Abstract
In yeast, the transcriptional adaptor yeast Ada2 (yAda2) is a part of the multicomponent SAGA complex, which possesses histone acetyltransferase activity through action of the yGcn5 catalytic enzyme. yAda2, among several SAGA proteins, serves to recruit SAGA to genes via interactions with promoter-bound transcription factors. Here we report identification of a new human Ada2 homologue, hAda2β. Ada2β differs both biochemically and functionally from the previously characterized hAda2α, which is a stable component of the human PCAF (human Gcn5 homologue) acetylase complex. Ada2β, relative to Ada2α, interacted selectively, although not stably, with the Gcn5-containing histone acetylation complex TFTC/STAGA. In addition, Ada2β interacted with Baf57 (a component of the human Swi/Snf complex) in a yeast two-hybrid screen and associated with human Swi/Snf in vitro. In functional assays, hAda2β (but not Ada2α), working in concert with Gcn5 (but not PCAF) or Brg1 (the catalytic component of hSwi/Snf complex), increased transcription via the B-cell-specific transcription factor Pax5/BSAP. These findings support the view that Gcn5 and PCAF have distinct roles in vivo and suggest a new mechanism of coactivator function, in which a single adaptor protein (Ada2β) can coordinate targeting of both histone acetylation and chromatin remodeling activities.
Activation of mRNA transcription is complex and dynamic. DNA-bound transcription factors recruit a number of proteins including basal transcription factors and regulatory adaptor/coactivator proteins. There are two generally accepted models for the role of adaptors: (i) to enzymatically alter the repressive structure of chromatin to promote binding of basal transcription factors and/or (ii) to provide a physical link between remotely bound sequence-specific activators and the basal transcriptional machinery.
In yeast, Ada2 is a transcriptional adaptor protein, identified through a genetic screen involving the chimeric activator GAL4DBD-VP16 (7). Yeast Ada2 (yAda2) potentiates transcription mediated by several transcription activators, such as herpesvirus VP16 and Gcn4 (2, 44). Deletion of yAda2 strongly inhibits the interaction between GAL4DBD-VP16 and TATA-binding protein (TBP) in vivo (2).
Ada2 is a member of a number of multiprotein complexes in yeast that contain histone acetyltransferase (HAT) activity (24, 41). In early studies, yAda2 was found in a trimeric module with two other adaptor proteins: Gcn5 and Ada3 (13, 26). Gcn5 was the first identified transcriptionally related HAT (11) and was subsequently found as the catalytic HAT subunit of the SAGA transcriptional regulatory complex. The 1.8-MDa SAGA complex also includes a number of other transcriptionally relevant subunits (24): Ada1, Ada5, the TBP-related subgroup of the Spt proteins (Spt3, Spt7, Spt8, and Spt20 which is identical to Ada5) (21, 40), a subset of the TATA binding protein-associated factors (TAFs) (TAFII17, TAFII25, TAFII60, TAFII68, and TAFII90) (22), and Tra1 (23, 42), the yeast homologue of the transcriptional coactivator TRRAP in higher eukaryotes (31).
The mouse and human homologues of yAda2 are identical to each other and are 31% identical and 53% similar to yAda2 in a 423-amino-acid (aa) overlap (14; the present study). Structure-function studies of human Ada2 (hAda2) suggested that, similar to yAda2, it was able to interact with both yeast and human Gcn5 (14, 55). Two human homologues of yGcn5 have been identified: hGcn5 and PCAF (14, 64), both of which contain an extended amino-terminal domain (47, 61). PCAF- and Gcn5-containing complexes were isolated by immunoaffinity purification from HeLa stable cell lines (10, 28, 37). These two large multiprotein complexes were similar in composition and contained the same corresponding groups of components (Adas, Spts, Tafs, and Tra1) as yeast SAGA. Two nearly identical Gcn5-containing complexes, TFTC (TBP-free TAF-containing complex) and STAGA (SPT3-TAF31-GCN5 acetyltransferase), respectively (28, 58), were purified by using different approaches. The polypeptide compositions of TFTC and STAGA were very similar: neither complex contained TBP, hTAF250, hTAF28, or hTAF18 (5), and both contained hTAF150, hTAF135, hTAF100, TAF80, TAF31, TAF30 (58), TRRAP, hAda3, hSPT3, hPAF65b, and SAP 130 (9, 10, 29). In addition, STAGA contained several proteins whose yeast homologues are present in the yeast SAGA complex, e.g., STAF67γ (ySPT7) and STAF42 (yAda1) (29). It is not clear how the PCAF and Gcn5 complexes differ from one another or what are their precise physiological roles. One interesting difference is that hAda2 is found in the PCAF complex (37) but may be absent from TFTC (10). Since Ada2 is an important structural and functional component of yeast SAGA, this potential difference in the human complexes suggests a possible regulatory role for hAda2. Possibly related to this is the finding that most cellular hAda2 exists as a monomer (20).
It has become clear in recent years that histone acetylation and chromatin remodeling work together to antagonize chromatin-mediated transcriptional repression (25). The most extensively characterized protein complex possessing chromatin remodeling activity is the 2-MDa ATP-dependent Swi/Snf complex in yeast. Swi/Snf is composed of 11 subunits (12), members of which were identified in numerous genetic screens as positive regulators of transcription (59). Biochemical studies have revealed that Swi2/Snf2 is the ATPase subunit of Swi/Snf. In mammals there are two related homologues of the SWI2/SNF2 gene, BRG1 and hBRM, and each is contained within a distinct protein complex. The complexes contain mammalian homologues of yeast SNF5 (INI1), SWI1 (BAF250), SWI3 (BAF170 and BAF155), SWP73 (BAF60a), ARP7 (β-actin), and ARP9 (BAF57) (33). Both Brg1 and hBrm human complexes appear to be essential for growth and development. Overexpression of dominant-negative forms of either Brg1 or hBrm led to significant decrease of transcriptional activation by estrogen, retinoid, or glucocorticoid receptors (16). Notably, both Swi/Snf- and Gcn5-containing complexes can bind the same transcriptional activators, including c-Myc, estrogen receptor, E2A (30, 63), and E2F (52).
The mammalian Pax5/BSAP transcription factor is a member of a highly conserved family of paired domain-containing factors and is essential for B lymphopoiesis and midbrain patterning (36). Pax5 (BSAP) functions as both a transcriptional activator and repressor. Pax5 activates CD19, Igα (mb-1), LEF-1, and N-myc expression and represses M-CSF-R gene, PD-1, and the 3′ immunoglobulin heavy-chain enhancer hs1,2 (35, 36, 45, 46). The dual role of Pax5 in transcriptional regulation may be caused by interactions with other proteins. Pax5 interacts with TBP and Rb in vitro (17) and with corepressor Grg4, a member of the Groucho family (18), and the Daxx protein in vivo (19). Interestingly, in the latter case, Pax5 formed a trimeric complex with Daxx and CBP, an important coactivator and HAT, suggesting that Pax5 requires HAT activity to stimulate transcription of target genes.
Here we describe the identification of a new homologue of Ada2 in humans, hAda2β. Our results suggest that Ada2β functions with Gcn5 and Swi/Snf complexes to coactivate Pax5-regulated transcription. The selectivity of Ada2β, compared to Ada2α, suggests that HATs may coactivate different promoters based, at least in part, on their Ada2 interaction.
MATERIALS AND METHODS
Cell lines.
The human cell lines HEK 293, HeLa, and H1299 were grown in Dulbecco modified Eagle’s medium supplemented with 10% fetal bovine serum. The human lymphoblastoid cell line BL-2 (Burkitt's lymphoma) was grown in RPMI 1640 medium (Fisher). Media were supplemented with 10% fetal bovine serum, 50 U of penicillin/ml, and 50 mg of streptomycin/liter.
Two-hybrid screens.
To screen for novel P/CAF interacting proteins, PCAF (positions 444 to 695) was cloned into the LexA DNA-binding domain vector pBTM116 (bait) and transformed into L40 yeast strain. A yeast two-hybrid screen was done, as previously described (54), by using a 10.5-day-postcoitus mouse library cloned in the pVP16 prey vector. A total of 3 × 108 transformants were screened, and positive colonies were selected on minimal medium plates, minus histidine, supplemented with 10 mM 3-aminotriazole (3-AT). The yeast two-hybrid screen for Ada2β interacting proteins was done as described above, except that Ada2β (aa 35 to 226) was used as bait, 108 colonies were screened and positive colonies were selected on 0.5 mM 3-AT plates, minus histidine. Approximately 15% of positive clones were BAF57.
Cloning of the Ada2β gene.
The cDNA clone corresponding to the full-length hAda2β gene was generated by reverse transcription-PCR (RT-PCR) with mRNA isolated from U2OS cells (human osteosarcoma). The following primers were used in PCR amplification: forward, 5′-ATGGGAGGCCGCGAGCTGACGATA-3′; and reverse, 5′-TCAAGACGCGTCCCTGGAGATCCA-3′.
Antibodies.
Monoclonal anti-Pax5 antibody was obtained from Pharmingen (San Diego, Calif.). Monoclonal anti-Gal4DBD (RK5C1), polyclonal anti-Brg1 (H88), and normal mouse and rabbit immunoglobulin G (IgG) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Monoclonal anti-Flag M2 (F3165) antibody was obtained from Sigma. The anti-TAF10, the anti-TAF6, and anti-TAF5 monoclonal mouse antibodies (4, 58), and the monoclonal antibody raised against hGCN5 (9) were described previously.
The following custom-made anti-peptide sera (Invitrogen) were used for immunoblottings and immunoprecipitations: anti-Gcn5, N-SVSAAVVPSTPIFSPSMGG-C; anti-hAda2α, N-VNKTRKIYDFLIREGYITKG-C; anti-hAda2β, N-KVLKKRILNFLTESGWISRD-C; and anti-TRRAP (3).
