Abstract
The general stress regulon of Bacillus subtilis is induced by the activation of the σB transcription factor. Activation of σB occurs when one of two phosphatases (RsbU and RsbP), each responding to a unique type of stress, actuates a positive regulator of σB by dephosphorylation. Nutritional stress triggers the RsbP phosphatase. The mechanism by which RsbP becomes active is unknown; however, its activation coincides with culture conditions that are likely to reduce the cell's levels of high-energy nucleotides. We now present evidence that RelA, a (p)ppGpp synthetase and the key enzyme of the stringent response, plays a role in nutritional stress activation of σB. An insertion mutation that disrupts relA blocks the activation of σB in response to PO4 or glucose limitation and inhibits the drop in ATP/GTP levels that normally accompanies σB induction under these conditions. In contrast, the activation of σB by physical stress (e.g., ethanol treatment) is not affected by the loss of RelA. RelA's role in σB activation appears to be distinct from its participation in the stringent response. Amino acid analogs which induce the stringent response and RelA-dependent (p)ppGpp synthesis do not trigger σB activity. In addition, neither a missense mutation in relA (relA240GE) nor a null mutation in rplK (rplK54), either of which is sufficient to inhibit the stringent response and RelA-dependent (p)ppGpp synthesis, fails to block σB activation by PO4 or glucose limitation.
The general stress regulon of Bacillus subtilis is a collection of approximately 127 genes (15, 26, 27, 37) whose expression is elevated when the bacterium is exposed to physical (e.g., heat or osmotic shock, ethanol treatment) or nutritional (e.g., glucose or PO4 limitation, azide treatment) stress (7, 8, 15, 19, 37, 39, 40). The regulon is controlled by the activity state of the σB transcription factor. As illustrated in Fig. 1, σB is inactive in unstressed B. subtilis due to an inhibitor protein (RsbW) that binds and holds σB unavailable to RNA polymerase (6, 11). σB is released from RsbW when a second regulatory protein (RsbV) binds to RsbW in lieu of σB (11). RsbV is normally inactive due to a RsbW-catalyzed phosphorylation (3, 11, 39). When B. subtilis is exposed to stress, one of two stress-specific phosphatases (RsbP and RsbU) is activated to dephosphorylate phosphorylated RsbV, thereby allowing RsbV to displace σB from the RsbW-σB complex (36, 44).
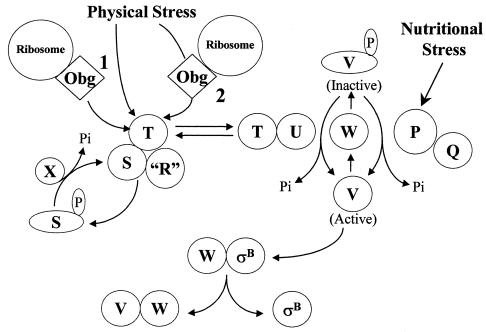
FIG. 1.
Model of σB activation. σB is held inactive in unstressed B. subtilis as a complex with an anti-σB protein, RsbW (W). σB is freed from RsbW when a release factor, RsbV (V), binds to RsbW. In unstressed B. subtilis, RsbV is inactive due to an RsbW-catalyzed phosphorylation (V-P). Physical stress activates an phosphorylated RsbV phosphatase, RsbU (U), which reactivates RsbV. RsbT (T) is the RsbU activator. RsbT is normally bound to a negative regulator, RsbS (S), which inhibits its activity. RsbR and a family of related proteins (R) also bind to RsbS and RsbT and are believed to facilitate their interactions. Upon exposure to stress, RsbT phosphorylates and inactivates RsbS and then activates the RsbU phosphatase. A ribosome-mediated process and Obg, a ribosome associated GTPase that can bind to RsbT, are required for stress to trigger the activation of RsbT. It is unknown whether ribosome- and Obg-dependent processes serve as separate facilitators for stress to activate RsbT (1) or comprise a linear pathway through which stress directly communicates to RsbT (2). RsbS-P is dephosphorylated and reactivated by a phosphatase, RsbX (X), that is encoded by one of the genes downstream of the sigB operon's σB-dependent promoter. RsbX levels become elevated when σB is active, which may facilitate a return of RsbT to an inactive complex with RsbS. Energy depletion activates a separate pathway in which a novel phosphorylated RsbV phosphatase (P) and its associated activator (Q) are triggered, by unknown means, to reactivate RsbV. The model is based on references 1, 4, 6, 8, 11, 18, 19, 30, 39, 40, 42, 44, and 46.
RsbP is the nutritional stress phosphatase. In vivo activation of RsbP requires RsbQ, a predicted hydrolase or acyltransferase, whose gene is cotranscribed with rsbP (9, 36). The metabolic inducer of the RsbP/Q system is unknown. Activation of σB by this system is commonly initiated by allowing B. subtilis cultures to enter stationary phase in rich (i.e., Luria-Bertani) medium, but it can also be brought on by any of a number of treatments which are likely to reduce the cell's energy charge level (e.g., glucose or phosphate limitation, azide treatment) (40). This common feature of the inducing conditions suggested that changes in ATP levels might be the signal that activates RsbP/Q.
RsbU is the phosphatase that responds to physical stress (19, 40, 44). Exposure to environmental insult empowers a positive regulator of this pathway, RsbT, to inactivate its inhibitor (RsbS) by phosphorylation and induce the RsbU phosphatase to reactivate phosphorylated RsbV (44). The RsbS-RsbT interactions are thought to be modulated by RsbR and a family of related proteins; however, the actual roles of the RsbR-like proteins in this process remain undefined (1, 2, 12). As is the case with the nutritional stress pathway, the signal that physical stress generates and the mechanism by which it is conveyed to RsbT are unknown. There is evidence, however, that a ribosome-mediated process may be involved in sensing or signaling physical stress. This notion is based on the failure of physical but not nutritional stress to activate σB in B. subtilis strains that are either deficient in a ribosome-associated GTP-binding protein (Obg) or missing ribosome protein L11 (30, 31, 36).
