κB only in the membrane-bound form (7). Deletion of the regulatory domain of PKCθ, similar to the mutation of calcium binding ligands in the regulatory domain of PKCα, abolishes binding to the cell membrane. This results in the complete incapability of either the constitutively active form of PKCα or catalytic domain of PKCθ to activate NF-κB in T cells. Taking into consideration that the IKK complex is recruited to the cell membrane following CD3/CD28 stimulation (34), it is possible that membrane-bound PKCα interacts in a similar way to PKCθ with the recruited IKK complex and subsequently activates it.
While the precise mechanism of IKK and NF-κB activation by PKCα remains to be defined, two important steps in PKCα signaling may be distinguished based on our kinetic data of the CD3/CD28-induced IKK complex activation in the presence of PKC inhibitors. First, there is an early phase (up to 5 min) of IKK complex activation that is rottlerin insensitive and is inhibited by either Gö6976 or by suppression of the PKCα gene. This represents the step at which PKCα acts independently of PKCθ. This possibility is supported by the previously published observation (37) that PKCα physically interacts with and activates IKKβ. However, this transient PKCα-mediated IKK activation is not sufficient to provide adequate NF-κB transcription (Fig. 5C).
The question of interest is the mechanism of PKCα signaling in the second or late phase of IKK activation following CD3/CD28 stimulation. Our data demonstrate that this step is inhibited by both rottlerin and Gö6976. Moreover, selective suppression of either PKCα or PKCθ expression also abrogated this phase of IKK activation. Taken together with the observation that PKCαA25E-mediated activation of IKKβ and NF-κB transcription is inhibited by rottlerin, suppression of PKCθ or expression of PKCθK409R, our results suggest that in the late phase of the IKK complex activation, PKCα activates through the PKCθ-dependent pathway. Whereas the detailed mechanism of PKCθ upregulation by PKCα falls short in this study, two possible scenarios are under investigation. One is direct phosphorylation of PKCθ by PKCα, which takes place on the plasma membrane following CD3/CD28 stimulation. Another scenario can include PKCα-mediated Lck release from the intracytoplasmic domain of CD4 (51). Since activation of PKCθ requires recruitment by Lck to rafts (7) and the subsequent phosphorylation by Lck (41), it is likely that PKCα sustains Lck activation and therefore provides the prolonged stimulus required for optimal PKCθ activation. This possibility is supported by the observation that inhibition of either Lck or Src kinases results in a partial or complete inhibition of PKCαA25E-mediated NF-κB activation (data not shown). Therefore, PKCα may augment or extend PKCθ activity through an Src kinase-dependent pathway (unpublished data).
The identification of PKCα as another PKC isoform that mediates TCR-induced NF-κB activation is, in part, supported by the few facts previously known about PKCα regulation. PKCα translocates to the plasma cell membrane with similar kinetics to PKCθ following CD3/CD28 cross-linking (59), and specific inhibition of either PKCα or PKCθ abrogates the expression of IL-2Rα following TCR ligation (59). Our data demonstrate that inhibition of either PKCα or PKCθ abrogates NF-κB activation in T lymphocytes. Our results indicate that participation of both PKC isoforms can provide prolonged IKK activation and subsequently efficient NF-κB transcription. Indeed, PKCα alone cannot activate NF-κB in PKCθ-deficient peripheral blood lymphocytes following CD3/CD28 ligation unless the lack of contribution of PKCθ is bypassed by phorbol ester and Ca2+ ionophore treatment (58). This observation suggests that PKCα-mediated activation of the IKK complex in mature T lymphocytes is too brief to provide adequate NF-κB transcription. However, stimulation with phorbol ester and ionomycin provides prolonged activation of PKCα that results in efficient IKK activation and NF-κB transcription in PKCθ-deficient lymphocytes. This is in contrast to immature PKCθ-deficient T cells, which demonstrate normal NF-κB activation following CD3/CD28 stimulation. Since the Ca2+ levels are more transient in immature T cells than in mature T cells (23), it remains possible that the time period of the direct activation of the IKK complex by PKCα can depend on the amplitude and duration of the Ca2+ influx in these two distinct cell populations.