Immunopurification of Flag-PCAF, hSwi/Snf, and TFTC.
HeLa Tet-off cells (Clontech) were stably transfected with Tet-regulated vector containing the Flag-PCAF sequence. Cells were selected in Dulbecco modified Eagle’s medium supplemented with 200 μg of hygromycin and 1 μg of tetracycline (to repress the expression of Flag-PCAF)/ml for 10 days. Cells were then propagated to the total number of 5 × 108 in medium lacking tetracycline. Nuclear extract was prepared and dialyzed against immunoprecipitation buffer (IP buffer; 20 mM HEPES [pH 7.8], 150 mM NaCl, 15% glycerol, 0.1% NP-40, protease inhibitors). Dialyzed nuclear extract was incubated with 300 μl of prewashed Flag-agarose beads (Sigma). After incubation, beads were consecutively washed three times with IP buffer, containing increasing amounts of NaCl (200, 300, and 400 mM). The bead-bound material was eluted with a Flag-peptide (0.5 mg/ml) dissolved in IP buffer and was analyzed by immunoblotting with specific antisera: anti-Flag monoclonal antibody for PCAF, custom-made polyclonal antibodies against Ada2α, Gcn5, and Ada2β (see the description above).
TFTC was prepared as described previously (4). Routinely, 500 to 1000 μl of HeLa cell nuclear extract was immunoprecipitated with 50 μl of protein G-Sepharose (Pharmacia) and ca. 2 to 5 μg of the different antibodies. Antibody-protein G-Sepharose-bound protein complexes were washed three times with IP buffer (25 mM Tris-HCl [pH 7.9], 10% [vol/vol] glycerol, 0.1% NP-40, 0.5 mM dithiothreitol [DTT], 5 mM MgCl2) containing 0.5 M KCl and twice with IP buffer containing 100 mM KCl. After being washed, proteins were eluted by an excess of the corresponding epitope peptide. Proteins were boiled in sodium dodecyl sulfate (SDS) sample buffer and separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membrane, and probed with the indicated primary antibodies. Chemiluminescence detection was performed according to manufacturer's instructions (Amersham).
Fractionation of HeLa nuclear extract on p11 column.
A total of 400 mg of HeLa nuclear extract was dialyzed against the binding buffer DB100 (20 mM Tris [pH 7.8], 100 mM KCl, 10% glycerol, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride [PMSF]) and was fractionated on p11 phosphocellulose column, prepared according to the manufacturer's instructions (Whatman). After an extensive washing with DB100, bound material was step eluted with five column volumes of DB buffer containing 0.3, 0.5, and 1.0 M KCl.
In vitro interactions.
Glutathione S-transferase (GST)-hAda2α was constructed as described previously (55). GST-hAda2β was constructed by inserting the Ada2β gene as a 5′-BamHI/BglII-3′ fragment into PGEX5X-1 vector (Amersham Biosciences), linearized with BamHI. GST proteins were prepared as described previously (55). Then, 5 μg of each fusion protein were incubated with 500 μl of 0.5 M HeLa fraction after p11 column for 3 h at 4°C. After incubation, glutathione beads were added to samples, followed by incubation for an additional 1 h with rotation. After several washes, bead-bound material was eluted from beads by boiling in the SDS-PAGE loading buffer.
As a template for the coupled in vitro transcription-translation reaction, the full-length murine Pax5 cDNA in pSP72 vector (Promega) was used (19). Binding of in vitro translated Pax5 to GST-Ada2β was carried out at 4°C for 2 h with rotation. Briefly, 1 to 2 μg of purified GST-Ada2β protein or GST alone coupled to glutathione-Sepharose beads was incubated with 5 μl of [35S]methionine-labeled Pax5 protein in the transcription-translation (TnT) mix supplemented with 50 mM Tris-HCl (pH 7.9), 120 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1% bovine serum albumin (BSA), and 1 mM DTT. After incubation, beads were washed five times with binding buffer containing 500 mM NaCl, and the bound proteins were eluted from the beads, resolved in SDS-PAGE, and exposed to film.
Immunoprecipitations in vivo.
HEK 293 cells were transiently transfected with pEBB-Pax5 construct (19) by using the GenePorter transfection reagent (Gene Therapy Systems, San Diego, Calif.). After 48 h, cells (1 × 106 to 2 × 106) were resuspended in lysis buffer (50 mM HEPES [pH 7.5], 250 mM NaCl, 0.1% NP-40, 1 mM EDTA, 1 mM DTT) supplemented with protease inhibitors (1 mM PMSF, 2 μg of leupeptin/ml, 2 μg of pepstatin/ml, 5 μg of aprotinin/ml) and incubated for 30 min on ice. Precleared lysates were incubated for 2 h at 4°C with anti-Pax5 monoclonal antibody (Pharmingen). Immunocomplexes were precipitated with 20 μl of protein G-agarose (Sigma Chemical Co.) by incubating them for 2 h at 4°C. After several washes with the lysis buffer, the bead-bound material was eluted with SDS loading buffer and analyzed by Western blotting with anti-Pax5, Ada2β, and Gcn5 sera.
Endogenous Gcn5 and Brg1 were immunoprecipitated from 500 μl of dialyzed HeLa nuclear extracts with 10 μl of Gcn5- or 2 μg of Brg1-specific antibody, respectively. After several washes with the lysis buffer (50 mM HEPES [pH 7.5], 250 mM NaCl, 0.1% NP-40, 1 mM EDTA, 1 mM DTT) supplemented with protease inhibitors (1 mM PMSF, 2 μg of leupeptin/ml, 2 μg of pepstatin/ml, 5 μg of aprotinin/ml), the bound proteins were eluted with SDS loading buffer lacking β-mercaptoethanol to protect the integrity of the antibodies bound to beads. The eluted material was analyzed by immunoblotting for the presence of indicated proteins.
Endogenous Pax5 was immunoprecipitated from BL-2 as described previously (19). After dialysis, Pax5 was immunoprecipitated with anti-Pax5 monoclonal antibody (Pharmingen), and the immunoprecipitated material was analyzed by Western blotting.
Luciferase assays.
Transient reporter assays were performed as reported previously using E1bLuc and pGL3 (CD19-2) reporter constructs (19). The Flag-tagged full-length Ada2β expression construct was generated by cloning an Ada2β PCR product digested with SalI into the XhoI site of pcDNA 3.1 vector (Invitrogen). Luciferase activity was measured according to the manufacturer's instructions (Promega). All readings were taken by AutoLumat LB953 luminometer (EG&G Berthold). Standard deviations were calculated based on three independent series of experiments where each sample was done in triplicate.
RT-PCR of CD19 in human BL-2 cells.
BL-2 cells in mid-log phase were electroporated (107 cells/cuvette) with 5 μg of pEGFP vector (Clontech) and 15 μg of the specific expression construct by using a Gene Pulser I apparatus (Bio-Rad) at 250 V and 960 μF. In control experiments, the total DNA content was kept constant by addition of an empty vector. At 48 h postelectroporation, green fluorescent protein (GFP)-positive cells were sorted on a Vantage SE fluorescence-activated cell sorter (Becton Dickinson). GFP-positive cells (5 × 105 to 2 × 106) were washed once in phosphate-buffered saline, and total RNA was extracted by using Trizol (Life Technologies) according to the manufacturer's instructions. A total of 5 μg of total RNA was used to prepare cDNA by using Ready-to-Go First-Strand beads (Amersham Pharmacia Biotech, Inc.). PCR amplification was performed with a ThermoHybaid cycler, by using 37 cycles of 94°C for 30 s, 54°C for 90 s, and 72°C for 60 s. The following primers were used for PCR amplification: CD19, 5′-ATCTTCTGCCTGTGTTCCCTTGTG and 3′-CGTCGCTGCTCGGGTTTCCATAAG; and GAPDH, 5′-TCCACCACCCTGTTGCTGTAG and 3′-GACCACAGTCCATGCCATCACT. The appropriate amounts of cDNA used for amplification were defined experimentally by serial dilutions. Identity of PCR products was confirmed by subcloning them into PCRII vector (Invitrogen) and sequencing. The experiments were repeated several times, and the error between experiments was <30%.
RESULTS
Identification of Ada2β as a PCAF-interacting protein by yeast two-hybrid assay.
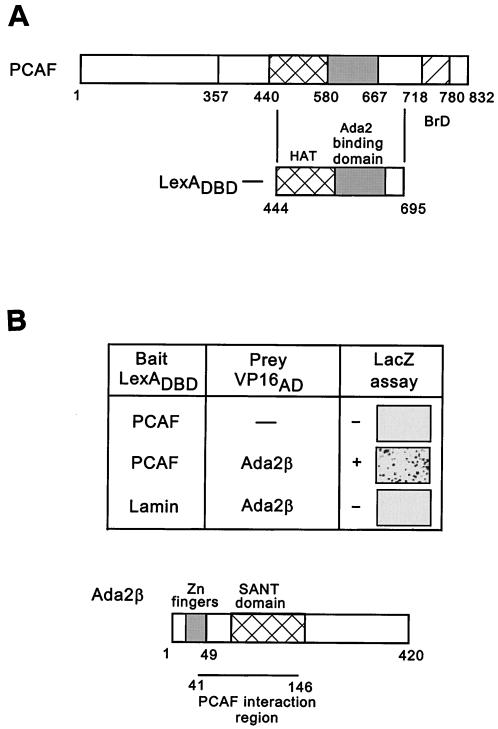
PCAF and Gcn5 are the catalytic subunits of at least three multiprotein HAT complexes in humans (PCAF, TFTC, and STAGA). To gain more information about PCAF/Gcn5 interacting partners, we performed a yeast two-hybrid screen. A region of PCAF encompassing the HAT and Ada2 interacting regions (residues 444 to 695) was fused to LexADBD and used as bait (Fig. 1A, upper panel). A cDNA expression library from mouse mid-gestation embryos fused to the VP16 activation domain was screened for interaction. Among 3.9 × 106 primary transformants, 40 cDNA clones were isolated that interacted specfically with LexA-PCAF, since no interaction was observed with either LexA DBD alone or LexA-Lamin (Fig. 1A, lower diagram, and data not shown). Sequence analysis of the interacting clones revealed three overlapping sequences with homology to yAda2 and hAda2. Based on comparison of these sequences, the minimal region of interaction with PCAF was deduced. The PCAF interacting region within the identified Ada2 homologue encompassed the SANT-domain (Fig. 1B). The SANT domain is an evolutionary conserved motif found in many chromatin-related factors, and in yAda2, containing both the yGcn5-interacting region and a region of interaction with histone acetylation substrates (8, 48).