Strains lacking ribosome protein L11 are not only blocked in the activation of σB by physical stress, but also have an impaired growth rate and are unable to activate the stringent response (33, 43, 46). The stringent response is a process in which amino acid deficiencies can trigger a ribosome-associated protein (RelA) to initiate the synthesis of (p)ppGpp and modulate transcription (10). The loss of RelA has been found by others to be accompanied by lowered induction of some B. subtilis stress response proteins (41). This observation, when taken with the requirement for protein L11 in both the activation of σB by physical stress and the activation of RelA by amino acid starvation, prompted us to examine the potential role of RelA in the activation of σB. We find that RelA does play a role in the activation of σB. However, unlike L11, RelA is not involved in physical stress induction of σB, but is instead an element of σB activation in response to nutritional stress.
MATERIALS AND METHODS
Bacterial strains.
All strains and plasmids used in this study are listed in Table 1. The BSA, BSJ, and BSZ strains are derivatives of PY22. BSA46 carries a specialized SPβ prophage, encoding a translational fusion of the σB-dependent gene ctc to the Escherichia coli lacZ gene (SPβ ctc::lacZ). This fusion allows β-galactosidase activity to be monitored as a measure of σB activity (4). BSZ7 is BSA46 transformed to RelA− by means of a plasmid (pUKRE1) that is incapable of autonomous replication in B. subtilis but carries an internal fragment of the B. subtilis relA gene.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype | Construction, reference, or source |
|---|---|---|
| Bacillus strains | ||
| PY22 | trpC2 | Laboratory strain |
| IS58 | trpC2 lys | 33 |
| IS56 | trpC2 lys relA240GE | 33 |
| BSJ18 | trpC2 lys SPβ ctc::lacZ | SPβ ctc::lacZ→IS58 |
| BSJ19 | trpC2 lys relA240EG SPβ ctc::lacZ | SPβ ctc::lacZ→IS56 |
| BSA46 | trpC2 SPβ ctc::lacZ | 5 |
| BSZ7 | trpC2 SPβ ctc::lacZ relA::pUK-RE1 | pUK-RE1→BSA46 |
| BSZ5 | trpC2 SPβ ctc::lacZ relA::pUS-RE1 | pUS-RE1→BSA46 |
| BSZ28 | trpC2 SPβ ctc::lacZ relA::pUS-RCT | pUS-RCT→BSA46 |
| BSZ66 | trpC2 amyE::spc::relA | pSp-RelA→PY22 |
| BSZ67 | trpC2 relA::pUK-RE1 amyE::spc::relA | BSZ7→BSZ66 |
| BSZ68 | trpC2 relA::pUK-RE1 amyE::spc::relA SPβ ctc::lacZ | BSA46→BSZ67 |
| BSZ9 | trpC2 SPβ ctc::lacZ rplK57 | 46 |
| Plasmids | ||
| pUS-19 | Apr Spcr | 5 |
| pUK-19 | Apr Kmr | 17 |
| pUS-RCT | Apr SpcrrelA 1987-2202 | This study |
| pUK-RE1 | Apr KmrrelA3-542 | This study |
| pUS-RE1 | Apr SpcrrelA3-542 | This study |
| pSp-RelA | ApramyE::Spc::relA | This study |
pUKRE1 was constructed by ligating a PCR-amplified portion of the relA sequence (nucleotides 3 to 542 of the 2,202-nucleotide relA gene [http://www.pasteur.fr/Bio/SubtiList]) from PY22 into pUK19 (17). Transformation of this plasmid into B. subtilis, followed by selection for a plasmid-encoded antibiotic resistance, yields transformants in which the plasmid integrated into the B. subtilis chromosome disrupting relA. relA is the promoter-proximal gene of a bicistronic operon (i.e., relA yrvI). Therefore, BSZ7 is not only RelA− but also has the downstream open reading frame (yrvI) separated from the relA promoter by the vector sequence. BSZ28 is BSA46 transformed with a plasmid (pUS-RCT) that carries the carboxy-terminal sequence (nucleotides 1987 to 2202) of relA in PUS-19 (5). BSZ28 is RelA+ but, like BSZ7, has a plasmid sequence separating yrvI from its putative promoter.
To create a copy of relA for complementation, relA and its promoter were amplified from PY22 with a pair of oligonucleotides that hybridize 146 bases upstream of the relA open reading frame and at the coding sequence for the relA carboxy terminus. The resulting 2.35-kbp fragment was cloned into an amyE integrating vector (pLD30 amyE::spec) and subsequently transformed into PY22, selecting for spectinomycin resistance and Amy−. The resulting strain (BSZ66) was then sequentially transformed with chromosome DNA from BSZ6 (relA::pUK-RE1) with selection for kanamycin resistance (relA3-542), and from BSA46 (SPβ ctc::lacZ) with selection for chloramphenicol resistance to yield BSZ68 (relA::pUK-RE1[relA3-542] amyE::relA SPβ ctc::lacZ). Test transformations of chromosome DNA from BSZ68 into wild-type B. subtilis verified the presence of the relA3-542 allele (small-colony Rel− phenotype linked to kanamycin resistance, AmyE− linked to spectinomycin resistance, and no linkage between kanamycin resistance and spectinomycin resistance).
Culture conditions.
Strains to be stressed by ethanol were grown in LB (29). Glucose and phosphate limitation was investigated in a synthetic medium (35) in which the glucose concentration was lowered from 0.2% to 0.05% or the PO4 concentration was lowered from 0.6 mM to 0.15 mM.
Analysis of (p)ppGpp GTP and ATP levels.
