Abstract
Conjugal transfer of Ti plasmids of Agrobacterium tumefaciens is controlled by a quorum-sensing system composed of the transcriptional activator TraR and its acyl-homoserine lactone quormone N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL). The population density dependence of quorum-sensing systems can often be circumvented by addition of the quormone to cultures at low cell numbers. However, the quorum-dependent activation of Ti plasmid conjugal transfer exhibited a lag of almost 8 h when the quormone was added to donor cells at low population densities (Piper and Farrand, J. Bacteriol. 182:1080-1088, 2000). As measured by activation of a TraR-dependent traG::lacZ reporter fusion, TraR in cells exposed to the cognate signal for 5 min showed detectable activity, while exposure for 15 min resulted in full activity. Thus, the lag in activation is not due to some intrinsic property of TraR. Cells exposed to the agonistic analog N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL) exhibited similar induction kinetics. However, activation of the reporter in cells exposed to the poorly effective alkanoyl acyl-HSL N-hexanoyl-l-homoserine lactone (C6-HSL) required the continued presence of the signal. As measured by an in vivo repressor assay, TraR activated by 3-oxo-C6-HSL or by 3-oxo-C8-HSL remained active for as long as 8 h after removal of exogenous signal. However, TraR activated by the alkanoyl quormone C6-HSL rapidly lost activity following removal of the signal. In quormone retention assays, which measure signal binding by TraR, cells grown with either of the two 3-oxo-acyl-HSL quormones retained the ligand after washing, while cells grown with C6-HSL lost the alkanoyl-HSL concomitant with the rapid loss of TraR activity. We conclude that TraR rapidly binds its quormone and that, once bound, the cognate signal and its close homologs are tightly retained. Moreover, in the absence of other regulatory factors, activated TraR remains functional after removal of the signal. On the other hand, poorly active signals are not tightly bound, and their removal by washing leads to rapid loss of TraR activity.
The Ti plasmids of Agrobacterium tumefaciens are conjugal elements, mediating their own transfer from donors to bacterial recipients (7). Transfer is strongly regulated and is responsive to two environmental cues, the presence of signal molecules produced by crown gall tumors, plant neoplasias induced by the pathogenic bacteria, and signal molecules produced by the donor bacteria themselves (9, 29). The tumor-produced signals, called opines, communicate to the Ti plasmids that the environment in which the host bacterium is located is conducive to horizontal dissemination (7). The bacterial signals, called quormones, control transfer by quorum sensing, ensuring that the transfer system is not activated until the donors have reached a critical population size. The two regulatory systems are linked in a hierarchical manner, with quorum sensing being controlled by opine availability (10, 25, 29).
Expression of the three Ti plasmid transfer (tra) operons requires the quorum-sensing transcriptional activator TraR (9, 28). TraR, in turn, requires its acyl-homoserine lactone (acyl-HSL) quormone ligand N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL) (38). TraR binds the acyl-HSL, forming active homodimers that bind target promoters containing an 18-bp inverted repeat called the tra box (11, 18, 19, 30, 40). Once bound, TraR in its ligand-bound dimer form activates transcription (40), presumably by interacting with one or more components of RNA polymerase.
Acyl-HSL-mediated quorum-sensing systems rely on accumulation of the quormone in the environment as an indicator of the bacterial population size. The signal is produced intracellularly (9, 15), transits out of the cell by diffusion (16) or by active efflux systems (6, 26), and reenters the cell, also probably by diffusion (16). In the absence of other mitigating elements, three primary factors working in concert establish the sensitivity, and therefore the population size, that sets the quorum for a given system. In the first, the rate of synthesis of the acyl-HSL signal determines the rate at which the quormone accumulates relative to the rate of increase of the bacterial population. Second, the level of expression of the gene coding for the quormone-responsive transcription factor determines the amount of target available to the acyl-HSL. Third, the affinity of the transcription factor for its cognate acyl-HSL coupled with the intracellular concentrations of the signal and the activator determine the point at which the quormone effectively converts the transcription factor to its active form.
Results from in vivo analyses suggest that TraR is extraordinarily sensitive to its cognate acyl-HSL; concentrations of exogenous signal as low as 1 to 2 nM are sufficient for TraR to activate an appropriate reporter (15, 38). Moreover, specially constructed reporter strains of A. tumefaciens can detect femtomole amounts of N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL) (3, 32). Consistent with these observations, purified TraR binds its quormone very tightly; the pure protein retains 3-oxo-C8-HSL even after extensive dialysis (30, 40). In addition to activating TraR, quormone binding plays a second role; ligand-bound dimers of TraR are 20-fold more resistant to proteolysis than their inactive monomers (40, 41).
This high affinity, coupled with the stabilization of active TraR, raises two questions concerning the Ti plasmid quorum-sensing system. First, when the quormone has accumulated to its critical concentration, how long does it take for TraR to bind the ligand and activate its target operons? Second, given its high affinity for 3-oxo-C8-HSL and the attendant stability of the dimer, how long does TraR remain active upon removal of the acyl-HSL signal from the environment? This question gains added interest with the recent report that at the end of exponential growth, A. tumefaciens strains produce a potent acyl-HSL lactonase (37). This intracellular enzyme rapidly deactivates free quormone by hydrolyzing the lactone ring.
In this study, we examined the rate at which exogenous 3-oxo-C8-HSL activates TraR and subsequent expression of its target genes in situ. We also examined the functional stability of activated TraR and, using an in vivo assay, correlated stability with retention by the activator of its acyl-HSL ligand. Our studies indicate that while the genes of the Ti plasmid conjugal transfer system can be activated rapidly, quickly shutting down the system following disappearance of the opine and acyl-HSL signals is more difficult and most likely requires additional factors.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Agrobacterium tumefaciens strain NTL4 is a Ti plasmid-cured derivative of the nopaline-agrocinopine type strain C58 in which the tetAR locus was removed by deletion (20). Escherichia coli DH5α (31) was used in assays in which the activity of TraR was measured by repression (19). The plasmid-mobilizing E. coli strain S17-1 was described by Simon et al. (33). A. tumefaciens strains were grown at 28°C in L broth (LB), MG/L (2), or ABM minimal medium (4). E. coli strains were grown at 28 or 37°C in LB or minimal medium A (A medium) (24). All liquid cultures were grown with agitation to ensure adequate aeration. Antibiotics were used at the following concentrations: for E. coli, kanamycin, 50 μg/ml; tetracycline, 10 μg/ml; ampicillin, 100 or 50 μg/ml for minimal medium; for A. tumefaciens, kanamycin, 50 μg/ml; carbenicillin, 50 μg/ml; and tetracycline, 2 μg/ml. When required, expression of traR from the trc promoter of pZLQR, which also expresses lacIq, was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 100 μM.