Our data suggest that PKCα can interpret the amplitude and duration of Ca2+ levels in T cells. Indeed, the fact that only membrane-bound PKCα can activate the IKK complex and induce NF-κB transcription suggests that the time of PKCα interaction with the cell membrane may define the kinetics of IKK activation and the subsequent proficiency of NF-κB transcription. PKCα translocation to the plasma cell membrane requires a Ca2+ concentration higher than 600 nM (33, 47). Therefore, Ca2+ concentrations that are lower than 600 nM should fail to translocate PKCα to the plasma membrane and hence to activate NF-κB. In fact, Ca2+ concentrations higher than 600 nM are required to induce NF-κB (20, 21). Moreover, only Ca2+ influx, but not the release of Ca2+ from intracellular stores, results in NF-κB activation in T cells (32). Finally, we observed that inhibition of Ca2+ influx by EGTA resulted in a defect in the kinetics of IKK activation similar to that seen with PKCα RNA suppression or pretreatment with Gö6976. This defect in the kinetics of IKK activation abrogated the NF-κB-dependent transcription following CD3/CD28 stimulation (Fig. 5C and E) (data not shown).
The fact that PKCα activates IL-2, RE/AP, NF-κB, and NF-AT, but not AP-1, transcription in a rottlerin-sensitive manner, suggests the divergence of PKCα-mediated pathways in T lymphocytes. Normal MAP kinase (MAPK) activation in PKCθ−/− deficient cells also suggests this possibility of PKCα-dependent pathways in the activation of MAPK (58). We observed that suppression of PKCα not only abrogates NF-κB and IL-2 transcription (Fig. 5E and 7C), but also inhibits RE/AP, NF-AT, and AP-1 activation (unpublished observations). Therefore, PKCα may initiate two different signaling pathways: one that leads to NF-κB activation in a PKCθ-dependent manner and another that activates AP-1-mediated gene transcription independently of PKCθ. However, our kinetic data indicate that the first pathway, which is characterized by IKK activation in a PKCθ-dependent manner, occurs during the late phase of TCR activation (>5 min). Thus, we hypothesize that this pathway, which leads to the prolonged IKK activation and subsequent efficient NF-κB transcription, can represent a novel mechanism of TCR amplification that is mediated by PKCα. Therefore, the level of PKCα-mediated TCR amplification will depend on the amplitude and duration of the rise in Ca2+ following TCR engagement. This hypothesis is currently being investigated.
In summary, this study characterizes a novel role for PKCα in mediating the CD3/CD28 signaling pathways that lead to the activation of both the IKK complex and NF-κB in T lymphocytes. Our data suggest that PKCα represents a Ca2+-dependent component of the TCR/CD28 pathways, which can act upstream of PKCθ in the activation of NF-κB.
Acknowledgments
This work was supported by the National Institutes of Health grant R01 AI36076 to C.V.P. and the Mayo Foundation. D.D.B. is supported by a Leukemia and Lymphoma Society Special Fellows Award and an Investigator Award from the Cancer Research Institute.
We thank Gary Bren for outstanding technical assistance and all the members of C. V. Paya's laboratory for thoughtful discussions. We also thank Teresa Hoff for excellent manuscript preparation.
REFERENCES
- 1.Baeuerle, P. A., and D. Baltimore. 1989. A 65-kD subunit of active NF-κB is required for inhibition of NF-κB by IκB. Genes Dev. 3:1689-1698. [DOI] [PubMed] [Google Scholar]
- 2.Baier, G., D. Telford, L. Giampa, K. M. Coggeshall, G. Baier-Bitterlich, N. Isakov, and A. Altman. 1993. Molecular cloning and characterization of PKCθ, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J. Biol. Chem. 268:4997-5004. [PubMed] [Google Scholar]
- 3.Baier-Bitterlich, G., F. Überall, B. Bauer, F. Fresser, H. Wachter, H. Grunicke, G. Utermann, A. Altman, and G. Baier. 1996. Protein kinase C-θ isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol. Cell. Biol. 16:1842-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beg, A. A., S. M. Ruben, R. I. Scheinman, S. Haskill, C. A. Rosen, and A. S. Baldwin, Jr. 1992. IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 6:1899-1913. [DOI] [PubMed] [Google Scholar]