Cells were grown in the synthetic medium (35) used for the glucose/PO4 limitation studies and incubated for at least two generations in the presence of 100 μCi/ml [32P]H3PO4 before the first sample was taken. Cultures were allowed to grow without inhibitor or treated at an optical density of approximately 0.3 with amino acid analogs (norvaline [1.5 mg/ml] or norvaline [1.0 mg/ml] and serine hydroxamate [1 mg/ml]) to trigger amino acid starvation and induce the stringent response. The medium for the norvaline treatment contained tryptophan but lacked Casamino Acids. The 100-μl samples were pelleted, resuspended in 100 μl of 3 M formic acid, subjected to two cycles of freezing and thawing, and centrifuged in an Eppendorf microfuge for 2 min to pellet debris. Supernatant samples (1 to 5 μl) extracted from equivalent numbers of cells (based on optical density), were spotted onto polyethylenemine-cellulose plates (Selecto Scientific) for separation by thin-layer chromatography in 1.5 M KH2PO4 (pH 3.4) (13, 41). Labeled nucleotides were visualized following chromatography by autoradiography and quantified by densitometry with an Alpha Imager 2000 (Alpha Innotech Corp., San Leandro, Calif.) and its associated software. Unlabeled ATP and GTP was spotted on the plates as markers and visualized after chromatography by UV light induced fluorescence. The identities of the labeled pppGpp and ppGpp were inferred by their positions in the chromatograph relative to the origin and GTP (13).
RNA analysis.
Cells were grown in synthetic medium with Casamino Acids to an optical density of 0.5, pelleted, and resuspended in a similar medium either lacking PO4 or without Casamino Acids but containing norvaline (0.05%). Samples were harvested at 2, 40, and 70 min after resuspension and processed for RNA extraction with the Q-BioGene Fast RNA Pro Blue kit (Q-BioGene) following the manufacturer's instructions.
RNA samples were subjected to reverse transcription-PCR with avian myeloblastosis virus reverse transcriptase/Taq DNA polymerase and oligonucleotide primer pairs that would hybridize to nucleotides 44 to 61 and 285 to 304 of the PA transcript region and nucleotides 33 to 52 and 171 to 210 of the PB transcript region. Amplified DNAs were analyzed by polyacrylamide gel electrophoresis (6% acrylamide) and ethidium bromide staining. To verify that the PCR products were the result of RNA amplification and not contaminating DNAs, the amplifications were repeated either without reverse transcriptase or by attempting to PCR amplify the reaction mixtures, reverse transcribed with the oligonucleotide for one of the transcripts (e.g., PA), with the oligonucleotides specific for the second transcript (e.g., PB). In neither case was a DNA product detected.
General methods.
B. subtilis transformation was carried out as described by Yasbin (45). β-Galactosidase assays were performed on chloroform-permeabilized cells, as described by Kenny and Moran (20).
RESULTS
RelA is a component of the σB nutritional stress pathway.
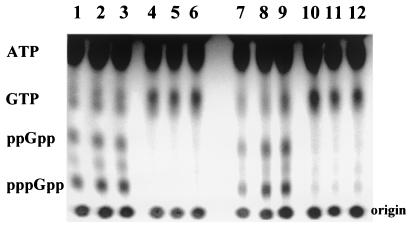
During a characterization of RelA in B. subtilis, it was noted that a RelA− strain had reduced levels of a number of σB-dependent proteins (41). Given RelA's association with ribosomes and our previous evidence for possible ribosome involvement in σB's activation (31, 46), we sought to determine whether RelA could influence either of the σB activation pathways. For this purpose, a null mutation was created in relA (relA3-542) by targeting an integrating plasmid into the interior of relA. RelA− transformants (BSZ5, BSZ7), selected on the basis of the plasmid-encoded antibiotic resistance, had a growth rate that was approximately one third that of their congenic parent (BSA46) (data not shown) and failed to accumulate (p)ppGpp following amino acid starvation (Fig. 2, lanes 4 to 6).
FIG. 2.
Induction of (p)ppGpp synthesis by amino acid limitation. Congenic B. subtilis strains BSA46 (RelA+) [lanes 1 to 3] and BSZ7 (relA3-542) [lanes 4 to 6] and BSJ18 (RelA+) [lanes 7 to 9] and BSJ19 (relA240GE) [lanes 10-12] were grown to optical density 0.3 in synthetic medium with Casamino Acids and 32[P]H3PO4 (100 μCi/ml), pelleted and resuspended in the same medium without Casamino Acid but with norvaline (1 mg/ml). Samples were taken at 20 min (lanes 1, 4, 7, and 10), 40 min (lanes 2, 5, 8, and 11), and 60 min (lanes 3, 6, 9, and 12) after resuspension and analyzed by thin-layer chromatography as described in Materials and Methods. The positions of ATP and GTP were determined with nucleotide markers. The (p)ppGpp positions were inferred from their mobilities relative to GTP (13).
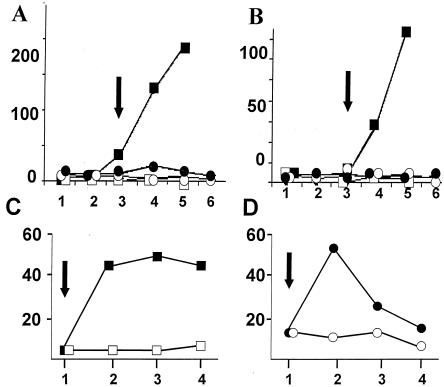
RelA+ and RelA− cultures were subjected to conditions that activate either the nutritional stress (PO4 or glucose limitation) or physical stress (4% ethanol) pathways for σB induction (40). As seen in Fig. 3, RelA+ B. subtilis but not the RelA− variant activated σB-dependent transcription when growth slowed in response to PO4 (Fig. 3A) or glucose (Fig. 3B) limitation. In contrast to the failure of nutritional stress to activate σB in RelA− B. subtilis, ethanol treatment resulted in rapid induction of σB activity in both wild-type and mutant B. subtilis (Fig. 3C and D). These results, as well as the β-galactosidase measurements that follow, depict representative data from assays that were repeated three or more times.
FIG. 3.