Acyl-homoserine lactones.
Chemically synthesized 3-oxo-C8-HSL, N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL), and N-hexanoyl-l-homoserine lactone (C6-HSL) were the generous gifts of David Lynn, Emory University, or synthesized according to published procedures (5). All acyl-HSLs were stored as concentrated stock solutions in high-pressure liquid chromatography-grade ethyl acetate at −20°C.
Plasmids.
Plasmid pPBL1, which contains a quormone-dependent, TraR-repressible lacZ reporter fusion, and the traR expression plasmid pZLQR, in which traR is expressed from the trc promoter, have been described previously (19). The acyl-HSL reporter plasmid pZLR4 contains the traR gene from pTiC58 expressed from its own weak constitutive promoter located immediately upstream of the gene (28, 29), and the TraR-activatable I41 traG::lacZ fusion (8) all cloned in pBBR1MCS5 (17) as described by Cha et al. (3).
Genetic manipulations.
Plasmid DNA was introduced into E. coli by CaCl2-mediated transformation (31) and into A. tumefaciens strains by electroporation (2) or by biparental matings with E. coli S17-1 conducted at 28°C (8).
Gene expression assays.
Production of β-galactosidase from lacZ reporter fusions was measured semiquantitatively on solid medium containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, 40 μg/ml). β-Galactosidase activity, expressed as units per 109 viable cells, was quantified as previously described (19). All assays were repeated at least twice, and values shown represent means or the results from representative experiments.
Culture manipulations.
Cells grown in medium containing an acyl-HSL were freed of removable quormone as follows. For repression assays, a culture of E. coli DH5α harboring the appropriate plasmids in 15 ml of A medium was subjected to centrifugation at 3,000 rpm for 5 min at 4°C in the SS34 rotor of a Sorvall RC5 refrigerated centrifuge. The supernatant was carefully removed, the cells were resuspended in 45 ml of fresh sterile A medium, and the process was repeated five times. Preliminary experiments showed that no acyl-HSL was detectable in the supernatants following the third wash. Following the fifth wash, the cells were resuspended in 15 ml of fresh sterile A medium containing the appropriate antibiotics and recultured as described in Results.
To remove the quormone from cells in which TraR was functioning as an activator, cultures of A. tumefaciens NTL4 harboring the appropriate plasmids in 4 ml of ABM medium were washed six times by centrifugation with 2-ml volumes of ABM medium as described above. Preliminary experiments indicated that no acyl-HSL was detectable in supernatants following the fourth wash. Following the final wash, the cells were resuspended in 4 ml of fresh ABM medium containing the appropriate antibiotics and recultured as described in Results.
Preparation of cell lysates and extraction of intracellular quormone.
Following growth in 15-ml cultures, cells were harvested by centrifugation and washed up to six times, depending upon the experiment, with 5-ml volumes of AB medium lacking a carbon source. Cells collected after the final wash were either lysed directly, as described below, or washed once by centrifugation with a 3-ml volume of modified Agrowash (20 mM Tris-HCl [pH 8.0], 0.5 M NaCl, 1% sodium Sarkosyl) and twice with the same buffer lacking Sarkosyl prior to lysis. For lysis, cells were collected by centrifugation, the pellets were resuspended in 200 μl of a buffer containing 0.2 M Tris-HCl (pH 8.0), 0.4 M disodium EDTA, and 0.7 M sucrose, and incubated on ice for 30 min to sensitize the cells to lysozyme. A volume of 800 μl of distilled water was added, and the cells were recovered by centrifugation for 5 min at 4°C and resuspended in 1.5 ml of TraR extraction buffer containing 30 mM Tris-HCl (pH 8.0), 0.2 mM disodium EDTA, 10% glycerol, 0.2 mM dithiothreitol, 1% Tween 20, and 2 mg of lysozyme per ml. Following incubation on ice for 20 min, the cells were disrupted by sonication, and unbroken cells, cell debris, and protein inclusion bodies were removed by centrifugation at 50,000 × g for 15 min at 4°C. The cleared lysates were extracted twice with 200-μl volumes of ethyl acetate, and the organic phases were pooled and taken to dryness under vacuum. Residues were dissolved in 50 μl of ethyl acetate and stored at −20°C.
Detecting acyl-HSLs.
Acyl-HSLs extracted from culture supernatants, from cell washes, and from cleared cell lysates were separated by thin-layer chromatography on analytical C18 reversed-phase thin-layer chromatography plates developed with a solvent of 60% (vol/vol) methanol-40% (vol/vol) water (32). The quormones were detected by using as bioreporters A. tumefaciens NTL4(pZLR4) (3) for 3-oxo-acyl-HSLs and Chromobacterium violaceum CV026blu (23) for alkanoyl acyl-HSLs as described elsewhere (3, 32). Following development, the plates were dried, and the images were archived by digital scanning (32).
RESULTS
Kinetics of TraR activation in situ.
Although TraR requires its acyl-HSL quormone for activity, there is no information concerning how long cells expressing this activator must be exposed to functional levels of signal to activate the transcription factor. To assess these temporal parameters, we constructed a derivative of A. tumefaciens strain NTL4 harboring pZLR4 (3), a plasmid that expresses traR from its native, low-activity promoter and also carries a TraR-dependent traG::lacZ reporter fusion (28, 29). This strain lacks a Ti plasmid and does not produce its own quormone or the TraR-specific antiactivator TraM (12, 13). However, TraR can activate the reporter if an active acyl-HSL is added exogenously to the culture (3).