FIG. 1.
LexADBD-PCAF two-hybrid screen. (A) PCAF domain structure. Nucleosomal recognition, aa 1 to 357; HAT domain, aa 440 to 580; Ada2-binding domain, aa 580 to 667; bromodomain, aa 718 to 780. The region of PCAF (aa 444 to 695) used as bait in the two-hybrid screen is shown below. (B) Two-hybrid interaction between PCAF and Ada2β. In the upper part of the panel, LexADBD-PCAF444-695 or LexADBD-Lamin were cotransformed into yeast with the amino terminus of Ada2β fused to the VP16 activation domain. Interaction was tested by using a LacZ assay of colonies transferred to filters. Positively interacting blue colonies are shown after 30 min of color development at 30°C. In the lower part of the panel is a diagram of Ada2β. Domains conserved between different Ada2 species are indicated. The region of interaction with PCAF is indicated. (C) Sequence alignment of hAda2β, mAda2β, and hAda2α. Alignment was performed by using CLUSTAL W. Identical amino acids are shown in black boxes, and similar amino acids are shown in gray boxes.Continued.
The full-length mouse cDNA sequence for the PCAF-interacting protein was retrieved from the mouse genomic database. The overall similarity in its sequence to both mouse Ada2 (mAda2) and hAda2, as well as the presence in its sequence of previously identified yAda2 domains (Cys-His-rich Zn finger sequence at the amino terminus and SANT domain; Fig. 1B), indicated that this was another Ada2-related gene. The gene was named Ada2β to distinguish it from the previously described human (GenBank accession no. KL0P4) and mouse (XP126372) Ada2, which were renamed Ada2α. Since the mAda2β (XP132031) and hAda2β proteins were 100% identical (Fig. 1C; true also of mAda2α and hAda2α; data not shown), we focused on the human version of Ada2β. Comparison of hAda2α and hAda2β at the amino acid level showed 59% similarity over the 467 aa (Fig. 1C and Table 1), a finding comparable to the level of similarity between yAda2 (NP010736) and either hAda2α (64%) or hAda2β (60%) (Table 1). A more extensive database search revealed the existence of apparent Ada2α and Ada2β orthologues in various species, including human, mouse, and Drosophila melanogaster (dAda 2α[AC009394] and dAda2β [CG9638]) (Table 1). In each species there is clearly higher conservation between one form and either Ada2α or Ada2β. Therefore, based on the level of homology between Ada2 in various species, the proteins were separated into two groups: Ada2α-like proteins with 68% overall homology and Ada2β-like proteins with 63% overall homology, whereas the α/β conservation within each organism was 52% overall. These results suggest that Ada2α and Ada2β proteins in these organisms may exert different, yet evolutionary conserved functions.
TABLE 1.
Comparison of Ada2 homologues at the amino acid level
| Protein | % Similaritya with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| hAda2β | hAda2α | mAda2β | mAda2α | dAda2β | dAda2α | aAda2a | aAda2b | |
| hAda2β | 100 | 59 | 100 | 59 | 68 | 57 | 55 | 54 |
| mAda2β | 100 | 59 | 100 | 59 | 68 | 57 | 55 | 53 |
| dAda2β | 68 | 51 | 68 | 51 | 100 | 48 | 55 | 50 |
| aAda2a | 55 | 47 | 55 | 47 | 53 | 50 | 100 | 74 |
| yADa2 | 60 | 64 | 60 | 64 | 57 | 57 | 59 | 53 |
Includes identical and homologous amino acid residues.
Recently, two forms of Ada2 were also identified in Arabidopsis spp. (dAda2a [AAK31319] and aAda2b [AAK31320]) (49). The two Ada2 proteins from Arabidopsis shared similar levels of homology with members of both Ada2α and Ada2β families (ca. 50%) and were more similar to each other (74%) (Table 1). The Arabidopsis Ada2 proteins exhibited the highest conservation with the yAda2 prototype (59 and 53% similarity) rather than with other members of the Ada2 families. Thus, these two Arabadopsis Ada2 proteins appear to have diverged in sequence and function from Ada2α and Ada2β.
Ada2β is not part of the PCAF complex.
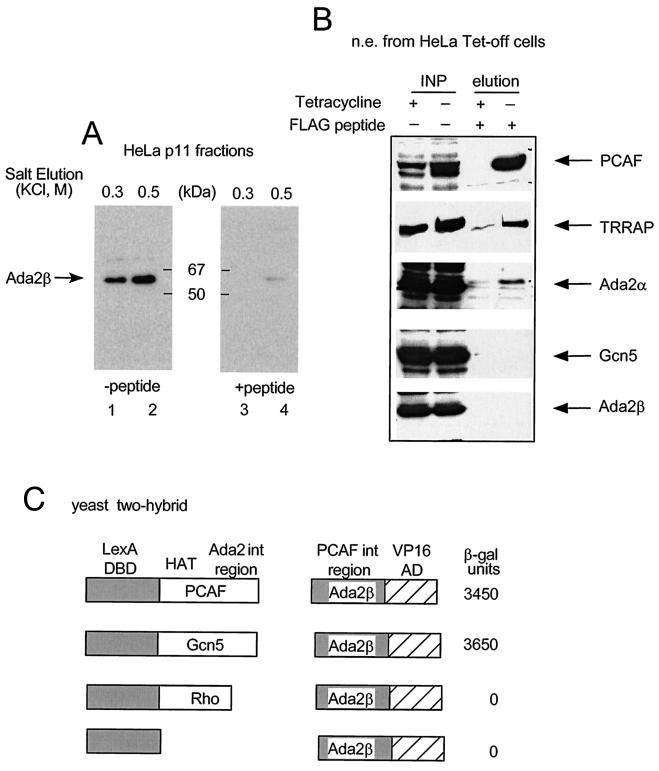
To facilitate biochemical characterization of Ada2β, we generated a rabbit polyclonal antibody against an amino-terminal Ada2β peptide (residues 40 to 59). Western blot analysis of the 0.5 M HeLa cell nuclear fraction), obtained after step elution from phosphocellulose chromatography, detected a protein of 55 to 62 kDa, in agreement with the predicted size of the mAda2β expressed sequence tag clone (Fig. 2A). The Western blot pattern was specific for the Ada2β antibody, because the Ada2β band disappeared when the antibody was preincubated with the corresponding peptide (Fig. 2A).
FIG. 2.
Examination of Ada2β association with PCAF. (A) Ada2β peptide antibodies. HeLa nuclear extract was fractionated on phosphocellulose p11 column, and bound material was step-eluted with increasing KCl (lanes 1 and 3 are a 0.3 M KCl elution, and lanes 2 and 4 are a 0.5 M KCl elution). The fractions were probed with anti-Ada2β sera either mock treated (left panel) or preincubated with the corresponding peptide (right panel). The position of the Ada2β-specific signal is shown. (B) Test of Ada2β association with PCAF in vivo. Flag-PCAF expression was induced in HeLa Tet-off cells by withdrawal of tetracycline (+). Cells treated with tetracycline served as negative control (−). Flag-agarose was used to immunoprecipitate FLAG-PCAF. Bound material was eluted with excess Flag-peptide. Immunoblotting was done with indicated antibodies. (C) Test of Ada2β interaction with PCAF or Gcn5 in the yeast two-hybrid assay. Yeast were cotransformed with PCAF, Gcn5, or Rho fused to LexADBD or LexADBD alone, along with the PCAF interacting region of Ada2β fused to VP16 activation domain. The LacZ reporter contained LexA binding sites. β-Galactosidase activity was measured in units per milligram of protein. The numbers are averages of three independent experiments.
It has been previously reported that Ada2α is associated with PCAF in a stable complex (37). Because we discovered Ada2β as a PCAF interacting protein, we predicted that PCAF could interact with either Ada2α or Ada2β and endogenous PCAF could be contained in a mixture of complexes. Ada2β-specific antibodies were used to test whether Ada2β physically interacts with PCAF. PCAF-containing complexes were immunopurified from engineered HeLa cells that expressed Flag-tagged PCAF under control of the Tet-off promoter (Fig. 2B) and examined for the presence of Ada2β. The identity of the PCAF complex was verified by probing the immmunorecipitated material with various antibodies: β-Flag (FLAG-PCAF), α-TRRAP, α-Ada2α, and α-Gcn5. As expected, Flag-PCAF, Ada2α, and TRRAP (37, 53) coprecipitated, whereas Gcn5 was absent, in concordance with previous data showing that Gcn5 is a component of a different complex (37). However, Ada2β did not coimmunoprecipitate with the PCAF complex, indicating that Ada2β does not associate with PCAF in vivo.
Since the PCAF and Gcn5 proteins share 81% homology, we tested whether Ada2β interacted with Gcn5 in vivo by using a yeast two-hybrid interaction assay (Fig. 2C). Bait plasmids encoding either LexA-PCAF or LexA-Gcn5 were cotransformed with the prey plasmid encoding Ada2β fused to the VP16 activation domain (Ada2β-VP16). Both PCAF and Gcn5 interacted strongly and specifically with Ada2β but not with a LexA-Rho fusion protein, or LexADBD alone (Fig. 2C). This suggests that the binary interaction is similar between Ada2β and either PCAF or Gcn5. Further examination of interaction between Ada2β and Gcn5 is described below.