Induction of σB by phosphate limitation or ethanol treatment in Rel+/− B. subtilis. BSA46 (RelA+ □, ▪) or BSZ5 (RelA− ○, •) were grown in a synthetic medium containing 0.15 mM (▪, •) or 0.6 mM (□, ○) PO4 (panel A) or 0.05% (▪, •) or 0.2% (□, ○) glucose (panel B). Samples were taken at 30-min intervals and assayed for σB-dependent β-galactosidase activity. The arrows show the times at which the cultures left exponential growth. In panels C and D, BSA46 (C) and BSZ5 (D) were grown continuously in LB (≤) or exposed to 4% ethanol (▪, •) at the point indicated by the arrows. Vertical axes, β-galactosidase (Miller units). Horizontal axes, time (30-min intervals).
relA is the promoter-proximal gene in a bicistronic operon (41; http://www.pasteur.fr/Bio/SubtiList). The function of the second gene of the operon, yrvI, is unproven, but based on homologies, it appears to be a d-tyrosyl-tRNA deacylase. Although it seemed unlikely that a block in yrvI expression could account for the failure of BSZ7 to activate σB, we tested this possibility with a second integrating plasmid, whose cloned relA element included the 3′ end of the relA gene. B. subtilis strains with this plasmid integrated into relA would express an intact relA, but carry the transcriptional disruption of yrvI present in the RelA− strain. RelA+ B. subtilis, with the relA operon interrupted by this plasmid, grew normally and responded like the wild-type strain to either phosphate (Fig. 4A) or glucose limitation (data not shown). To further verify that the loss of RelA was responsible for the block in σB induction, a relA3-542 strain (BSZ68) was constructed which contained a cloned copy of the B. subtilis relA gene and its promoter at the amyE locus. The second copy of relA corrected both the slow-growth phenotype and the σB activation defect (Fig. 4B) of the relA3-542 allele. We conclude that RelA is dispensable for σB induction by physical stress, but it is necessary for the activation of σB's nutritional stress pathway when triggered by either PO4 or glucose limitation.
FIG. 4.
σB activation by PO4 limitation in relA::pUS-RCT and relA3-542 amyE::spc::relA strains. BSA46 (RelA+ [Δ], panels A and B), BSZ28 (RelA+ YrvĪ [▴], panel A), and BSZ68 (relA3-542 amyE::relA [▴], panel B) were grown in low-phosphate synthetic medium, with samples taken at the indicated times for β-galactosidase analyses. The arrows depict the times at which the cultures left exponential growth. Vertical axes, β-galactosidase (Miller units). Horizontal axes, time (minutes).
The stringent response doesn't activate σB.
A requirement for RelA in activating the nutritional stress pathway of σB was unexpected. We and others had previously found no induction of σB following amino acid limitation, the classical activator of RelA (23, 40). Two experiments were undertaken to verify this finding. The first involved examining the ability of a B. subtilis strain, lacking ribosome protein L11, to respond to PO4 limitation and activate σB. The gene encoding L11 (rplK) was originally designated relC based on the inability of strains with mutations at this locus to initiate the stringent response (32, 33, 43). As seen in Fig. 5, the rplK57 strain (BSZ9), unlike the RelA− strain, is still able to respond to PO4 limitation and activate σB. This result demonstrates that even though the nutritional stress pathway has a RelA-dependent component, this component does not require a ribosome protein that is necessary for RelA activation by amino acid limitation.
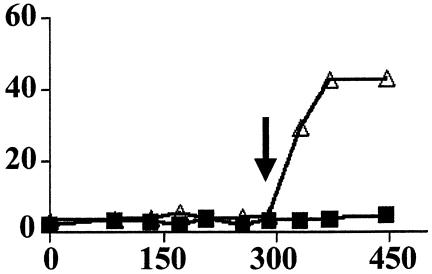
FIG. 5.
Induction of σB by PO4 limitation in RplK̄ B. subtilis. B. subtilis strain BSZ9 (rplK57) was grown in low (Δ) or high (▪) PO4 synthetic medium. Samples were taken at the indicated times (minutes) and assayed for σB-dependent β-galactosidase activity (Miller units). The arrow indicates the time as which the cultures left exponential growth.
To directly examine the possible connection between the traditional stringent response and σB activation, we compared the effects of norvaline, an inducer of the stringent response, to that of PO4 limitation on the activity of a σB-dependent promoter. The sigB operon has two promoters, a σA-dependent promoter providing basal expression of the operon, and an internal σB-dependent promoter that upregulates the expression of the downstream half of the operon (18, 42). With reverse transcription-PCR techniques to follow the activity of these promoters, we observed (Fig. 6) that the activity of σB-dependent promoter (PB) was stimulated by PO4 limitation, but diminished following induction of the stringent response. The activity of the σA promoter (PA) was reduced by both limitation for PO4 and norvaline treatment. Thus, the abundance of the σB-dependent transcript increases relative to the σA-dependent transcript following PO4 limitation, but not norvaline treatment. The observation that a mutation (rplK56), which blocks the RelA-dependent stringent response, fails to prevent σB induction by PO4 limitation, and our confirmation of the finding that amino acid limitation does not activate σB, argue that the RelA-dependent contribution to σB activation is distinct from its role in the stringent response.
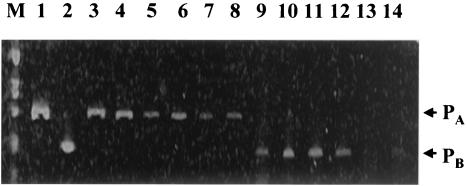
FIG. 6.
Effect of PO4 or amino acid limitation on the activities of the sigB operon promoters. BSA46 (wild-type B. subtilis) was grown in a complete synthetic medium to an optical density of 0.5, pelleted, and resuspended in either a medium without PO4 (lanes 3 to 5, 9 to 11) or lacking Casamino Acids but containing norvaline (0.05%) (lanes 6 to 8, 12 to 14). Samples were taken at 2 (lanes 3, 6, 9, and 12), 20 min (lanes 4, 7, 10, and 13), and 70 min (lanes 5, 8, 11, and 14) after resuspension and analyzed by reverse transcription-PCR with oligonucleotides that would amplify nucleotides +44 to +304 of the σA-dependent transcript (PA) and nucleotides +32 to +210 of the σB-dependent transcript (PB). After amplification, the DNAs were fractionated by polyacrylamide gel electrophoresis (6% acrylamide) and stained with ethidium bromide. Lanes 1 and 2 illustrate the PCR products amplified from B. subtilis chromosomal DNA with the PA- and PB-specific oligonucleotides, respectively. M, 1-kbp DNA ladder (Invitrogen).