An exponential-phase culture of NTL4(pZLR4) in ABM medium was grown to an optical density at 600 nm (OD600) of 0.15 (approximately 107 CFU/ml), 3-oxo-C8-HSL, the cognate quormone (Fig. 1), was added to a final concentration of 25 nM, a level about 10-fold higher than that required for maximal activation of the reporter (15, 38), and incubation was continued. At 25 s, 5 min, and 25 min, 4-ml volumes of the culture were removed, and the cells were washed six times as described in Materials and Methods, and resuspended in 3 ml of fresh ABM medium. Each culture was immediately split into three equal subcultures. Cells from one subculture were assayed immediately for expression of the reporter. Fresh signal in a 2-μl volume was added to one of the remaining cultures, and the paired subcultures were incubated at 28°C with shaking. After 4 h of growth, corresponding to about two doublings, samples of each culture were assayed for expression of the reporter gene. As controls, two subcultures, one continuously exposed and the other never exposed to 3-oxo-C8-HSL, were incubated without washing for the duration of the experiment.
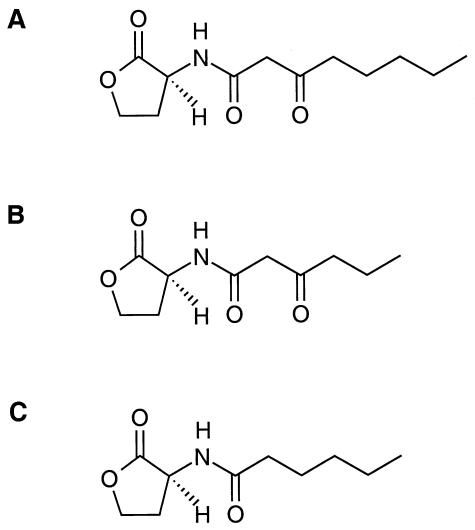
FIG. 1.
Structures of the acyl-homoserine lactone autoinducers used in this study. (A) N-(3-Oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL); (B) N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL); (C) N-hexanoyl-l-homoserine lactone (C6-HSL).
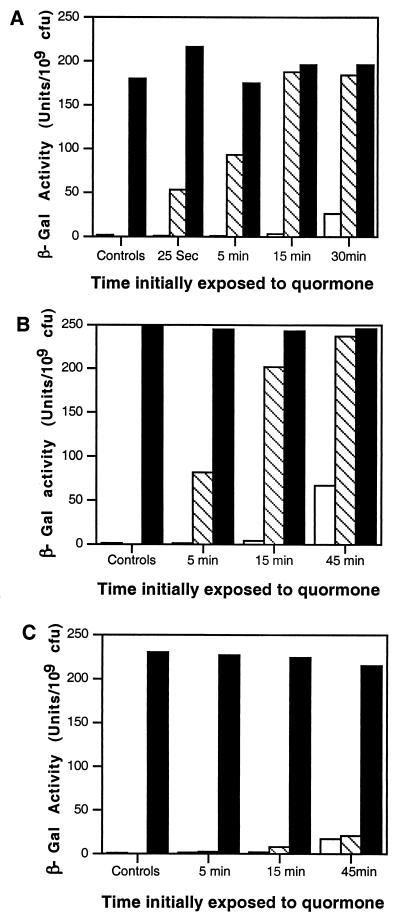
The culture to which no quormone was added did not detectably express the reporter, while the cells continuously exposed to the signal expressed the fusion at a high level (Fig. 2A). Washed cultures from each time point that were resuspended in medium containing the acyl-HSL for the remainder of the experiment also expressed the reporter at high levels (Fig. 2A). Cells that had been incubated with the quormone for 25 s prior to washing and resuspension in medium lacking signal showed a low but detectable level of activation (Fig. 2A). Incubation with signal for 15 or 30 min prior to washing resulted in maximum induction of the traG::lacZ reporter, while incubation with the quormone for 5 min prior to washing yielded activation to about 50% of the level of the fully induced control culture (Fig. 2A). The cultures that were assayed for expression directly after resuspension either failed to activate the reporter or, as exemplified by the cultures exposed to signal for 15 or 30 min, activated the reporter at very low levels. These results indicate that the level of expression that we observed in the cultures following the 4-h incubation period in medium lacking signal was not due to induction of the reporter that occurred during the period of growth with the signal prior to washing and resuspending the cells. We conclude that exposure of the cells to 3-oxo-C8-HSL for between 5 and 15 min is sufficient to activate amounts of TraR sufficient to fully express the reporter.
FIG. 2.
In situ activation of TraR by acyl-HSLs. 3-Oxo-C8-HSL (25 nM final concentration) (A), 3-oxo-C6-HSL (100 nM final concentration) (B), or C6-HSL (2.5 μM final concentration) (C) was added to exponential-phase cultures [OD600 = 0.15 (ca 107 CFU/ml)] of A. tumefaciens strain NTL4(pZLR4) growing in ABM minimal medium containing IPTG to induce expression of traR. The cultures were incubated, 4-ml samples were withdrawn at the indicated times, and the cells were harvested and washed extensively as described in Materials and Methods. The washed cells were resuspended in 4 ml of ABM lacking IPTG, and each culture was split into three subcultures. One subculture was assayed for β-galactosidase activity, expressed as unitsper 109 CFU, immediately after washing and resuspension (open bars). 3-Oxo-C8-HSL or 3-oxo-C6-HSL was added to the second subculture (solid bars), and an equal volume of sterile distilled water was added to the third subculture (hatched bars). The subcultures were reincubated in parallel with vigorous shaking for 4 h, at which time the cells from each culture were collected by centrifugation and assayed for β-galactosidase activity. Parallel cultures, not subjected to washing, were incubated continuously with the quormone (panels A, B, and C, controls, solid bars) or without the quormone (panels A, B, and C, controls, hatched bars) for the full duration of the experiment. Samples from each of these cultures were assayed for β-galactosidase activity. The experiments shown in panels A and B were repeated twice, and the experiment shown in panel C was repeated three times. In each case, data from a representative repetition are presented.