Ada2β interacts with Gcn5 and BAF57, a component of SWI/SNF complex.
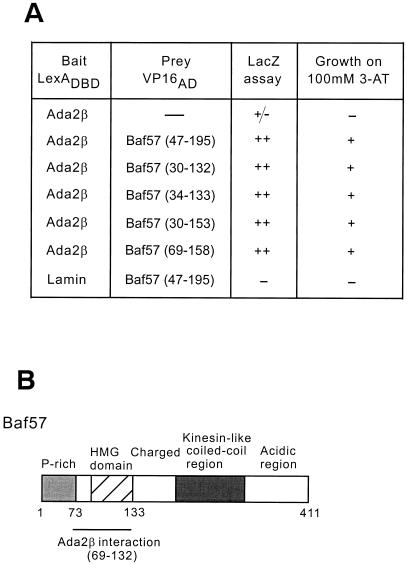
To gain insight into the function of Ada2β, a second two-hybrid screen was performed, by using as bait LexADBD fused to the longest clone of Ada2β recovered in the original screen (residues 35 to 150). A total of 108 colonies were screened, and one strongly interacting clone (representing 15% of the positives) was identified as Baf57 (Fig. 3A), a component of the Swi/Snf-related human Brg1 and Brm chromatin remodeling complexes (56). Examination of various overlapping clones of Baf57 obtained in the two-hybrid screen indicated that the HMG domain (residues 69 to 133) was sufficient for interaction with Ada2β (Fig. 3). This conserved domain is reported to mediate protein-protein contacts, as well as to bind to unusual DNA structures (such as cruciforms and hairpins) (51).
FIG. 3.
LexADBD-Ada2β two-hybrid screen. (A) Test of LexADBD-Ada2β interaction with Baf57. Either LexADBD-Ada2β or LexADBD-Lamin was cotransformed into yeast with deletion mutants of Baf57 (dimensions are indicated in parentheses) fused to the VP16 activation domain. The strength and specificity of interactions were assessed by two means: (i) color LacZ assay on filters and (ii) ability to grow on 100 mM 3-AT. (B) Schematic of Baf57. Functional domains of Baf57 are indicated. The Ada2β-interacting region is shown: P-rich (proline-rich), HMG domain (high-mobility-group domain), Charged, Kinesin-like coiled-coil region, and Acidic region.
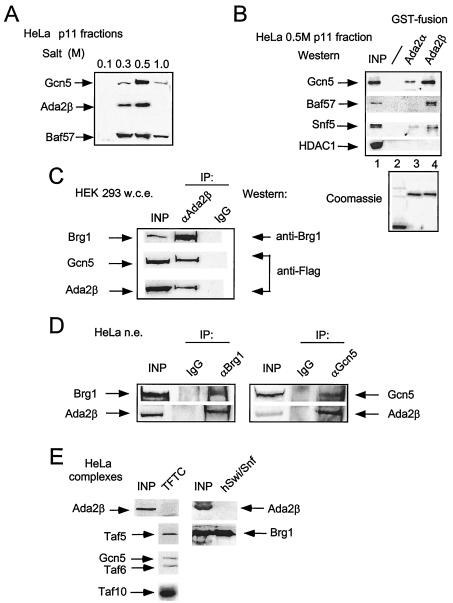
These data indicate that Ada2β interacts with Baf57 and with both Gcn5 and PCAF in the yeast two-hybrid assay; however, Ada2β does not appear to be in a stable complex with PCAF in vivo. Based on these results, a series of biochemical experiments was performed to test whether Ada2β associates with Gcn5 or Baf57. As a first step, HeLa nuclear extract was fractionated on a phosphocellulose column (p11). The proteins were step eluted by using increasing ionic strengths, and resulting fractions were analyzed for Ada2β, Gcn5, and Baf57 (Fig. 4A). The majority of Gcn5 and Ada2β proteins were eluted in the 0.5 M fraction, whereas the elution profile of Baf57 spread over the three step fractions (0.3, 0.5, and 1 M).
FIG. 4.
Analysis of physical interactions between Ada2β, Gcn5, and Baf57 proteins. (A) p11 column elution profiles of Gcn5, Ada2β, and Baf57. HeLa cell nuclear extract was fractionated by p11 chromatography. Flowthrough and salt elutions of bound material were analyzed by immunoblotting for Gcn5, Ada2β, and Baf57 with specific antisera. (B) GST-Ada2β interaction with Gcn5 and Baf57. In the upper panel, glutathione-beads bearing GST-Ada2α, GST-Ada2β, or GST proteins were incubated with HeLa 0.5 M fraction after p11 column chromatography. The bead-bound material was analyzed by Western blotting for indicated proteins. In the lower panel, a Coomassie blue stain of GST fusion proteins (20% of beads) used in the binding experiments is shown. (C) Coimmunoprecipitation of Brg1 and Gcn5 with Ada2β. HEK 293 cells were transiently transfected with Flag-Ada2β and Flag-Gcn5 constructs. At 48 h posttransfection, cell extracts were prepared, and Ada2β was immunoprecipitated with anti-Ada2β sera. The IgG fraction of an irrelevant polyclonal sera was used as a control for specificity. The Ada2β immunocomplexes were analyzed for the presence of Flag-Ada2β, Flag-Gcn5, and Brg1 by Western blotting with the indicated antibodies. (D) Endogenous Ada2β coimmunoprecipitates with Brg1 and Gcn5 in vivo. Endogenous Gcn5 and Brg1 were immunoprecipitated from dialyzed HeLa nuclear extract with Gcn5- or Brg1-specific polyclonal antibody. SDS-eluted material was analyzed for the presence of Brg1, Gcn5, and Ada2β with the indicated sera. The IgG fraction of an irrelevant polyclonal sera was used in mock immunoprecipitation to determine specificity of the interactions. (E) Analysis of Ada2β in purified TFTC and human Swi/Snf. The purified TFTC and hSwi/Snf (43) complexes (indicated as TFTC and hSwi/Snf, respectively) were analyzed for the presence of Ada2β. Input lane (INP) represents the starting HeLa nuclear extract material used in purification of the complexes. The positions of the TFTC- and hSwi/Snf-specific components are indicated.
To test whether Ada2β interacts physically with Gcn5 or Baf57 in vitro, a GST-Ada2 pull-down assay was performed by using the partially purified HeLa p11 fractions described above as a source of Gcn5 and Baf57. Similar amounts of affinity resins bearing either GST, GST-Ada2α, or GST-Ada2β proteins (Fig. 4B, bottom panel) were incubated with the HeLa cell p11 0.5 M fraction. GST-Ada2α was used to examine potential specificity of the Ada2β interactions. Resins were washed with binding buffer, and the material remaining bound to beads was examined by Western blotting. Resin bearing GST-Ada2β interacted more strongly with Gcn5, Baf57, and Snf5 (another subunit of hSwi/Snf complex) than did GST-Ada2α resin (Fig. 4B, top panel). Additional specificity was indicated by the absence of Gcn5, Baf57, and Snf5 interaction with GST. Furthermore, HDAC1 did not bind to either GST-Ada2α or GST-Ada2β (Fig. 4B, top panel). Thus, Ada2β, unlike Ada2α, interacts in vitro with both Gcn5- and Baf57-containing complexes.
In vivo association of Ada2β with Gcn5- and Baf57-containing complexes was then investigated. HEK 293 cells were cotransfected with plasmids expressing Flag-Ada2β and Flag-Gcn5, and the Ada2β protein was immunoprecipitated with Ada2β antiserum (Fig. 4C). The IgG fraction of an irrelevant rabbit serum was used as a control for specificity of immunoprecipitation. The presence of Gcn5 and Ada2β in the immunoprecipitates was detected with anti-Flag antibody, and the presence of the Baf57-containing complex was detected with antiserum against Brg1, the catalytic subunit of the Baf57-containing Swi/Snf complex (32). Both Gcn5 and Brg1 were detected in the Ada2β-immunoprecipitated material but not in the material precipitated with control IgG antibody, suggesting that Ada2β interacts with both Gcn5- and Baf57/Brg1-containing complexes in vivo.
To determine whether endogenous proteins associate under physiological conditions, Ada2β was tested for association with either Gcn5- or Baf57/Brg1-containing complexes. Either Brg1 or Gcn5 was immunoprecipitated from HeLa cells by using the corresponding specific antisera (Fig. 4D) and tested for the presence of Ada2β. Both Gcn5 and Brg1 immunoprecipitated Ada2β, suggesting that Ada2β interacts with Gcn5 and Baf57/Brg1 in cells at normal endogenous levels.
To test whether Ada2β stably associates with either TFTC/STAGA or hSwi/Snf, we examined purified TFTC and hSwi/Snf complexes for the presence of Ada2β (Fig. 4E). Ada2β was not associated with either complex, suggesting that high-stringency treatment during purification caused dissociation of Ada2β from TFTC/STAGA and Swi/Snf complexes. The structural integrity of both TFTC/STAGA and hSwi/Snf was confirmed by immunoblotting against individual subunits of each complex (Taf5, -6, and -10 for TFTC; Brg1 for hSwi/Snf).
Ada2β, and not Ada2α, mediates transcriptional activation of Pax5, together with either Gcn5 or Brg1.
One function of yAda2 or hAda2α as an adaptor-coactivator is to interact with DNA-bound transactivators (2, 44) to stimulate transcription (14). We tested whether hAda2β similarly regulates transcriptional activation. We chose Pax5/BSAP activator as a model to examine for several reasons. First, mouse Gcn5 (but not PCAF) expression level is very high in spleen, a significant B-cell milieu. Second, Pax5 is a B-cell-specific DNA-binding transcription factor, which enhances transcription through histone acetylation (19). Finally, Pax5 is a substrate for CBP- and Gcn5-mediated acetylation in vitro (A. V. Emelyanov and B. K. Birshtein, unpublished data). These observations led us to test whether Pax5 utilizes the Ada2α/β and Gcn5/PCAF coactivators for transcription regulation.