- 5.Bell, M. P., C. J. Huntoon, D. Graham, and D. J. McKean. 2001. The analysis of costimulatory receptor signaling cascades in normal T lymphocytes using in vitro gene transfer and reporter gene analysis. Nat. Med. 7:1155-1158. [DOI] [PubMed] [Google Scholar]
- 6.Berridge, M. J. 1993. Inositol trisphosphate and calcium signalling. Nature 361:315-325. [DOI] [PubMed] [Google Scholar]
- 7.Bi, K., Y. Tanaka, N. Coudronniere, K. Sugie, S. Hong, M. J. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat. Immunol. 2:556-563. [DOI] [PubMed] [Google Scholar]
- 8.Brockman, J. A., D. C. Scherer, T. A. McKinsey, S. M. Hall, X. Qi, W. Y. Lee, and D. W. Ballard. 1995. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol. Cell. Biol. 15:2809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 10.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 11.Cao, Y., E. M. Janssen, A. W. Duncan, A. Altman, D. D. Billadeau, and R. T. Abraham. 2002. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 21:4809-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazaubon, S., F. Bornancin, and P. J. Parker. 1994. Threonine-497 is a critical site for permissive activation of protein kinase C α. Biochem. J. 301:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, D., and E. V. Rothenberg. 1993. Molecular basis for developmental changes in interleukin-2 gene inducibility. Mol. Cell. Biol. 13:228-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapham, D. E. 1995. Calcium signaling. Cell. 80:259-268. [DOI] [PubMed] [Google Scholar]
- 15.Corbalan-Garcia, S., J. A. Rodriguez-Alfaro, and J. C. Gomez-Fernandez. 1999. Determination of the calcium-binding sites of the C2 domain of protein kinase C α that are critical for its translocation to the plasma membrane. Biochem. J. 337:513-521. [PMC free article] [PubMed] [Google Scholar]
- 16.Coudronniere, N., M. Villalba, N. Englund, and A. Altman. 2000. NF-κB activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-θ. Proc. Natl. Acad. Sci. USA 97:3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crabtree, G. R., and N. A. Clipstone. 1994. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu. Rev. Biochem. 63:1045-1083. [DOI] [PubMed] [Google Scholar]
- 18.DiDonato, J., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16:1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 20.Dolmetsch, R. E., R. S. Lewis, C. C. Goodnow, and J. I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855-858. [DOI] [PubMed] [Google Scholar]
- 21.Dolmetsch, R. E., K. Xu, and R. S. Lewis. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392:933-936. [DOI] [PubMed] [Google Scholar]
- 22.Frantz, B., E. C. Nordby, G. Bren, N. Steffan, C. V. Paya, R. L. Kincaid, M. J. Tocci, S. J. O'Keefe, and E. A. O'Neill. 1994. Calcineurin acts in synergy with PMA to inactivate IκB/MAD3, an inhibitor of NF-κB. EMBO J. 13:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman, B. D., Q. H. Liu, S. Somersan, M. I. Kotlikoff, and J. A. Punt. 1999. Receptor avidity and costimulation specify the intracellular Ca2+ signaling pattern in CD4+CD8+ thymocytes. J. Exp. Med. 190:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Paramio, P., Y. Cabrerizo, F. Bornancin, and P. J. Parker. 1998. The broad specificity of dominant inhibitory protein kinase C mutants infers a common step in phosphorylation. Biochem. J. 333:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genot, E. M., P. J. Parker, and D. A. Cantrell. 1995. Analysis of the role of protein kinase C-α, -ɛ, and -ζ in T cell activation. J. Biol. Chem. 270:9833-9839. [DOI] [PubMed] [Google Scholar]
- 26.Harhaj, E. W., and S. C. Sun. 1998. IκB kinases serve as a target of CD28 signaling. J. Biol. Chem. 273:25185-25190. [DOI] [PubMed] [Google Scholar]
- 27.Hoyos, B., D. W. Ballard, E. Bohnlein, M. Siekevitz, and W. C. Greene. 1989. κB-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science 244:457-460. [DOI] [PubMed] [Google Scholar]
- 28.Imboden, J. B., and J. D. Stobo. 1985. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J. Exp. Med. 161:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwamoto, T., M. Hagiwara, H. Hidaka, T. Isomura, D. Kioussis, and I. Nakashima. 1992. Accelerated proliferation and interleukin-2 production of thymocytes by stimulation of soluble anti-CD3 monoclonal antibody in transgenic mice carrying a rabbit protein kinase C α. J. Biol. Chem. 267:18644-18648. [PubMed] [Google Scholar]