TraR responds to other acyl-HSLs but requires considerably higher concentrations of these analogs to activate transcription (3, 32, 38, 42). We examined, using the same experimental protocols, the kinetics of TraR activation in cells exposed to two noncognate acyl-HSLs. One, 3-oxo-C6-HSL, differs from the cognate only by the length of the acyl side chain (Fig. 1) and is a relatively good coactivator (38). The second, C6-HSL, differs from 3-oxo-C8-HSL in the length of and chemistry at the acyl C-3 position of the side chain (Fig. 1) and is a very poor signal (3, 32, 38, 42).
Cultures grown with 100 nM 3-oxo-C6-HSL, a concentration sufficient to maximally induce the reporter, exhibited response kinetics similar to those observed with 3-oxo-C8-HSL, the cognate quormone (Fig. 2B). Cells that had been exposed for 5 min prior to washing and resuspension in medium lacking the 3-oxo-C6 signal exhibited a significant level of activation, while the reporter was fully induced in cells grown with the signal for 15 and 45 min (Fig. 2B).
Cultures grown with C6-HSL exhibited a significantly different response. The alkanoyl quormone at a concentration of 2.5 μM fully induced the reporter in cells exposed to this analogue continuously or over the 4 h of postwash incubation (Fig. 2C). However, cells exposed to the alkanoyl-HSL for 5 or 15 min failed to activate the reporter, and cells exposed for 45 min showed only very low levels of induction (Fig. 2C). Cells assayed at the time of the wash showed similar low levels of activity (Fig. 2C), suggesting that the low level of expression observed in the 45-min cultures is due to activation of the lacZ reporter that occurred during the prewash phase of the experiment.
We conclude from these results that cells expressing TraR require only a short exposure, as little as 5 to 10 min, to the cognate quormone and closely related acyl-HSLs such as 3-oxo-C6-HSL. Moreover, following such short exposures, activated TraR remains functional even after the extracellular quormone is removed. However, with less active signals such as C6-HSL, the acyl-HSL must be present continuously for TraR to remain active.
In situ quormone binding by TraR.
The contrast in persistence of reporter expression by TraR in cells exposed to the 3-oxo-acyl-HSLs compared to cells exposed to C6-HSL suggests that the functional activator is much more stable in cells grown with the 3-oxo-forms. To test this hypothesis, we developed an in vivo assay for quormone binding based on the cellular retention of these signal molecules. The assay, described in Materials and Methods, is similar to that used in several studies assessing the intracellular interaction between the quormone and LuxR (1, 16) and LasR (26) and is predicated on the observation that purified dimer TraR contains 1 mol of tightly bound quormone per mol of protomer (40). In our assay, we detected the TraR-bound quormone by using a bioreporter following separation by thin-layer chromatography (30, 32). While not as sensitive as the radiometric assays (16), the bioreporter will detect active acyl-HSLs in pico- to femtomole amounts (32).
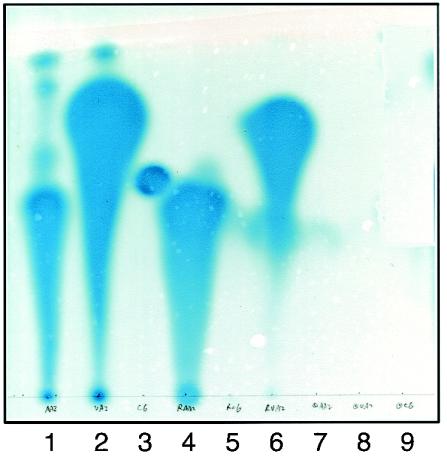
To validate the assay, we assessed the ability of cells expressing and lacking TraR to retain the cognate quormone after extensive washing. Cultures of NTL4 harboring pZLQ, which lacks traR, or pZLQR, which expresses traR, were grown to late exponential phase in ABM containing IPTG and 50 nM 3-oxo-C8-HSL. The cells were harvested and washed six times, and cell-free lysates were prepared as described in Materials and Methods. Ethyl acetate extracts of these lysates were chromatographed on C18 reversed-phase thin-layer chromatography plates, and the acyl-HSL was detected by overlaying the plates with a culture of the bioreporter strain NTL4(pZLR4). Extracts from NTL4(pZLQ) did not contain detectable levels of quormone (Fig. 3, lane 7) while extracts prepared from NTL4(pZLQR) contained an easily detectable amount of the acyl-HSL (Fig. 3, lane 4). Moreover, the active species chromatographed with a mobility and spot shape indistinguishable from those of authentic 3-oxo-C8-HSL (Fig. 3, compare lanes 1 and 4). Thus, cells expressing TraR strongly retain the acyl-HSL, apparently in unchanged form, while the quormone can be washed out completely from cells lacking TraR.
FIG. 3.
3-Oxo-acyl-HSLs but not C6-HSL are retained by A. tumefaciens cells expressing TraR. Cultures of A. tumefaciens NTL4(pZLQ), which does not express traR, and NTL4(pZLQR), which does express the activator from the trc promoter, were grown in ABM minimal medium to a density of about 107 CFU/ml. The two cultures were each split into three subcultures, and 3-oxo-C8-HSL (50 nM) was added to one, 3-oxo-C6-HSL (150 nM) was added to a second, and C6-HSL (2.5 μM) was added to the third. At the same time IPTG was added to all cultures of NTL4(pZLQR) to induce expression of traR. The six cultures were grown to late exponential phase (OD600 of about 1.2), at which time the cells were harvested, washed, and broken, and the cell extracts were assayed for intracellular quormone by thin-layer chromatography with A. tumefaciens NTL4(pZLR4) as the bioindicator, all as described in Materials and Methods. The thin-layer chromatography plates were spotted with the following samples: 1, pure 3-oxo-C8-HSL standard; 2, pure 3-oxo-C6-HSL standard; 3, pure C6-HSL standard; extracts of NTL4(pZLQR) grown with: 4, 3-oxo-C8-HSL; 5, C6-HSL; 6, 3-oxo-C6-HSL; and extracts of NTL4(pZLQ) grown with: 7, 3-oxo-C8-HSL; 8, 3-oxo-C6-HSL; and 9, C6-HSL.