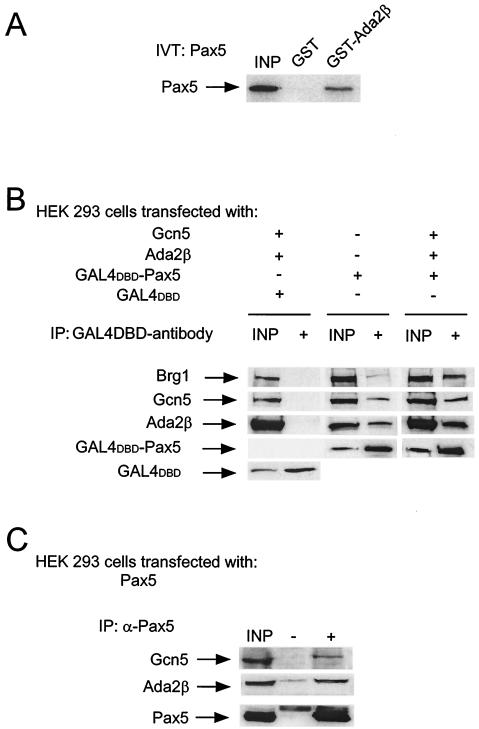
We examined whether Ada2β interacts with Pax5. Initially, interaction of Pax5 with Ada2β was tested in vitro by using GST pull-down. Beads containing GST or GST-Ada2β were incubated with in vitro-translated Pax5 (Fig. 5A). Pax5 bound to GST-Ada2β but not to GST, suggesting a direct interaction between Pax5 and Ada2β. Second, since Ada2β interacts with Gcn5 and Baf57/Brg1 in vivo (Fig. 4C and D), physical interactions between these latter proteins and Pax5 were tested (Fig. 5B). HEK 293 cells (Pax5−) were transfected with plasmids expressing Gal4DBD or Gal4DBD-Pax5, along with Gcn5 and Ada2β in various combinations (Fig. 5B). Gal4DBD or Gal4DBD-Pax5 was immunoprecipitated by using anti-Gal4 monoclonal antibody, and the bound proteins were analyzed for the presence of Brg1, Gcn5, and Ada2β. None of these proteins were coimmunoprecipitated with the Gal4DBD protein alone (Fig. 5B, left row). In contrast, the Gal4DBD-Pax5 immunoprecipitate contained all three proteins (Fig. 5B, middle and right row). Interestingly, Gal4DBD-Pax5 was also able to bind endogenously expressed Ada2β, Gcn5, and Brg1, albeit more weakly than the overexpressed proteins (Fig. 5B, compare middle and right panels). This result was confirmed by a second immunoprecipitation experiment in which HEK 293 cells were transfected with Pax5-expressing plasmid, and the Pax5 protein was immunoprecipitated with Pax5-specific antiserum (Fig. 5C). Transfected Pax5 coimmunoprecipitated Gcn5 and Ada2β, but no signal was detected for either Gcn5 or Ada2β in mock immunoprecipitation (Fig. 5C).
FIG. 5.
Pax5 physical interactions with Ada2β, Gcn5, and Brg1. (A) Physical interaction of Pax5 with GST-Ada2β in vitro. In vitro-translated Pax5 was incubated with beads bearing GST or GST-Ada2β. After several washes, bound material was eluted and resolved by SDS-PAGE. The gel was enhanced and exposed to X-ray film for 24 h to detect S35-labeled Pax5. (B) Coimmunoprecipitations of GAL4DBD-Pax5 with Gcn5, Ada2β, and Brg1. Whole-cell extract from HEK 293 cells transfected with GAL4DBD, GAL4DBD-Pax5, Gcn5, and Ada2β (as indicated) were subjected to immunoprecipitations with anti-GAL4 monoclonal antibody. The immunoprecipitated samples were analyzed by Western blotting for the presence of the indicated proteins with specific antisera. The input lanes (INP) represent 10% of the starting material. (C) Pax5 immunoprecipitation from HEK 293 cells. Whole-cell extract from cells transfected with Pax5 was immunoprecipitated with anti-Pax5 monoclonal antibody or mock precipitated with the IgG fraction of unrelated monoclonal antibodies. Immunoblotting was done for Gcn5, Ada2β and Pax-5.
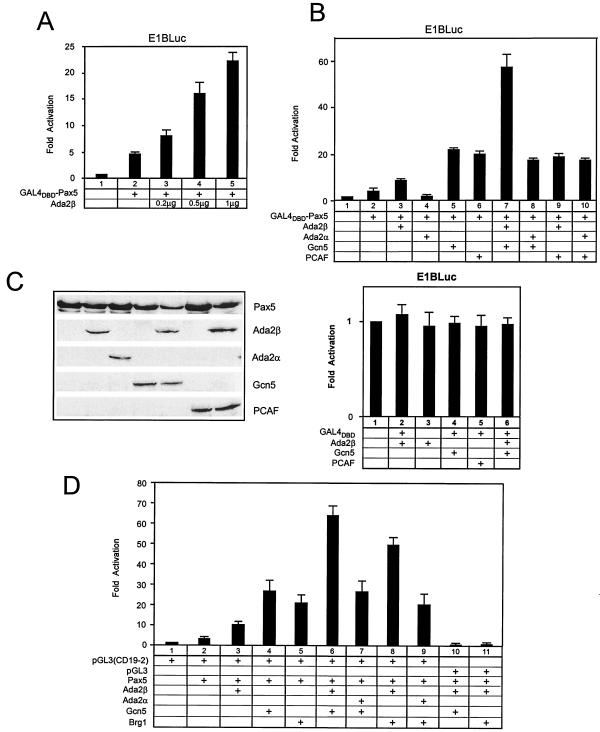
Thus, binding experiments indicate that Ada2β interacts with Gcn5 and Brg1 and that these proteins interact with the Pax5 activator. These findings led us to test whether Ada2β modulates Pax5-mediated transcriptional activation. First, a transient reporter assay with Gal4DBD-Pax5 was carried out by using a luciferase reporter driven by the E1B promoter bearing five Gal4-binding sites upstream of the TATA box (E1Bluc) (19). HEK 293 cells were transfected with Gal4DBD-Pax5 and increasing amounts of a plasmid expressing Ada2β (Fig. 6A). Gal4DBD-Pax5 activated transcription three- to fourfold greater than the background level (compare columns 1 and 2). Transactivation by Gal4DBD-Pax5 increased linearly with increasing amounts of Ada2β (columns 3 to 5). Ada2β by itself did not have any effect on transcription (Fig. 6B, lower panel). The largest amount of transfected Ada2β-expressing plasmid yielded 14-fold activation over Gal4-Pax5 alone. Thus, Ada2β coactivates Gal4DBD-Pax5.
FIG. 6.
Pax5 functional interactions with Ada2α/Ada2β, Gcn5/PCAF, and Brg1. (A) Effect of increasing amounts of Ada2β on Gal4DBD-Pax5. HEK 293 cells were transfected with Gal4DBD-Pax5 expression vector and various amounts (0.2, 0.5, and 1 μg) of pCDNA3-Ada2β vector. Cells were cotransfected with a reporter bearing the luciferase gene under control of Gal4-binding sites integrated into the E1B promoter region (E1Bluc). The basal activity of E1Bluc was ∼1,000 light units. Error bars indicate the standard deviation. (B) Effect of coactivators on Gal4DBD-Pax5-dependent transcription. For the upper part of panel B, HEK 293 cells were transiently transfected with 200 ng of the Gal4DBD-Pax5 mammalian expression vector in various combinations with the indicated expression vectors, together with the Gal4-responsive luciferase reporter, E1Bluc. Typically, 0.5 μg of Ada2β or Ada2α expression vectors and 1 μg of Gcn5 or PCAF expression vectors were used in transfections. As shown in the lower section of panel B, Gal4DBD alone, or in any combination with the indicated coactivators (Ada2β, Gcn5, or PCAF), did not activate transcription of the E1Bluc reporter. (C) Western analysis of transfected proteins. Immunoblot analysis of ectopically expressed proteins in HEK 293 cells was done. Cells were transfected as for the upper panel and then immunoblotted. The protein expression levels were determined with specific antibodies: anti-Pax5 (Pharmingen) and anti-Flag (Sigma) for detection of Ada2β and PCAF, anti-HA (Babco) for detection of Ada2α, and anti-Gcn5 custom-made polyclonal serum. (D) Pax5 cotransfections with coactivators in H1299 cells. Pax5 DNA was transfected in different combinations with the indicated DNAs. Cells were cotransfected with the luciferase reporter pGL3 (no Pax5-binding sites) or pGL3 (CD19-2), bearing two Pax5-binding sites derived from the CD-19 promoter, a gift of James Hagman. The basal activity of pGL3 was ∼10,000 light units. Error bars are shown.
Next, the effect of Ada2β and Gcn5 coexpression on Gal4DBD-Pax5-driven transcription was tested. To examine potential specificity in vivo, both homologues of Ada2 (α and β) and of Gcn5 (Gcn5 and PCAF) were tested. (Fig. 6B, upper panel). In these cotransfections, an intermediate amount of Ada2α or Ada2β was used (0.5 μg) relative to the amounts used in the experiment in Fig. 5A. Coexpression of Gal4DBD-Pax5 and Ada2β (Fig. 6B, column 3) enhanced transcription over background (column 1) and over Gal4DBD-Pax5 alone (column 2). In contrast, coexpression of Gal4-Pax5 and Ada2α did not increase transactivation (column 4). Coexpression of either Gcn5 or PCAF similarly increased Gal4-Pax5 activation (columns 5 and 6). The striking result was that coexpression of Gal4-Pax5, Ada2β, and Gcn5 was the only triple combination that exhibited synergy (column 7). That is, in cotransfection with Gal4DBD-Pax5, none of the combinations (Ada2β/PCAF, Ada2α/Gcn5, and Ada2α/PCAF) activated transcription above the levels achieved with Gcn5/PCAF alone (columns 8, 9, and 10). These differences were not due to protein levels, since levels of protein expression for Ada2β by itself and with either Gcn5 or PCAF were comparable (Fig. 5D). As expected, Gal4DBD alone was transcriptionally inert in any combination tested (Fig. 6B, lower panel). These results indicate that Ada2β functions selectively with Gcn5 to coactivate Gal4DBD-Pax5 transcription.