Nucleotide changes during activation of σB.
RelA's role as a component of the nutritional stress pathway for σB activation is likely to be tied to a unique aspect of its activity during glucose and PO4 starvation and not the accumulation of (p)ppGpp. Previous models of the induction of σB by nutritional stress envisioned a drop in high energy nucleotides as a possible signal for σB activation. We therefore asked whether this might be the case in the RelA directed activation and if nucleotide levels are differentially altered during PO4 limitation in RelA+/− strains.
To examine this, RelA+ and RelA− strains of B. subtilis were grown to stationary phase in PO4-limited medium. Parallel cultures with or without 32PO4 were sampled for changes in ATP/GTP abundance by thin layer chromatography and σB activity by σB-dependent β-galactosidase activity, respectively. As illustrated in Fig. 7, coincident with a cessation of growth in PO4-limited medium, the RelA+ strain displayed an increase in σB activity and a drop in ATP/GTP (Fig. 7B). Neither the drop in ATP/GTP nor the activation of σB was evident when the Rel+ strain entered stationary phase in a medium with adequate glucose and phosphate (Fig. 7A). Continued incubation of this culture did however lead to a decrease in nucleotide levels and a rise in σB activity after 1.5 h in stationary phase. As expected, the RelA− strain failed to induce σB activity upon entry into stationary phase in the PO4-deficient medium. In addition, it did not display the dramatic decline in ATP/GTP levels that occurred in the RelA+ strain (Fig. 7C).
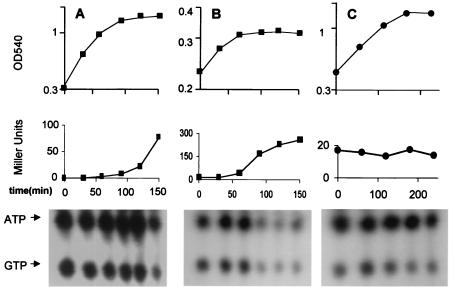
FIG. 7.
Changes in ATP/GTP levels by RelA+/− B. subtilis in low-PO4 medium. Parallel cultures of wild-type B. subtilis (BSA46) or a congenic RelA− strain (BSZ7) were grown in synthetic medium with [32P]H3PO4 (100 μCi/ml), for labeling of nucleotides, or without [32P]H3PO4, for growth and σB-dependent β-galactosidase measurements. (A) BSA46 grown in synthetic medium with high glucose and phosphate. (B) BSA46 grown in low-phosphate medium, (C) BSZ7 grown in low-phosphate medium. Samples were taken and analyzed for growth (optical density) (top panels), β-galactosidase activity (Miller units) (middle panels), and ATP/GTP (thin-layer chromatography) (bottom panels). The positions of ATP and GTP in the thin-layer chromatography system employed were determined with markers as in Fig. 2.
To estimate the degree to which ATP and GTP declined in these cultures, we quantitated several representative chromatograms by densitometry. In PO4-limited medium, the levels of ATP and GTP, present in the stationary phase RelA+ extract dropped to around 20% of that seen in the exponentially growing culture, (Table 2). In contrast, the ATP/GTP levels of either the RelA+ culture in high PO4 medium, or the RelA− culture in either low or high-PO4 medium, persisted at levels that were approximately 80% to 90% of that seen in exponentially growing cultures. Activation of the stringent response by the addition amino acid analogs triggered a predictable drop in GTP levels, due to ppGpp synthesis, but no decline in ATP (Table 2). These results reveal a coincidence between the RelA+ phenotype, nutritional conditions that trigger σB activation, and a drop in ATP/GTP levels.
TABLE 2.
Changes in ATP and GTP
| Strain | Culture conditionsa | Relative nucleotide levelsb (%)
|
|
|---|---|---|---|
| ATP | GTP | ||
| BSA46 (RelA+) | Stationary phase 0.6 mM PO42− | 104.9 ± 2.9 | 79.25 ± 5.25 |
| Stationary phase 0.15 mM PO42− | 19.6 ± 9.3 | 14.8 ± 3.2 | |
| Norvaline (1 mg/ml) + serine hydroxamate (1 mg/ml) | 114.5 ± 5.5 | 31.75 ± 4.75 | |
| BSZ-7 (RelA−) | Stationary phase 0.6 mM PO42− | 82.8 ± 16 | 75 ± 7 |
| Stationary phase 0.15 mM PO42− | 87.3 ± 9 | 88.5 ± 12 | |
B. subtilis grown in [32PO4] was harvested 1 h after growth slowed in either high or low PO4 medium or 40 min after the addition of the indicated amino acid analogs.
Samples were extracted with 3 M formic acid and analyzed as in Fig. 3. Autoradiograms of the separated nucleotides were quantitated by densitometry (Alpha Imager 2000) and compared to the amount of that nucleotide extracted from a similar concentration of cells (based on the O D540) harvested during exponential growth. The data (average of two separate experiments) are expressed as a percentage of the labeled nucleotide in a mid-log-phase culture growing in the same medium.
RelA's activation of σB does not correlate with (p)ppGp accumulation.
PO4 and glucose limitation can induce both σB activity and the synthesis of (p)ppGpp. E. coli has two enzymes, RelA and SpoT, that are capable of synthesizing (p)ppGpp (10). RelA responds to amino acid starvation, while SpoT is triggered by glucose/PO4 limitation. In B. subtilis (p)ppGpp synthesis appears to be solely dependent on a single RelA/SpoT homolog (41). Given that the synthesis of (p)ppGpp has been reported to cause a significant drop in GDP/GTP levels in B. subtilis (16, 25), it seemed plausible that the RelA dependent drop in ATP/GTP levels seen following PO4 limitation might involve such a reaction.