Similar cultures of the two strains were grown in ABM containing IPTG and 150 nM 3-oxo-C6-HSL or 2.5 μM C6-HSL. The 3-oxo-C6 quormone was retained by cells expressing TraR (Fig. 3, lane 6) but not by cells lacking TraR (Fig. 3, lane 8). However, the alkanoyl HSL was not retained at a detectable level by either culture (Fig. 3, lanes 5 and 9). The alkanoyl-HSLs are only weakly detected by the A. tumefaciens bioreporter (3, 32). We reexamined the extracts from cells grown with C6-HSL with the Chromobacterium violaceum bioreporter, which is particularly sensitive to these unsubstituted acyl-HSLs (3, 23). While the reporter easily detected our sample of pure C6-HSL, after exhaustive washing, no signal was seen in extracts of either A. tumefaciens strain grown with the alkanoyl quormone (data not shown). We conclude from these studies that 3-oxo-C8- and 3-oxo-C6-HSL are bound tightly by TraR. However, while C6-HSL is bound by and can activate TraR, it is not tightly retained by the protein.
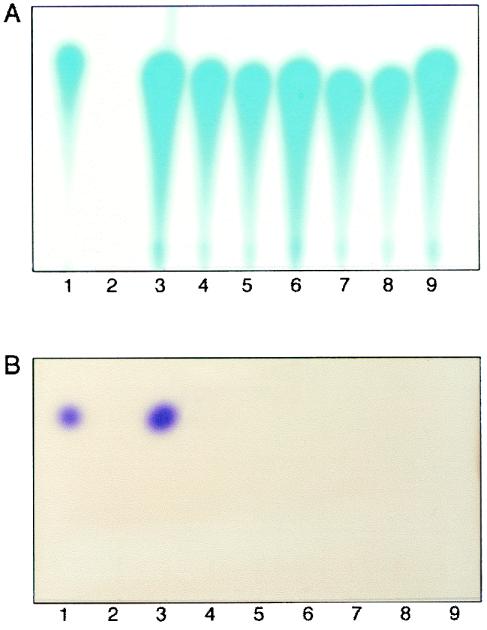
We assessed the long-term stability of active TraR-ligand complexes by assaying for the retention of the acyl-HSLs in NTL4(pZLQR) that had been grown with IPTG and quormone, washed, and then reincubated without signal or IPTG for up to 22 h following the wash. In cells preincubated with 3-oxo-C8-HSL, the quormone was detected in cells sampled at each time point following resuspension (Fig. 4A). However, in cells grown with C6-HSL, the quormone was not detectably retained by cells in any sample after the washing regimen (Fig. 4B). The alkanoyl quormone was retained at detectable levels by cells washed once with AB minimal medium (Fig. 4B, lane 3), and retention was dependent upon the presence of TraR (Fig. 4B, compare lanes 2 and 3). However, a second wash with AB minimal medium reduced the retained alkanoyl quormone to below detectable levels (Fig. 4B, lane 4).
FIG. 4.
Quormone retention by cells expressing traR after removal of exogenous acyl-HSL. Cultures of NTL4(pZLQ) and NTL4(pZLQR) were grown to late exponential phase in ABM medium containing IPTG to induce traR and 3-oxo-C8-HSL (panel A) or C6-HSL (panel B). Cells of NTL4(pZLQ) were harvested, washed once with ABM, and lysed, and the cell extracts were assayed for acyl-HSLs (panels A and B, lanes 2) by thin-layer chromatography as described in Materials and Methods. Cells of NTL4(pZLQR) were harvested, and a sample of each culture was assayed for retention of the acyl-HSL after one (panels A and B, lanes 3), two (panels A and B, lanes 4), and four (panels A and B, lanes 5) washes with AB minimal medium. A portion of the six-times-washed cultures of NTL4(pZLQR) originally grown with 3-oxo-C8-HSL and with C6-HSL were diluted into fresh ABM medium lacking quormone and IPTG, and incubation was continued. One-milliliter samples were removed at time zero (lanes 6) and after 4 h (lanes 7), 12 h (lanes 8), and 22 h (lanes 9) of further incubation. Cells in samples taken at each time point were washed and lysed, and ethyl acetate extracts of the lysates were assayed for the acyl-HSLs by thin-layer chromatography with the A. tumefaciens (panel A) or C. violaceum (panel B) reporter strain as described in Materials and Methods. Lane 1 contains samples of pure 3-oxo-C8-HSL (panel A) or C6-HSL (panel B) as standards.
Activated TraR remains functional when the quormone is removed.
The preceding experiments suggested that TraR rapidly binds the quormone in situ and that, once bound, the cognate acyl-HSL is strongly retained. The tight ligand binding and the relative stability of the protein (30, 40, 41) in turn suggest that, once activated, TraR will retain activity even when the free acyl-HSL signal drops below its critical concentration. To examine this hypothesis and to assess the functional stability of TraR, we designed an experimental system in which we could remove the quormone from the medium and monitor the decay of functional TraR. To maximize the sensitivity of this assay, we chose to use a reporter in which active TraR functions as a repressor (19). Thus, a decrease in the level of active ligand-bound TraR could be monitored by an increase in reporter gene activity.