We then examined Pax5-mediated activation to determine whether Ada2β works with Gcn5 or Brg1 (the catalytic subunit of the hSwi/Snf-containing complex) in transcriptional activation (Fig. 6D). Pax5 activity was assayed by using a vector bearing two Pax5-binding sites derived from the human CD19 promoter compared to a reporter lacking Pax5-binding sites. Similar results to those observed for Gal4DBD-Pax5 were obtained for Pax5 with respect to specificity of Ada2β compared to Ada2α in cooperating with Gcn5. Thus, Ada2β/Gcn5 (Fig. 6D, column 6) but not Ada2α/Gcn5 (column 7) activated more than Gcn5 alone (column 4). In addition, coexpression of Pax5 and Brg1 with Ada2β (column 8) but not with Ada2α (column 7) significantly enhanced transcription beyond Pax5 and Brg1 alone (column 5). These stimulatory effects of coactivators were Pax5 dependent since no transactivation was observed with a reporter lacking Pax5-binding sites but retaining the basal promoter (columns 10 and 11). Thus, Ada2β, but not Ada2α, specifically coactivated Pax5-dependent transcriptional activation. This Ada2β-specific coactivation occurs for both Gcn5 and Brg1.
Ada2β associates with Pax5 and mediates transcriptional activation of the endogenous CD19 gene, together with either Gcn5 or BRG1, in a B-cell line.
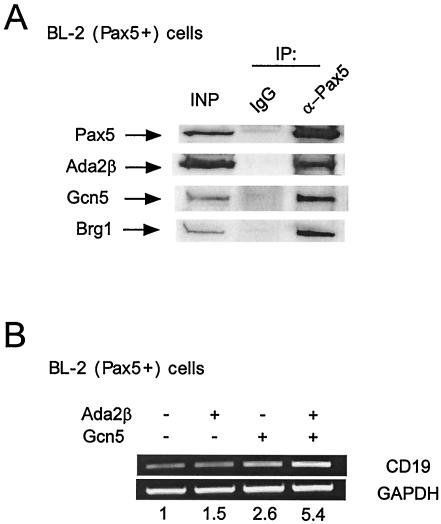
We next determined whether Pax5 physically and functionally associated with Ada2β and Gcn5 in B cells. First, to assess physical interaction between these proteins, the coimmunoprecipitation experiment was performed in BL-2 extract. BL-2 is a human Burkitt lymphoma B-cell line that expresses endogenous Pax5. Anti-Pax5 antibody immunoprecipitated Pax5 with Ada2β, Gcn5, and Brg1 in B cells compared to IgG alone (Fig. 7A). Next, activation of a Pax5-dependent endogenous gene (CD19) was examined. BL-2 cells were transfected with Ada2β- and/or Gcn5-expressing plasmids. The transfections contained a GFP-expressing vector for positive selection. Transcription of the CD19 gene in GFP-positive cells was detected by RT-PCR (Fig. 7B). Transfection of Ada2β resulted in a modest but reproducible elevation of CD19 transcription (1.5-fold above the background level), and expression of Gcn5 resulted in an ∼2.5-fold increase. When Ada2β was cotransfected in combination with Gcn5, CD19 transcription increased further, showing a slightly more than additive response (ca. 5.5-fold versus 4.0-fold if additive). These data, examining the endogenous Pax5-dependent CD19 gene in B cells, are consistent with the results in HEK 293 cells by using reporters and transfected Pax5, indicating that Ada2β works in concert with Gcn5 in gene activation.
FIG. 7.
Interaction of Pax5 with Ada2β, Gcn5, and Brg1 in Burkitt lymphoma cells. (A) Coimmunoprecipitation of Pax5 with Ada2β, Gcn5, and Brg1 in BL-2 cells. Pax5 was immunoprecipitated from nuclear extracts with Pax5 monoclonal antibodies. Nonspecific monoclonal IgG was used as a negative control. Immunoprecipitates were examined for Ada2β, Gcn5, and Brg1 by Western blotting. (B) Effect of Ada2β and Gcn5 on endogenous CD19 expression in BL-2 cells. Cells were cotransfected with GFP vector for positive selection of transfected cells. At 48 h posttransfection, cells were sorted by using GFP and lysed, and then total RNA was prepared. RT-PCR was performed with CD19-specific primers. GAPDH signal was used as a loading control. The numbers below represent the fold activation of CD19 transcription in coactivator-transfected cells versus cells transfected with GFP only. The CD19 transcription signal in GFP-only cells was arbitrarily set to 1 (basal). Experiments were repeated at least three times with essentially the same fold activation.
DISCUSSION
In this study, we describe a new human Ada2 (hAda2) homologue called hAda2β to distinguish it from the previously identified Ada2α. Whereas Ada2α is a stable component of the PCAF complex, Ada2β appears not to be a stable constituent of either PCAF or Gcn5 macromolecular complexes. However, Ada2β does interact physically and functionally with Gcn5, the catalytic subunit of HAT complex TFTC/STAGA, and with subunits of the chromatin remodeling complex Swi/Snf, Baf57 and Brg1 (15, 57). In addition, Ada2β and Gcn5 or Brg1 interacted with Pax5 transcription factor, leading to enhancement of Pax5-dependent transcription. In contrast, Pax5-dependent transcription was not similarly enhanced by the two other human homologues of Ada2 and Gcn5, i.e., Ada2α and PCAF, respectively. Thus, it appears that there is selectivity of activator cooperation with specific hAda2 and Gcn5 homologues and that the acetyltransferases Gcn5 and PCAF have distinct functions in vivo.
Yeast Gcn5 was the first transcriptional coactivator to be identified as a HAT. Unlike yeast, higher eukaryotes contain two homologues of Gcn5, called hGcn5 and PCAF. One hypothesis for this duplication is that the homologues have unique functions either during development or in specific adult tissues. Indeed, gene knockout studies of Gcn5 and PCAF in mice point to different roles in development (60): PCAF is dispensable in mice, whereas loss of Gcn5 caused embryonic death at ca. 10.5 days after fertilization. In addition, PCAF and Gcn5 display different expression patterns in adult tissues of mice (60). PCAF expression is pronounced in heart and skeletal muscles, where it functions to activate muscle-specific genes (39).
In contrast, Gcn5 expression is high in brain, kidney, thymus, and spleen, the latter perhaps consistent with a role in regulating B-cell-specific transcription as shown in the present study. In PCAF-null cells, Gcn5 shows ubiquitous tissue expression except in muscle, suggesting that Gcn5 may compensate for loss of PCAF (62). Interestingly, two other highly related HATs, p300 and CBP, members of a distinct acetyltransferase family, also have overlapping and distinct functions both in vivo and in vitro (19, 27, 50, 65). Our results predict that similar differences in phenotypic severity will be observed in Ada2α versus Ada2β mouse knockouts.
Gcn5 and PCAF are components of at least three large multisubunit complexes in mammals: STAGA, TFTC, and PCAF (28, 37, 58). These complexes are large and have many subunits, not all of which have been identified. Several reports on biochemical characterization of these complexes suggest that they are highly similar in composition. Direct comparison is difficult because most of them have been affinity immunopurified by using different tagged and overexpressed subunits. Indeed, in one study using overexpressed PCAF or Gcn5, Ada2α was found in stable association with either HAT complex (37), and in a second study Ada2α coimmunoprecipitated with overexpressed tagged Spt3 in the STAGA complex (29). In our study PCAF associated with only Ada2α in vivo. In contrast, in previous studies, TFTC, which contains Gcn5, was purified as a native complex and Ada2α was not detected (10). These findings are consistent with our observations that Gcn5 is not stably associated with either Ada2 homologue.
Clearly, Ada2α and Ada2β are capable of binary association with either PCAF or Gcn5. Indeed, we discovered Ada2β by using PCAF as a bait in two-hybrid interaction screening. Also, in direct two-hybrid comparisons, pairwise interactions between Gcn5/PCAF and Ada2α/β occurred with similar affinities (Fig. 2C and data not shown). However, the Ada2 homologues are clearly distinct, and this distinction manifests in their sequence, biochemistry, and function. Sequence homology between hAda2α/β is not higher than between either hAda2α/yAda2 or hAda2β/yAda2 (Fig. 1C). This indicates that the two human homologues likely diverged from a common ancestral gene to acquire different functions. This deduction is supported by the existence of clear Ada2α and Ada2β homologues in mouse and Drosophila (and two Ada2 genes in Arabidopsis which, however, do not clearly match hAda2α/β), suggesting that the two separate Ada2 lineages are evolutionarily conserved and may therefore play distinct roles in multicellular organisms.