With thin-layer chromatography, we asked whether B. subtilis, subjected to PO4 limitation, with its accompanying decrease in ATP/GTP levels, would display a concomitant accumulation of (p)ppGpp. If an increase in (p)ppGpp paralleled the decrease in ATP/GTP, it would suggest a mechanism by which RelA might directly reduce the cell's ATP/GTP levels. Extracts of 32PO4-labeled B. subtilis entering stationary phase in either a low PO4 or a high PO4 medium, or exposed to an amino acid analogs to activate the stringent response, were separated by thin-layer chromatography and examined for ATP, GTP and (p)ppGpp by autoradiography. The PO4-limited culture (Fig. 8 lanes 1 to 4) displayed the anticipated decrease in ATP/GTP, but failed to accumulate (p)ppGpp at levels comparable to the decline in ATP/GTP or the amount detected in the culture exposed to an amino acid analog (Fig. 8 lanes 9 to 11). Extended (5x) exposure of the chromatogram detected small amounts of ppGpp in both the low and high PO4 cultures (not shown). The failure of (p)ppGpp to accumulate at levels equivalent to the levels of ATP/GTP decline argues that either the ATP/GTP decline isn't a direct result of (p)ppGpp synthesis or that the (p)ppGpp, formed under these conditions, is being rapidly turned over.
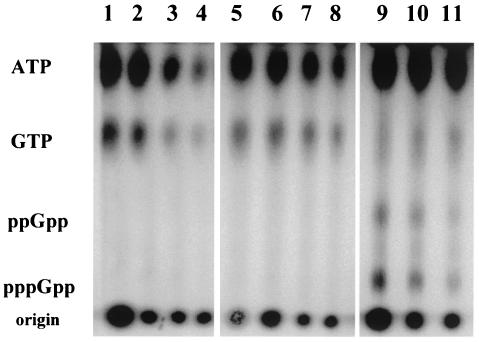
FIG. 8.
Changes in ATP, GTP, and (p)ppGpp by B. subtilis following entry into stationary phase in low or high PO4 medium or amino acid limitation. Wild-type B. subtilis (BSA46) grown in synthetic medium with [32P]H3PO4 (100 μCi/ml) for labeling nucleotides and either limiting (lanes 1 to 4) or nonlimiting PO4 (lanes 5 to 11). At an optical density of 0.4, norvaline (1 mg/ml) and serine hydroxamate (1 mg/ml) were added to a portion of the culture with nonlimiting PO4 (illustrated in lane 5) to mimic amino acid starvation. Samples were taken at 20-min intervals for both the untreated (lanes 6 to 8) and amino acid-limited (lanes 9 to 11) cultures. The low-PO4 and high-PO4 cultures ceased exponential growth at the times represented by lanes 3 and 7, respectively. Growth of the amino acid-limited cultures slowed by the first sample taken after the addition of the amino acid analogs (lane 9). Samples were analyzed by thin-layer chromatography as in Fig. 2.
BSJ19 (IS56) is a relaxed B. subtilis strain. It carries a relA mutation in which a glycine residue, conserved in both RelA and SpoT, is replaced by glutamate (41). B. subtilis carrying this relA allele (relA240GE) has approximately 2% of the (p)ppGpp synthetase activity of wild-type strains (41). BSJ19's inability to synthesize (p)ppGpp while still encoding a RelA protein allows us to ask whether the RelA-dependent drop in ATP/GTP and the inducibility of σB is linked to RelA's (p)ppGpp synthetase activity. In a preliminary experiment to verify the strain's Rel̄ phenotype, we subjected BSJ19 and its congenic parent (BSJ18) to amino acid limitation and examined their accumulation of (p)ppGpp by thin-layer chromatography. As illustrated in Fig. 2, the RelA+ strain (lanes 7 to 9) readily accumulated (p)ppGpp, while these nucleotides were barely detected in the relA240GE strain (lanes 10 to 12). We also noted that, unlike our relA null strain, whose growth rate was only one third of that of its RelA+ parent, the growth rates of BSJ18 and BSJ19 were indistinguishable in rich (LB) medium.
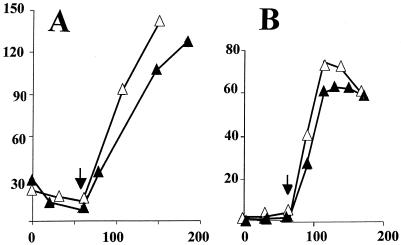
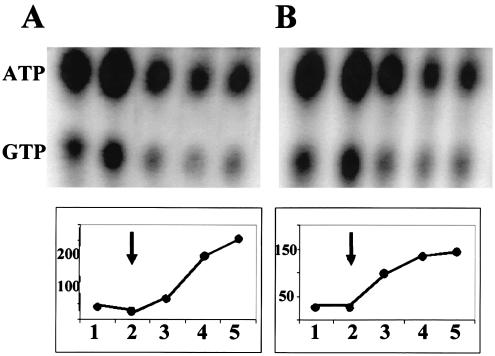
The BSJ18/19 strains were cultured in PO4-limited medium with samples taken to assess both σB induction and possible ATP/GTP changes. As illustrated in Fig. 9, the strain with the relA240GE allele (Fig. 9B) activated σB to a level that was similar (70%) to that of its wild-type counterpart (Fig. 9A) and displayed a reduction in ATP/GTP that was virtually indistinguishable from the RelA+ strain as the cultures' growth slowed. This result suggests that RelA's role in activating the nutritional stress pathway of σB, is not only distinct for its known role in triggering the stringent response, but may involve an activity other than (p)ppGpp synthesis.
FIG. 9.
σB induction and ATP/GTP levels in relA240GE B. subtilis. BSJ18 (RelA+) (A) and its congenic relA240GE strain (BSJ19) (B) were grown in a synthetic medium with limiting PO4. [32P]H3PO4 (100 μCi/ml) was present in parallel cultures for labeling of nucleotides. Samples were taken at 20-min intervals (represented by the numbers on the x axis) and analyzed as in Fig. 7 for σB-dependent β-galactosidase activity (Miller units) and ATP/GTP. The arrows indicate the onset of stationary phase for each culture.