Cultures of E. coli DH5α(pPBL1) harboring pZLQR, in which traR is expressed from the trc promoter of the vector, or pZLQ, the vector without traR, were grown in A medium. pPBL1 contains lacZ expressed from a promoter that is strongly repressed by TraR in a quormone-dependent manner (19). When the exponential-phase cultures had reached a density of about 107 CFU/ml, traR was induced by addition of IPTG, and incubation was continued for an hour. At this time, 3-oxo-C8-HSL (25 nM) was added to the culture of DH5α(pPBL1, pZLQ). The culture of DH5α(pPBL1, pZLQR) was divided into three subcultures, and 3-oxo-C8-HSL was added to one and 3-oxo-C6-HSL was added to the second. The third culture received no quormone. The four cultures, including DH5α(pPBL1, pZLQ), were incubated for 4 h, at which time the three cultures of DH5α(pPBL1, pZLQR) were harvested, and the cells of each were washed six times as described in Materials and Methods and resuspended in the original volume of culture medium lacking IPTG. Each of these cultures was split into two subcultures, to one of which was added the quormone to which the cells had first been exposed. Incubation was continued, and samples from each culture were taken at timed intervals and assayed for β-galactosidase activity.
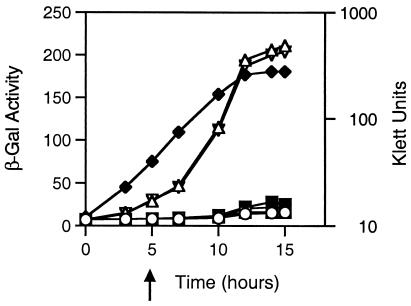
The culture containing the reporter but no copy of traR as well as the culture containing traR but never exposed to an acyl-HSL both strongly expressed the reporter (Fig. 5). On the other hand, the reporter remained repressed in cultures exposed to either quormone throughout the course of the experiment. In cultures in which the exogenous quormone was removed, the reporter remained repressed throughout the 8 h of postwash incubation (Fig. 5).
FIG. 5.
TraR retains in situ activity after removal of the 3-oxo-acyl-HSL quormones. Cultures of E. coli DH5α(pPBL1, pZLQ), which lacks traR, and of E. coli DH5α(pPBL1, pZLQR), which expresses traR, were grown in A medium to a density of about 107 CFU/ml as determined by Klett colorimetry (♦, red filter). At time zero, IPTG (100 μM) was added to induce expression of traR in DH5α(pPBL1, pZLQR), and the two cultures were split into two and three subcultures, respectively. After 1 h of incubation, 3-oxo-C8-HSL (25 nM) (□) or 3-oxo-C6-HSL (100 nM) [▿, DH5α(pPBL1, pZLQ); ○, DH5α(pPBL1, pZLQR)] was added. One subculture of DH5α(pPBL1, pZLQR) received no quormone (▵). After 4 h of reincubation (arrow), cultures of DH5α(pPBL1, pZLQR) that were grown with a quormone were harvested and washed extensively as described in Materials and Methods, and each culture was further split into two subcultures, both of which lacked IPTG. 3-Oxo-C8-HSL (25 nM, □) or 3-oxo-C6-HSL (100 nM, ○) was added to one such washed subculture, and an equal volume of sterile water was added to the second (▪, •). The cultures were reincubated, and samples were taken from each culture at the indicated times and assayed for β-galactosidase activity. The experiment was repeated twice; the results from one experiment are shown.
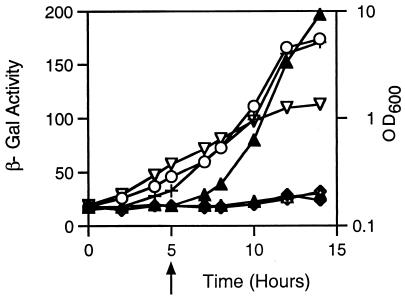
The results described above suggest that TraR bound with 3-oxo-C8- or 3-oxo-C6-HSL can remain active for extended periods after removal of exogenous signal. As indicated by our retention assays, while the activator binds the two 3-oxo-substituted quormones very tightly, it does not retain the alkanoyl quormone C6-HSL (Fig. 3 and 4B). We repeated the in situ stability experiment, this time comparing cells grown with 3-oxo-C8-HSL with cells grown with C6-HSL. As in the previous experiment, cells not expressing traR and cells expressing traR but not exposed to either acyl-HSL strongly expressed the reporter (Fig. 6). Cells exposed throughout the experiment to 25 nM C6-HSL, an ineffective concentration, continued to express the reporter, while cells exposed to the alkanoyl HSL at 2.5 μM or to 3-oxo-C8-HSL at 25 nM strongly repressed expression of the reporter fusion (Fig. 6). The reporter remained repressed in cells in which the exogenous 3-oxo-C8-HSL had been removed by washing. However, in cells in which the exogenous C6-HSL was removed, expression of the reporter was detectable 2 h after washing and became strongly derepressed during the remainder of the incubation period (Fig. 6).
FIG. 6.
TraR rapidly loses in situ activity after removal of C6-HSL. A culture of E. coli DH5α(pPBL1, pZLQR), which expresses traR, was incubated in A medium, and growth was followed turbidometrically (▿, OD600) as described in the legend to Fig. 5. At time zero, IPTG (100 μM) was added to induce expression of traR, the culture was split into four subcultures, and each was incubated for 1 h. 3-Oxo-C8-HSL (25 nM) (♦) was added to one subculture, and C6-HSL at two concentrations (○, 25 nM; ▵, 2.5 μM) was added to two subcultures. The fourth subculture (+) received no quormone. After 4 h of reincubation (arrow), cultures that received 3-oxo-C8-HSL or the high concentration of C6-HSL were harvested and washed extensively as described in Materials and Methods, and the resuspended cells from each culture were split into two subcultures. 3-Oxo-C8-HSL (♦, 25 nM) or C6-HSL (▵, 2.5 μM) was added to one of each of the subcultures, an equal volume of sterile water was added to the second (⋄, ▴), and growth was continued. Samples were taken from each culture at the indicated times and assayed for β-galactosidase activity. The experiment was repeated twice; results from one experiment are shown.
DISCUSSION
We reported previously that, when grown with the conjugal opines, strains harboring a wild-type Ti plasmid exhibited a delay of 8 h in tra gene activation by TraR even when the acyl-HSL was added at saturating levels to low-density cultures (27). This delay contrasts sharply with the induction of luciferase activity in Vibrio fischeri within 30 min after addition of the cognate quormone to low-density cultures (5, 35). We considered two mechanisms that could account for this lag. In the first, some property of TraR itself could prevent rapid activation at saturating quormone levels. Alternatively, some additional element could delay TraR-mediated gene activation even when quormone levels are high.