There are functional distinctions between the Ada2 homologues as well. By analogy to membership of Gcn5 and PCAF in separate complexes, we reasoned that the two hAda2 homologues may be subunits of separate complexes. Since Ada2α is a component of the PCAF complex (20; the present study), it was logical to predict that Ada2β is a subunit of the Gcn5 complex. To our surprise, Ada2β was not a stable component of the Gcn5 complex and in fact fractionated in a biochemically separable complex (N. A. Barlev and S. L. Berger, unpublished observations). However, Ada2β interacted transiently and selectively, compared to Ada2α, with Gcn5 and with Baf57 (discussed below). Consistent with our previous observations (55), Ada2α was also able to interact with Gcn5 in a GST pull-down assay, although more weakly than Ada2β interaction with Gcn5. A clear distinction was observed in that Ada2β, but not Ada2α, interacted with elements of the human Swi/Snf complex in the GST pull-down (Fig. 4C). It is interesting that Ada2β specifically interacted in the two-hybrid assay with the HMG-box region of Baf57, which has been implicated in DNA binding (56). Several DNA-binding proteins, including transcription factors Oct-1 and -2, p53, steroid hormone receptors, and recombination factors RAG1 and RAG2, interact with HMG boxes (51). Although there is no evidence that Ada2 can bind DNA, Ada2 contains one copy of the SANT domain. The SANT domain is related to the Myb motif, which may serve as a DNA-binding surface when present in three to four copies (1).
Chromatin remodeling and histone acetylation activities have been extensively documented to cooperate in gene regulation (6, 25). The first evidence for this came from genetic studies in yeast, where mutations in either Swi2 or Gcn5 were modest in their phenotypic severity, but double mutations were very deleterious (40). In addition, yeast Ada2 and Gcn5 were found in the same genetic screen as many of the Swi/Snf complex proteins (38). There is similar initial recruitment via association between DNA-bound activators and components of each type of complex. Direct and specific interactions have been demonstrated between acidic activation domains, such as those derived from herpesvirus VP16, yeast Gcn4 and Gal4 with either the Gcn5 complex SAGA or the Swi/Snf complex. Within SAGA both Ada2 and the large ATM-related protein Tra1 interact with activators, whereas in Swi/Snf three proteins (Swi5, Swi1, and Swi2/Snf2 [34]) interact with activators. Our findings suggest a novel variation on this recruitment theme, where Ada2β, although not a stable component of either a SAGA-type or Swi/Snf-type complex, interacts with an activator (Pax5) and hence serves to recruit both varieties of chromatin effector enzymes by transient association. Thus, association with a common protein, Ada2β, could serve to coordinate the recruitment of histone modification and nucleosome remodeling activities.
Notable in our study is the specificity of functional coactivator synergy between Ada2β and Gcn5 or Brg1, compared to Ada2α, in Pax5-mediated transcriptional activation. Importantly, in B cells the Pax5-dependent gene CD19 is also induced by Ada2β functioning in concert with Gcn5.
Pax5 is known to regulate transcription both as an activator and a repressor. Apparently, Pax5 represses transcription via interactions with proteins from the Groucho family and histone deactyltranferases (18). Transcriptional activation by Pax5 is less well studied, although the HAT activity of CBP, but not p300, seems to be important for this process (19). Our study sheds light on aspects of the mechanism of Pax5-mediated activation. Ada2β binds Pax5 directly in vitro (Fig. 5C), and Ada2β coimmunoprecipitated with Gcn5 or Baf57 when Pax5 was present in the sample (Fig. 5C, lower panel, and 6A), suggesting that Pax5 may serve as a scaffold for these multiple interactions. Transcriptional stimulation of Pax5 by simultaneous expression of Ada2β/Gcn5 or Ada2β/Brg1 may result from sequential recruitment of histone acetylation and chromatin remodeling complexes. Ada2β, by interacting with both Pax5 and one of the chromatin-directed complexes, may stabilize these interactions.
An alternative model is that Ada2β by itself, or as a part of a complex, may recruit another, yet unidentified, chromatin-directed enzymatic activity. This unknown activity may positively affect histone acetylation and/or remodeling. In this scenario, Ada2β may trigger initial enzymatic changes to local chromatin to enhance the binding and/or activity of the Gcn5- and Brg1-containing complexes. Possibly relevant to this is the finding that another of the Ada2β-interacting partners in the two-hybrid screen was topoisomerase II (P. Zegerman and T. Kouzarides, data not shown), which is known to relax DNA conformation to increase accessibility of transcriptional factors to their cognate sites. In this respect, it will be interesting to examine the order of chromatin changes at Pax5-dependent promoters during activation.
Pax5 appears to require both acetylation and chromatin remodeling activities to promote transcription. The transcriptional role of Pax5 is still enigmatic. Pax5 by itself is a weak activator, at least in reporter assays, although transcription of several genes, including CD19, is critically dependent on the presence of Pax5 (35, 36). It is possible that the major role of Pax5 in transcriptional activation is not to recruit basal transcription machinery to the promoters but rather, in accordance with our observations, to modulate the structure of local chromatin, allowing other sequence-specific factors to bind the promoter and activate transcription. Thus, the requirement of Pax5 for acetylation and remodeling activities may be promoter dependent. In agreement with this, we observed no effect of Ada2β/Gcn5/Brg1 coexpression on transcription of the mb-1 gene (data not shown), which is also Pax5 dependent. Further studies are necessary to establish the role of histone modifications and chromatin remodeling, as well as the importance of Ada2β in the process of gene-specific transactivation mediated by Pax5.
In summary, hAda2β functions selectively with Gcn5 and Brg1 in vitro and in vivo. These findings indicate that Gcn5 and PCAF have distinct functions in vivo in gene activation. In addition, Ada2β may have a unique role in promoter recruitment of representatives of the two major chromatin altering activities.
Acknowledgments
We thank Paul Moore for initial help in identifying the hAda2β gene and Gavin Schnitzler for the gift of purified hSwi/Snf. We also thank G. Moore, D. Sterner, and members of the Berger lab for helpful discussions and critical reading of the manuscript.
The research was supported by National Institute of Health grants to S.L.B. (CA78831) and to B.K.B. (AI 13509 and AI 41572).
N.A.B. and A.V.E. contributed equally to this study.
REFERENCES
- 1.Aasland, R., A. F. Stewart, and T. Gibson. 1996. The SANT domain: a putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem. Sci. 21:87-88. [PubMed] [Google Scholar]
- 2.Barlev, N. A., R. Candau, L. Wang, P. Darpino, N. Silverman, and S. L. Berger. 1995. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J. Biol. Chem. 270:19337-19344. [DOI] [PubMed] [Google Scholar]
- 3.Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. [DOI] [PubMed] [Google Scholar]
- 4.Bell, B., E. Scheer, and L. Tora. 2001. Identification of hTAF(II)80 delta links apoptotic signaling pathways to transcription factor TFIID function. Mol. Cell 8:591-600. [DOI] [PubMed] [Google Scholar]
- 5.Bell, B., and L. Tora. 1999. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp. Cell Res. 246:11-19. [DOI] [PubMed] [Google Scholar]
- 6.Belotserkovskaya, R., and S. L. Berger. 2003. Interplay between chromatin modifying and remodeling complexes in transcriptional regulation. Crit. Rev. Eukaryot. Gene Expr. 9:221-230. [DOI] [PubMed]
- 7.Berger, S. L., B. Piña, N. Silverman, G. A. Marcus, J. Agapite, J. L. Regier, S. J. Triezenberg, and L. Guarente. 1992. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell 70:251-265. [DOI] [PubMed] [Google Scholar]
- 8.Boyer, L. A., M. R. Langer, K. A. Crowley, S. Tan, J. M. Denu, and C. L. Peterson. 2002. Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol. Cell 10:935-942. [DOI] [PubMed] [Google Scholar]
- 9.Brand, M., J. G. Moggs, M. Oulad-Abdelghani, F. Lejeune, F. J. Dilworth, J. Stevenin, G. Almouzni, and L. Tora. 2001. UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation. EMBO J. 20:3187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand, M., K. Yamamoto, A. Staub, and L. Tora. 1999. Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J. Biol. Chem. 274:18285-18289. [DOI] [PubMed] [Google Scholar]
- 11.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a transcriptional co-activator linking gene expression to histone acetylation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 12.Burns, L. G., and C. L. Peterson. 1997. The yeast SWI-SNF complex facilitates binding of a transcriptional activator to nucleosomal sites in vivo. Mol. Cell. Biol. 17:4811-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candau, R., and S. L. Berger. 1996. Structural and functional analysis of yeast putative adaptors: evidence for an adaptor complex in vivo. J. Biol. Chem. 271:5237-5345. [DOI] [PubMed] [Google Scholar]
- 14.Candau, R., P. A. Moore, L. Wang, N. Barlev, C. Y. Ying, C. A. Rosen, and S. L. Berger. 1996. Identification of human proteins functionally conserved with the yeast putative adaptors ADA2 and GCN5. Mol. Cell. Biol. 16:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi, T. H., M. Wan, K. Zhao, I. Taniuchi, L. Chen, D. R. Littman, and G. R. Crabtree. 2002. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature 418:195-199. [DOI] [PubMed] [Google Scholar]
- 16.Chiba, H., M. Muramatsu, A. Nomoto, and H. Kato. 1994. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucleic Acids Res. 22:1815-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhard, D., and M. Busslinger. 1999. The partial homeodomain of the transcription factor Pax-5 (BSAP) is an interaction motif for the retinoblastoma and TATA-binding proteins. Cancer Res. 59:1716s-1725s. [PubMed] [Google Scholar]