- 30.Jain, J., C. Loh, and A. Rao. 1995. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7:333-342. [DOI] [PubMed] [Google Scholar]
- 31.Jamieson, C., P. G. McCaffrey, A. Rao, and R. Sen. 1991. Physiologic activation of T cells via the T cell receptor induces NF-κB. J. Immunol. 147:416-420. [PubMed] [Google Scholar]
- 32.Kanno, T., and U. Siebenlist. 1996. Activation of nuclear factor-κB via T cell receptor requires a Raf kinase and Ca2+ influx. Functional synergy between Raf and calcineurin. J. Immunol. 157:5277-5283. [PubMed] [Google Scholar]
- 33.Keranen, L. M., and A. C. Newton. 1997. Ca2+ differentially regulates conventional protein kinase Cs' membrane interaction and activation. J. Biol. Chem. 272:25959-25967. [DOI] [PubMed] [Google Scholar]
- 34.Khoshnan, A., S. J. Kempiak, B. L. Bennett, D. Bae, W. Xu, A. M. Manning, C. H. June, and A. E. Nel. 1999. Primary human CD4+ T cells contain heterogeneous IκB kinase complexes: role in activation of the IL-2 promoter. J. Immunol. 163:5444-5452. [PubMed] [Google Scholar]
- 35.Kieran, M., V. Blank, F. Logeat, J. Vandekerckhove, F. Lottspeich, O. Le Bail, M. B. Urban, P. Kourilsky, P. A. Baeuerle, and A. Israel. 1990. The DNA binding subunit of NF-κB is identical to factor KBF1 and homologous to the rel oncogene product. Cell 62:1007-1018. [DOI] [PubMed] [Google Scholar]
- 36.Lai, J.-H., G. Horvath, J. Subleski, J. Bruder, P. Ghosh, and T.-H. Tan. 1995. RelA is a potent transcriptional activator of the CD28 response element within the interleukin 2 promoter. Mol. Cell. Biol. 15:4260-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lallena, M. J., M. T. Diaz-Meco, G. Bren, C. V. Payá, and J. Moscat. 1999. Activation of IκB kinase β by protein kinase C isoforms. Mol. Cell. Biol. 19:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitges, M., C. Schmedt, R. Guinamard, J. Davoust, S. Schaal, S. Stabel, and A. Tarakhovsky. 1996. Immunodeficiency in protein kinase cβ-deficient mice. Science 273:788-791. [DOI] [PubMed] [Google Scholar]
- 39.Lin, X., E. T. Cunningham, Jr., Y. Mu, R. Geleziunas, and W. C. Greene. 1999. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity 10:271-280. [DOI] [PubMed] [Google Scholar]
- 40.Lin, X., A. O'Mahony, Y. Mu, R. Geleziunas, and W. C. Greene. 2000. Protein kinase C-θ participates in NF-κB activation induced by CD3-CD28 costimulation through selective activation of IκB kinase β. Mol. Cell. Biol. 20:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, Y., S. Witte, Y. C. Liu, M. Doyle, C. Elly, and A. Altman. 2000. Regulation of protein kinase Cθ function during T cell activation by Lck-mediated tyrosine phosphorylation. J. Biol. Chem. 275:3603-3609. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Lago, M. A., J. Freire-Moar, and P. Barja. 1999. Inhibition of protein kinase C α expression by antisense RNA in transfected Jurkat cells. Eur. J. Immunol. 29:466-476. [DOI] [PubMed] [Google Scholar]
- 43.Martiny-Baron, G., M. G. Kazanietz, H. Mischak, P. M. Blumberg, G. Kochs, H. Hug, D. Marme, and C. Schachtele. 1993. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268:9194-9197. [PubMed] [Google Scholar]
- 44.Medkova, M., and W. Cho. 1998. Mutagenesis of the C2 domain of protein kinase C-α. Differential roles of Ca2+ ligands and membrane binding residues. J. Biol. Chem. 273:17544-17552. [DOI] [PubMed] [Google Scholar]
- 45.Mercurio, F., J. A. DiDonato, C. Rosette, and M. Karin. 1993. p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev. 7:705-718. [DOI] [PubMed] [Google Scholar]
- 46.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 47.Mosior, M., and R. M. Epand. 1994. Characterization of the calcium-binding site that regulates association of protein kinase C with phospholipid bilayers. J. Biol. Chem. 269:13798-13805. [PubMed] [Google Scholar]
- 48.Newton, A. C. 1995. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 270:28495-28498. [DOI] [PubMed] [Google Scholar]
- 49.Newton, A. C., and J. E. Johnson. 1998. Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochim. Biophys. Acta 1376:155-172. [DOI] [PubMed] [Google Scholar]