DISCUSSION
Induction of σB by the nutritional stress occurs via the activation of the RsbQ/P phosphatase (9, 36), a process that is triggered by culture conditions consistent with reducing the cells' overall energy level (40). It is plausible, although unproven, that the drop in nucleoside triphosphate that occurs concomitant with σB activation could itself be the signal that communicates nutritional stress to RsbP/Q. The current work reaffirms the coincidence of σB activation with a drop in nucleoside triphosphates, but adds a possible role for RelA in this process.
In an earlier study characterizing relA in B. subtilis, σB-dependent gene products were among a group of proteins whose levels appeared to increase following amino acid depletion in a RelA+ but not a RelA− strain of B. subtilis (41). Although we and others were not been able to induce σB by depletion of amino acids in past (23, 40) or current work (Fig. 6), we now find that RelA influences the RsbP-dependent pathway of σB activation in response to glucose or PO4 limitation. In the original RelA study (41), the authors raised the possibility that the observed induction of σB-dependent proteins might have been due to oxygen limitation rather than amino acid deprivation. Given that oxygen limitation is an inducing condition for σB's nutritional stress pathway (36), this circumstance could reconcile our findings. RelA is clearly involved in the activation of σB by nutritional stress, but, the mechanism by which RelA contributes to this process is not clear.
RelA's best characterized activity is that of a (p)ppGpp synthetase; however it is unlikely that (p)ppGpp itself is the signal that triggers σB induction. Amino acid limitation, an effective inducer of (p)ppGpp synthesis fails to activate σB. A notable difference between the RelA+/− strains is the decline in ATP/GTP levels in the RelA+ strain and the persistence of these nucleotides at relatively stable levels in the RelA− strain, when growth slowed in PO4-limiting medium. In light of the observation that inhibitors of GTP or ATP synthesis (i.e., decoynine, azide) can induce σB in otherwise rich medium (24, 40), this result raises the possibility that a RelA-dependent effect on nucleotide levels, rather than (p)ppGpp synthesis itself, could be the connection between glucose or phosphate limitation and the activation of σB. In support of the idea that RelA's role in σB activation involves facilitating a decline in energy charge, we have observed (S. Zhang, unpublished data) that azide treatment can activate σB in a RelA− strain.
Assuming that RelA can activate σB by directing a decline in ATP/GTP, can this decline be attributed to its activity as a (p)ppGpp synthetase? The synthesis of (p)ppGpp is known to result in a significant drop in GTP/GDP levels (10, 16, 22, 35). The GTP drop has two causes: the use of GTP as a substrate for (p)ppGpp synthesis, and a (p)ppGpp-dependent inhibition of IMP dehydrogenase, an enzyme in the guanine nucleotide biosynthetic pathway (22). This phenomenon is known to affect another stress-activated response of B. subtilis, entry into sporulation. GTP/GDP levels are the intracellular indicators of nutrient availability that trigger endospore formation (21, 22, 28, 34). An order of magnitude drop in GTP, but not ATP, occurs when B. subtilis cultures are induced to sporulate, with RelA's (p)ppGpp synthetase contributing to this GTP decline (21, 22).
It could be speculated that under the culture conditions which we use to activate σB, the synthesis of (p)ppGpp in glucose/PO4 starved cells drops GTP/ATP to a sufficiently low level so as to activate σB's nutritional stress pathway, while adequate carbon and PO4 reserves could prevent a sufficient drop in nucleotide pools during (p)ppGpp synthesis induced by amino acid limitation. This might allow σB activation under one (p)ppGpp inducing condition but not the other.
Two of our findings are not consistent with this model. First, the levels of (p)ppGpp that are formed in response to PO4 limitation are significantly less than the marked drop in ATP/GTP levels that occurs in the culture under this condition (Fig. 8). If the drop in ATP/GTP is a consequence of the channeling of these nucleotides into (p)ppGpp, ppGpp turnover under this condition will need to be invoked to explain its low abundance. Second, a relA variant (relA240GE), whose product has only 2% of the (p)ppGpp synthetase activity of the wild-type RelA (Fig. 2), displays near normal levels of σB activity and ATP/GTP decline following PO4 limitation (Fig. 9). A more likely model might envision a yet to be defined RelA function that affects nucleotide levels, but is not directly tied to RelA's activity as a (p)ppGpp synthetase. The existence of such an unspecified property is suggested by the slow growth phenotype of our “null” relA mutant. If RelA's function is limited to (p)ppGpp synthesis and decay, it is not obvious why relA null mutants, created by us and others (41), display impaired growth, while the missense mutant (relA240GE), whose lesion affects (p)ppGpp synthetase activity, does not.
In light of our previous finding of the likely involvement of a putative ribosome associated GTP binding protein (Obg) in activating the physical stress pathway of σB induction (30, 31, 46), it is intriguing that RelA, another ribosome associating protein, might be involved in activating σB's nutrient-dependent pathway. This coincidence raises the possibility that the ribosome associated processes could be common elements in both pathways of σB activation, with ribosome-associated proteins sensing unique stress-triggered changes in ribosome status and communicating this to the stress-activators of σB.
Acknowledgments
We thank A. L. Sonenshein for advice and helpful discussions.
This work was supported by NIH grant GM-48220 and a grant from the Texas Advanced Research Program.