- 18.Eberhard, D., G. Jimenez, B. Heavey, and M. Busslinger. 2000. Transcriptional repression by Pax5 (BSAP) through interaction with corepressors of the Groucho family. EMBO J. 19:2292-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emelyanov, A. V., C. R. Kovac, M. A. Sepulveda, and B. K. Birshtein. 2002. The interaction of Pax5 (BSAP) with Daxx can result in transcriptional activation in B cells. J. Biol. Chem. 277:11156-11164. [DOI] [PubMed] [Google Scholar]
- 20.Forsberg, E. C., L. T. Lam, X. J. Yang, Y. Nakatani, and E. H. Bresnick. 1997. Human histone acetyltransferase GCN5 exists in a stable macromolecular complex lacking the adapter ADA2. Biochemistry 36:15918-15924. [DOI] [PubMed] [Google Scholar]
- 21.Grant, P. A., L. Duggan, J. Côté, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 22.Grant, P. A., D. Schieltz, M. G. Pray-Grant, D. J. Steger, J. C. Reese, J. R. Yates III, and J. L. Workman. 1998. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcription stimulation. Cell 94:45-53. [DOI] [PubMed] [Google Scholar]
- 23.Grant, P. A., D. Schieltz, M. G. Pray-Grant, J. R. Yates, and J. L. Workman. 1998. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol. Cell 2:863-867. [DOI] [PubMed] [Google Scholar]
- 24.Grant, P. A., D. E. Sterner, L. J. Duggan, J. L. Workman, and S. L. Berger. 1998. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 8:193-197. [DOI] [PubMed] [Google Scholar]
- 25.Gregory, P. D., K. Wagner, and W. Horz. 2001. Histone acetylation and chromatin remodeling. Exp. Cell Res. 265:195-202. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi, J., N. Silverman, G. A. Marcus, and L. Guarente. 1995. ADA3, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA2 and GCN5 in a trimeric complex. Mol. Cell. Biol. 15:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, H., H. Lu, R. L. Schiltz, C. A. Pise-Masison, V. V. Ogryzko, Y. Nakatani, and J. N. Brady. 1999. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez, E., T. K. Kundu, J. Fu, and R. G. Roeder. 1998. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J. Biol. Chem. 273:23781-23785. [DOI] [PubMed] [Google Scholar]
- 29.Martinez, E., V. B. Palhan, A. Tjernberg, E. S. Lymar, A. M. Gamper, T. K. Kundu, B. T. Chait, and R. G. Roeder. 2001. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol. Cell. Biol. 21:6782-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massari, M. E., P. A. Grant, M. G. Pray-Grant, S. L. Berger, J. L. Workman, and C. Murre. 1999. A conserved motif present in a class of helix-loop-helix proteins activates transcription by direct recruitment of the SAGA complex. Mol. Cell 4:63-73. [DOI] [PubMed] [Google Scholar]
- 31.McMahon, S. B., H. A. Van Buskirk, K. A. Dugan, T. D. Copeland, and M. D. Cole. 1998. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 94:363-374. [DOI] [PubMed] [Google Scholar]
- 32.Muchardt, C., and M. Yaniv. 1993. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 12:4279-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muchardt, C., and M. Yaniv. 2001. When the SWI/SNF complex remodels. the cell cycle. Oncogene 20:3067-3075. [DOI] [PubMed] [Google Scholar]
- 34.Neely, K. E., A. H. Hassan, C. E. Brown, L. Howe, and J. L. Workman. 2002. Transcription activator interactions with multiple SWI/SNF subunits. Mol. Cell. Biol. 22:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nutt, S. L., A. M. Morrison, P. Dorfler, A. Rolink, and M. Busslinger. 1998. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J. 17:2319-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nutt, S. L., C. Thevenin, and M. Busslinger. 1997. Essential functions of Pax-5 (BSAP) in pro-B cell development. Immunobiology 198:227-235. [DOI] [PubMed] [Google Scholar]
- 37.Ogryzko, V. V., T. Kotani, X. Zhang, R. L. Schiltz, T. Howard, X.-J. Yang, B. H. Howard, J. Qin, and Y. Nakatani. 1998. Histone-like TAFs within the PCAF histone acetylase complex. Cell 94:35-44. [DOI] [PubMed] [Google Scholar]
- 38.Pollard, K. J., and C. L. Peterson. 1998. Chromatin remodeling: a marriage between two families? Bioessays 20:771-780. [DOI] [PubMed] [Google Scholar]
- 39.Puri, P. L., V. Sartorelli, X. J. Yang, Y. Hamamori, V. V. Ogryzko, B. H. Howard, L. Kedes, J. Y. Wang, A. Graessmann, Y. Nakatani, and M. Levrero. 1997. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell 1:35-45. [DOI] [PubMed] [Google Scholar]
- 40.Roberts, S. M., and F. Winston. 1997. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics 147:451-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleh, A., V. Lang, R. Cook, and C. J. Brandl. 1997. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J. Biol. Chem. 272:5571-5578. [DOI] [PubMed] [Google Scholar]
- 42.Saleh, A., D. Schieltz, N. Ting, S. B. McMahon, D. W. Litchfield, J. R. Yates III, S. P. Lees-Miller, M. D. Cole, and C. J. Brandl. 1998. Tra1p is a component of the yeast Ada-Spt transcriptional regulatory complexes. J. Biol. Chem. 273:26559-26565. [DOI] [PubMed] [Google Scholar]
- 43.Schnitzler, G., S. Sif, and R. E. Kingston. 1998. Human SWI/SNF interconverts a nucleosome between its base state and a stable remodeled state. Cell 94:17-27. [DOI] [PubMed] [Google Scholar]
- 44.Silverman, N., J. Agapite, and L. Guarente. 1994. Yeast ADA2 protein binds to the VP16 protein activation domain and activates transcription. Proc. Natl. Acad. Sci. USA 91:11665-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh, M., and B. K. Birshtein. 1996. Concerted repression of an immunoglobulin heavy-chain enhancer, 3′ alpha E(hs1,2). Proc. Natl. Acad. Sci. USA 93:4392-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh, M., and B. K. Birshtein. 1993. NF-HB (BSAP) is a repressor of the murine immunoglobulin heavy-chain 3′ alpha enhancer at early stages of B-cell differentiation. Mol. Cell. Biol. 13:3611-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, E. R., J. M. Belote, R. L. Schiltz, X. J. Yang, P. A. Moore, S. L. Berger, Y. Nakatani, and C. D. Allis. 1998. Cloning of Drosophila GCN5: conserved features among metazoan GCN5 family members. Nucleic Acids Res. 26:2948-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sterner, D. E., X. Wang, M. H. Bloom, G. M. Simon, and S. L. Berger. 2002. The SANT domain of Ada2 is required for normal acetylation of histones by the yeast SAGA complex. J. Biol. Chem. 277:8178-8186. [DOI] [PubMed] [Google Scholar]
- 49.Stockinger, E. J., Y. Mao, M. K. Regier, S. J. Triezenberg, and M. F. Thomashow. 2001. Transcriptional adaptor and histone acetyltransferase proteins in Arabidopsis and their interactions with CBF1, a transcriptional activator involved in cold-regulated gene expression. Nucleic Acids Res. 29:1524-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka, Y., I. Naruse, T. Maekawa, H. Masuya, T. Shiroishi, and S. Ishii. 1997. Abnormal skeletal patterning in embryos lacking a single Cbp allele: a partial similarity with Rubinstein-Taybi syndrome. Proc. Natl. Acad. Sci. USA 94:10215-10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas, J. O., and A. A. Travers. 2001. HMG1 and -2, and related “architectural” DNA-binding proteins. Trends Biochem. Sci. 26:167-174. [DOI] [PubMed] [Google Scholar]
- 52.Trouche, D., C. Le Chalony, C. Muchardt, M. Yaniv, and T. Kouzarides. 1997. RB and hbrm cooperate to repress the activation functions of E2F1. Proc. Natl. Acad. Sci. USA 94:11268-11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassilev, A., J. Yamauchi, T. Kotani, C. Prives, M. L. Avantaggiati, J. Qin, and Y. Nakatani. 1998. The 400-kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol. Cell 2:869-875. [DOI] [PubMed] [Google Scholar]
- 54.Vojtek, A., S. Hollenberg, and J. Cooper. 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 55.Wang, L., C. Mizzen, C. Ying, R. Candau, N. Barlev, J. Brownell, C. D. Allis, and S. Berger. 1997. Histone acetyltransferase activity is conserved between yeast and human GCN5 and required for complementation of growth and transcriptional activation. Mol. Cell. Biol. 17:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, W., T. Chi, Y. Xue, S. Zhou, A. Kuo, and G. R. Crabtree. 1998. Architectural DNA binding by a high-mobility-group/kinesin-like subunit in mammalian SWI/SNF-related complexes. Proc. Natl. Acad. Sci. USA 95:492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, W., J. Cote, Y. Xue, S. Zhou, P. A. Khavari, S. R. Biggar, C. Muchardt, G. V. Kaplana, S. P. Goff, M. Yaniv, J. L. Workman, and G. R. Crabtree. 1996. Purification and biochemical heterogeneity of the mammalian SWI/SNF complex. EMBO J. 15:5370-5382. [PMC free article] [PubMed] [Google Scholar]
- 58.Wieczorek, E., M. Brand, X. Jacq, and L. Tora. 1998. Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature 393:187-191. [DOI] [PubMed] [Google Scholar]
- 59.Winston, F., and M. Carlson. 1992. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 8:387-391. [DOI] [PubMed] [Google Scholar]
- 60.Xu, W., D. G. Edmondson, Y. A. Evrard, M. Wakamiya, R. R. Behringer, and S. Y. Roth. 2000. Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat. Genet. 26:229-232. [DOI] [PubMed] [Google Scholar]
- 61.Xu, W., D. G. Edmondson, and S. Y. Roth. 1998. Mammalian GCN5 and P/CAF acetyltransferases have homologous amino-terminal domains important for recognition of nucleosomal substrates. Mol. Cell. Biol. 18:5659-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamauchi, T., J. Yamauchi, T. Kuwata, T. Tamura, T. Yamashita, N. Bae, H. Westphal, K. Ozato, and Y. Nakatani. 2000. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl. Acad. Sci. USA 97:11303-11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagisawa, J., H. Kitagawa, M. Yanagida, O. Wada, S. Ogawa, M. Nakagomi, H. Oishi, Y. Yamamoto, H. Nagasawa, S. B. McMahon, M. D. Cole, L. Tora, N. Takahashi, and S. Kato. 2002. Nuclear receptor function requires a TFTC-type histone acetyl transferase complex. Mol. Cell 9:553-562. [DOI] [PubMed] [Google Scholar]
- 64.Yang, X.-J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral E1A oncoprotein. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 65.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]