- 50.Nolan, G. P., S. Ghosh, H. C. Liou, P. Tempst, and D. Baltimore. 1991. DNA binding and IκB inhibition of the cloned p65 subunit of NF-κB, a rel-related polypeptide. Cell 64:961-969. [DOI] [PubMed] [Google Scholar]
- 51.Parolini, I., S. Topa, M. Sorice, A. Pace, P. Ceddia, E. Montesoro, A. Pavan, M. P. Lisanti, C. Peschle, and M. Sargiacomo. 1999. Phorbol ester-induced disruption of the CD4-Lck complex occurs within a detergent-resistant microdomain of the plasma membrane. Involvement of the translocation of activated protein kinase C isoforms. J. Biol. Chem. 274:14176-14187. [DOI] [PubMed] [Google Scholar]
- 52.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90:373-383. [DOI] [PubMed] [Google Scholar]
- 53.Saijo, K., I. Mecklenbrauker, A. Santana, M. Leitger, C. Schmedt, and A. Tarakhovsky. 2002. Protein kinase C β controls nuclear factor κB activation in B cells through selective regulation of the IκB kinase α. J. Exp. Med. 195:1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sen, J., L. Venkataraman, Y. Shinkai, J. W. Pierce, F. W. Alt, S. J. Burakoff, and R. Sen. 1995. Expression and induction of nuclear factor-κB-related proteins in thymocytes. J. Immunol. 154:3213-3221. [PubMed] [Google Scholar]
- 55.Shao, X., B. A. Davletov, R. B. Sutton, T. C. Sudhof, and J. Rizo. 1996. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science 273:248-251. [DOI] [PubMed] [Google Scholar]
- 56.Soltoff, S. P. 2001. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J. Biol. Chem. 276:37986-37992. [DOI] [PubMed] [Google Scholar]
- 57.Su, T. T., B. Guo, Y. Kawakami, K. Sommer, K. Chae, L. A. Humphries, R. M. Kato, S. Kang, L. Patrone, R. Wall, M. Teitell, M. Leitges, T. Kawakami, and D. J. Rawlings. 2002. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3:780-786. [DOI] [PubMed] [Google Scholar]
- 58.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 59.Szamel, M., A. Appel, R. Schwinzer, and K. Resch. 1998. Different protein kinase C isoenzymes regulate IL-2 receptor expression or IL-2 synthesis in human lymphocytes stimulated via the TCR. J. Immunol. 160:2207-2214. [PubMed] [Google Scholar]
- 60.Thompson, J. E., R. J. Phillips, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1995. IκBα regulates the persistent response in a biphasic activation of NF-κB. Cell 80:573-582. [DOI] [PubMed] [Google Scholar]
- 61.Toullec, D., P. Pianetti, H. Coste, P. Bellevergue, T. Grand-Perret, M. Ajakane, V. Baudet, P. Boissin, E. Boursier, F. Loriolle et al. 1991. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266:15771-15781. [PubMed] [Google Scholar]
- 62.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human IκBα on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 14:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trushin, S. A., K. N. Pennington, A. Algeciras-Schimnich, and C. V. Paya. 1999. Protein kinase C and calcineurin synergize to activate IκaB kinase and NF-κB in T lymphocytes. J. Biol. Chem. 274:22923-22931. [DOI] [PubMed] [Google Scholar]
- 64.Valge, V. E., J. G. Wong, B. M. Datlof, A. J. Sinskey, and A. Rao. 1988. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell 55:101-112. [DOI] [PubMed] [Google Scholar]
- 65.Verdaguer, N., S. Corbalan-Garcia, W. F. Ochoa, I. Fita, and J. C. Gomez-Fernandez. 1999. Ca2+ bridges the C2 membrane-binding domain of protein kinase Cα directly to phosphatidylserine. EMBO J. 18:6329-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villalba, M., S. Kasibhatla, L. Genestier, A. Mahboubi, D. R. Green, and A. Altman. 1999. Protein kinase Cθ cooperates with calcineurin to induce Fas ligand expression during activation-induced T cell death. J. Immunol. 163:5813-5819. [PubMed] [Google Scholar]
- 67.Werlen, G., E. Jacinto, Y. Xia, and M. Karin. 1998. Calcineurin preferentially synergizes with PKC-θ to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 17:3101-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whiteside, S. T., M. K. Ernst, O. LeBail, C. Laurent-Winter, N. Rice, and A. Israël. 1995. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol. Cell. Biol. 15:5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 70.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]