REFERENCES
- 1.Akbar, S., C. M. Kang, T. A. Gaidenko, and C. W. Price. 1997. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol. Microbiol. 24:567-578. [DOI] [PubMed] [Google Scholar]
- 2.Akbar, S., T. A. Gaidenko, C. M. Kang, M. O'Reilly, K. M. Devine, and C. W. Price. 2001. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor σB of Bacillus subtilis. J. Bacteriol. 183:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper, S., A. Dufour, D. A. Garsin, L. Duncan, and R. Losick. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260:165-177. [DOI] [PubMed] [Google Scholar]
- 4.Benson, A. K., and W. G. Haldenwang. 1992. Characterization of a regulatory network that controls σB expression in Bacillus subtilis. J. Bacteriol. 174:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson, A. K., and W. G. Haldenwang. 1993. Regulation of σB levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson, A. K., and W. G. Haldenwang. 1993. Bacillus subtilis σB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. USA 90:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson, A. K., and W. G. Haldenwang. 1993. The σB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody, M. S., K. Vijay, and C. W. Price. 2001. Catalytic function of an α/β hydrolase is required for energy stress activation of the σB transcription factor in Bacillus subtilis. J. Bacteriol. 183:6422-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. C. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 2. ASM Press, Washington, D.C.
- 11.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-σ factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 176:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaidenko, T. A., X. Yang, Y. M. Lee, and C. W. Price. 1999. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the stress signaling pathway of Bacillus subtilis. J. Mol. Biol. 288:29-39. [DOI] [PubMed] [Google Scholar]
- 13.Gropp, M., E. Eizenman, G. Glaser, W. Samarrai, and R. Rudner. 1994. A relA(S) suppressor mutant allele of Bacillus subtilis which maps to relA and responds only to carbon limitation. Gene 140:91-96. [DOI] [PubMed] [Google Scholar]
- 14.Guérout-Fleury, A.-M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 15.Hecker, M., W. Schumann, and U. Voelker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 16.Inaoka, T., and K. Ochi. 2002. RelA protein is involved in induction of genetic competence in certain Bacillus subtilis strains by moderating the level of intercellular GTP. J. Bacteriol. 184:3923-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju, J., Luo, T., and W. G. Haldenwang. 1998. Forespore expression and processing of the SigE transcription factor in wild-type and mutant B. subtilis. J. Bacteriol. 180:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalman, S., M. L. Duncan, S. M. Thomas, and C. W. Price. 1990. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J. Bacteriol. 172:5575-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, C. M., M. S. Brody, S. Akbar, X. Yang, and C. W. Price. 1996. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor σB in response to environmental stress. J. Bacteriol. 178:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney, T. J., and C. P. Moran, Jr. 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor of Bacillus subtilis. J. Bacteriol. 169:3329-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez, J. M., A. Dromerick, and E. Freese. 1981. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J. Bacteriol. 146:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez, J. M., C. L. Marks, and E. Freese. 1979. The decrease in guanine nucleotides initiates sporulation in Bacillus subtilis. Biochim. Biophys. Acta 587:238-252. [DOI] [PubMed] [Google Scholar]
- 23.Maul, B., U. Voelker, S. Riethdorf, S. Engelmann, and M. Hecker. 1995. σB-dependent regulation of gsiB in response to multiple stimuli in Bacillus subtilis. Mol. Gen. Genet. 248:114-120. [DOI] [PubMed] [Google Scholar]
- 24.Mueller, J. P., G. Bukusoglu, and A. L. Sonenshein. 1992. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J. Bacteriol. 174:4361-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochi, K., J. Kandala, and E. Freese. 1982. Evidence that Bacillus subtilis sporulation induced by the stringent response is caused by the decrease in GTP or GDP. J. Bacteriol. 151:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Voelker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis with a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price, C. W., P. Fawcett, H. Cérémonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 28.Ratnayake-Lecamwasam, M., P. Serror, K.-W. Wong, and A. L. Sonenshein. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Scott, J. M., and W. G. Haldenwang. 1999. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor σB. J. Bacteriol. 181:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott, J. M., J. Ju, T. Mitchell, and W. G. Haldenwang. 2000. The Bacillus subtilis GTP binding protein Obg and regulators of the σB stress response transcription factor cofractionate with ribosomes. J. Bacteriol. 182:2771-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, I., P. Paress, and S. Pestka. 1978. Thiostrepton-resistant mutants exhibit relaxed synthesis of RNA. Proc. Natl. Acad. Sci. USA 75:5993-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, I., P. Paress, K. Cabane, and E. Dubnau. 1980. Genetics and physiology of the rel system of Bacillus subtilis. Mol. Gen. Genet. 178:271-279. [DOI] [PubMed] [Google Scholar]
- 34.Sonenshein, A. L. 2000. Control of sporulation initiation in Bacillus subtilis. Curr. Opin. Microbiol. 3:561-566. [DOI] [PubMed] [Google Scholar]
- 35.Stulke, J., R. Hanschke, and M. Hecker. 1993. Temporal activation of β-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J. Gen. Microbiol. 139:2041-2045. [DOI] [PubMed] [Google Scholar]
- 36.Vijay, K., M. S. Brody, E. Fredlund, and C. W. Price. 2000. A PP2C phosphatase containing a PA5 domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol. Microbiol. 35:180-188. [DOI] [PubMed] [Google Scholar]
- 37.Voelker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Voelker, R. Schmid, H. Mach, and M. Hecker. 1994. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 38.Voelker, U., A. Dufour, and W. G. Haldenwang. 1995. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of σB. J. Bacteriol. 177:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voelker, U., A. Voelker, and W. G. Haldenwang. 1996. Reactivation of the Bacillus subtilis anti-σB antagonist, RsbV, by stress or starvation-induced phosphatase activities. J. Bacteriol. 178:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voelker, U., A. Voelker, B. Maul, M. Hecker, A. Dufour, and W. G. Haldenwang. 1995. Separate mechanisms activate σB of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wendrich, T. M., and M. A. Marahiel. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbial. 26:65-79. [DOI] [PubMed] [Google Scholar]
- 42.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, X., and E. E. Ishiguro. 2001. Involvement of the N terminus of ribosomal protein L11 in regulation of the RelA protein of Escherichia coli. J. Bacteriol. 183:6532-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, X., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]
- 45.Yasbin, R. E., G. A. Wilson, and F. E. Young. 1973. Transformation and transfection of lysogenic strains of Bacillus subtilis 168. J. Bacteriol. 113:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, S., J. M. Scott, and W. G. Haldenwang. 2001. Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor σB. J. Bacteriol. 183:2316-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]