Our current studies rule out the first possibility. Under conditions in which expression of traR is induced, exposing the cells to the cognate acyl-HSL for as little as 5 min results in a significant level of activation, while a 15-min exposure is enough to activate sufficient amounts of the transcription factor to maximize expression of a TraR-dependent reporter (Fig. 2A). Given that the assay for activation of TraR requires transcriptional activation of a TraR-dependent reporter, which itself takes as long as 30 min (Fig. 1), it is likely that exposure of the cells to the quormone for well under 10 min is sufficient to convert the activator into its functional form. We conclude that the delay in induction observed with the wild-type Ti plasmid is due to some additional control mechanism.
The activity of TraR is antagonized by TraM, a small antiactivator protein (12, 13). TraM binds dimeric TraR and prevents the activator from binding its promoter target sites (14, 21, 34). Our test systems reported here lack TraM. However, in the intact tra regulatory system, TraM prevents activation of the tra regulon resulting from basal expression of traR that occurs in the absence of the conjugal opine (27). In this regard, we propose that the long lag in TraR activation that we observed in quormone-supplemented opine-induced cultures reflects the requirement to produce enough TraR protein to titrate the available TraM. Consistent with this hypothesis, overexpression of TraR overcomes antiactivation by TraM (12, 13).
Although required at a higher concentration, 3-oxo-C6-HSL activated TraR in situ with kinetics similar to those of the native acyl-HSL (Fig. 2B). This result is consistent with the observation that 3-oxo-C6-HSL, the acyl side chain of which is shorter than that of the cognate signal by two carbons (Fig. 1), is a reasonably effective coactivator of TraR (3, 32, 38, 42). In contrast, under the conditions of the induction assay, the alkanoyl quormone C6-HSL failed to activate the reporter to significant levels in cells that were subsequently washed free of the exogenously supplied signal (Fig. 2C). C6-HSL is a particularly ineffective coactivator (3, 32, 42) and a very poor antagonist (42), suggesting that it is not efficiently bound by TraR. However, the TraR-dependent reporter in the control culture that had been continuously incubated with very high concentrations of C6-HSL was activated to normal levels (Fig. 2C).
The contrast in activation levels in the washed cells versus the continuously exposed cells suggested that the intracellular alkanoyl-HSL was removed during the washing regimen. This proved to be the case; after the washing regimen, cells grown with C6-HSL failed to retain detectable levels of intracellular quormone (Fig. 3 and 4B). In contrast, cells grown with either of the 3-oxo forms retained the acyl-HSLs (Fig. 3 and 4A). Strong retention of the 3-oxo-C8 quormone is consistent with the observation that purified TraR in its active dimer form binds its cognate acyl-HSL tightly (30, 40). Since C6-HSL is not strongly retained, we speculate that while the alkanoyl quormone can bind to and activate TraR, the affinity of this signal for the activator is very low.
From these observations, we suggest that, like the radiometric retention assay (16), our bioindicator-based retention assay can provide a reasonable measure of the relative affinity of a given acyl-HSL for TraR. Strains lacking TraR fail to retain detectable amounts of 3-oxo-C8-HSL, while strains expressing wild-type TraR retain the quormone strongly (Fig. 3 and 4A). Moreover, cells expressing mutants of TraR with substitutions at amino acids predicted by crystal structure to play a role in quormone binding (36, 39) fail to retain the acyl-HSL (22).
The strong affinity of 3-oxo-C8 HSL for TraR (30, 40), coupled with its increased half-life (41), suggested that, once formed, ligand-bound dimers, in the absence of other factors, should persist in active form for some time after removal of the exogenous signal. This proved to be the case; as measured by an in vivo repression-based reporter assay (19), TraR activated by 3-oxo-C8-HSL retained DNA-binding activity for at least 8 h after removal of the exogenous acyl-HSL (Fig. 5). TraR activated with 3-oxo-C6-HSL also retained DNA-binding activity for the duration of the experiment. However, TraR from cells grown with effective concentrations of C6-HSL quickly lost DNA-binding activity after removal of the signal (Fig. 6). Taken together, these results are consistent with those of the kinetic analyses and support our suggestion that the two 3-oxo-acyl-HSLs, but not the alkanoyl-HSL, are bound very tightly by TraR.
Quormone binding and subsequent dimerization increase the half-life of TraR some 20-fold (40, 41). TraR is moderately overexpressed in our repression assays, accounting for the retention of TraR activity despite dilution of the activator on a per-cell basis associated with growth of the culture. However, the results suggest that even when expressed at biologically relevant levels from opine-induced Ti plasmids, TraR could persist in its active ligand-bound dimeric form for some period after dissipation of the opine signal and concomitant cessation of traR transcription.
The slow turnover of active TraR introduces the problem of how the Ti plasmid conjugal transfer system is efficiently shut down when the opine signal is no longer available. At the end of exponential growth, some A. tumefaciens strains produce an enzyme that hydrolyzes the homoserine lactone ring of acyl-HSLs (37). However, while the activity of this lactonase prevents the accumulation of newly made signal, it is unlikely to affect existent TraR dimers. Recent structural studies predict that the acyl-HSL ligand is completely enclosed within the interior of each protomer and is not accessible to solvent, much less to an enzyme (36, 39). We suggest that TraM participates in downregulating expression of the tra regulon when the opines become limiting. In this model, loss of the opine signal leads to repression of TraR production. Preformed TraR is then inactivated by TraM, thereby shutting down transcriptional activation of the tra regulon. This model is consistent with the observation that TraR activates expression of TraM (12, 13). Positive control of TraM ensures that the antiactivator will be expressed at levels sufficient to inactivate TraR after repression of expression of the activator gene.
In support of this model, induced expression of TraM results in the rapid inactivation of existing active TraR, as measured by both repression and activation assays (21). Our results suggest that elaborate and efficient regulatory measures exist for downregulating the Ti plasmid conjugation system as well as for activating the system in response to changing environmental conditions.
Acknowledgments
We thank Yinping Qin for helpful advice and the other members of the laboratory for discussions.
The studies reported in this paper were supported by grant R01 GM52465 from the NIH to S.K.F.
REFERENCES
- 1.Adar, Y. Y., and S. Ulitzur. 1993. GroESL proteins facilitate binding of externally added inducer by LuxR protein-containing E. coli cells. J. Biolumin. Chemilumin. 8:261-266. [DOI] [PubMed] [Google Scholar]
- 2.Cangelosi, G. A., E. A. Best, G. Marinetti, and E. W. Nester. 1991. Genetic analysis of Agrobacterium. Methods Enzymol. 204:384-397. [DOI] [PubMed] [Google Scholar]
- 3.Cha, C., P. Gao, Y.-C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 4.Chilton, M.-D., T. C. Currier, S. K. Farrand, A. J. Bendich, M. P. Gordon, and E. W. Nester. 1974. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci. USA 71:3672-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhard, A., C. A. Widrig, P. McBath, and J. Schineller. 1986. Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch. Microbiol. 146:35-40. [DOI] [PubMed] [Google Scholar]
- 6.Evans, K., L. Passador, R. Srikumar, E. Tsang, J. Nezezon, and K. Poole. 1998. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 180:5443-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrand, S. K. 1998. Conjugal plasmids and their transfer. p. 199-233. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae: molecular biology of model plant-associated bacteria. Kluwer Academic Press, Dordrecht, The Netherlands.
- 8.Farrand, S. K., I. Hwang, and D. M. Cook. 1996. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J. Bacteriol. 178:4233-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua, C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuqua, C., and S. C. Winans. 1996. Localization of OccR-activated and TraR-activated promoters that express two ABC-type permeases and the traR gene of Ti plasmid pTiR10. Mol. Microbiol. 20:1199-1210. [DOI] [PubMed] [Google Scholar]
- 11.Fuqua, C., and S. C. Winans. 1996. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J. Bacteriol. 178:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuqua, C., M. Burbea, and S. C. Winans. 1995. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J. Bacteriol. 177:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang, I., D. M. Cook, and S. K. Farrand. 1995. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J. Bacteriol. 177:449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang, I., A. J. Smyth, Z.-Q. Luo, and S. K. Farrand. 1999. Modulating quorum sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol. Microbiol. 34:282-294. [DOI] [PubMed] [Google Scholar]
- 15.Hwang, I., P.-L. Li, L. Zhang, K. R. Piper, D. M. Cook, M. E. Tate, and S. K. Farrand. 1994. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc. Natl. Acad. Sci. USA 91:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan, H. H., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. I. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS carrying different antibiotic resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 18.Li, P.-L., and S. K. Farrand. 2000. The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J. Bacteriol. 182:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo, Z.-Q., and S. K. Farrand. 1999. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc. Natl. Acad. Sci. USA 96:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo, Z.-Q., T. E. Clemente, and S. K. Farrand. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 14:98-103. [DOI] [PubMed] [Google Scholar]
- 21.Luo, Z.-Q., Y. Qin, and S. K. Farrand. 2000. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem. 275:7713-7722. [DOI] [PubMed] [Google Scholar]
- 22.Luo, Z.-Q., A. J. Smyth, P. Gao, Y. Qin, and S. K. Farrand. 2003. Mutational analysis of TraR. Correlating function with molecular structure of a quorum-sensing transcriptional activator. J. Biol. Chem. 278:13, 173-. 13:182. [DOI] [PubMed] [Google Scholar]
- 23.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Oger, P., and S. K. Farrand. 2001. Co-evolution of the agrocinopine opines and the agrocinopine-mediated control of TraR, the quorum-sensing activator of the Ti plasmid conjugation system. Mol. Microbiol. 41:1173-1185. [DOI] [PubMed] [Google Scholar]
- 26.Pearson, J. P., C. V. Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piper, K. R., and S. K. Farrand. 2000. Quorum sensing but not autoinduction of Ti plasmid conjugal transfer requires control by the opine regulon and the antiactivator TraM. J. Bacteriol. 182:1080-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 362:448-450. [DOI] [PubMed] [Google Scholar]
- 29.Piper, K. R., S. Beck von Bodman, I. Hwang, and S. K. Farrand. 1999. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum sensing by the opine regulon in Agrobacterium. Mol. Microbiol. 32:1077-1089. [DOI] [PubMed] [Google Scholar]
- 30.Qin, Y., Z.-Q. Luo, A. J. Smyth, P. Gao, S. Beck Von Bodman, and S. K. Farrand. 2000. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 19:5212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:37-45. [Google Scholar]
- 34.Swiderska, A., A. K. Berndtson, M.-R. Cha, L. Li, G. M. J. Beaudoin III, J. Zhu, and C. Fuqua. 2001. Inhibition of the Agrobacterium tumefaciens TraR quorum-sensing regulator — interactions with the TraM anti-activator. J. Biol. Chem. 276:49449-49458. [DOI] [PubMed] [Google Scholar]
- 35.Ulitzur, S., and J. W. Hastings. 1979. Autoinduction in a luminous bacterium: A confirmation of the hypothesis. Curr. Microbiol. 2:345-348. [Google Scholar]
- 36.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. Di Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, H. B., L.-H. Wang, and L.-H. Zhang. 2002. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:4638-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature (London). 362:446-448. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, R.-G., T. Pappas, J. L. Brace, P. C. Miller, T. Oulmassov, J. M. Molyneaux, J. C. Anderson, J. K. Bashkin, S. C. Winans, and A. Joachimiak. 2002. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature (London) 417:971-974. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, J., and S. C. Winans. 1999. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc. Natl. Acad. Sci. USA 96:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 98:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, J., J. W. Beaber, M. I. Moré, C. Fuqua, A. Eberhard, and S. C. Winans. 1998. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol. 180:5398-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]