Abstract
In a proteomic analysis of rpoS-deficient Vibrio vulnificus versus the wild type, one of the down-regulated proteins in the rpoS mutant strain was identified as a Fur protein, a ferric uptake regulator. The expression of a fur::luxAB fusion was significantly influenced by sigma factor S, the rpoS gene product, and positively regulated by Fur under iron-limited conditions.
Vibrio vulnificus, a causative agent of septicemia in humans, is a halophilic marine bacterium. Thus, it is thought that this microorganism has survived diverse environmental stresses and has developed efficient survival mechanisms, which involve sigma S (reviewed in reference 9). The biological functions of the rpoS gene were assessed by generating a knockout mutation of this gene in V. vulnificus (K.-J. Park et al., unpublished results). To identify the target genes regulated by this rpoS gene product, we compared the proteomic pattern of the rpoS knockout strain with that of the wild-type strain. Interestingly, one of proteins down-regulated in extracts of the rpoS mutant strain turned out to be Fur, which functions as a transcriptional repressor for the genes involved in iron acquisition and iron metabolism in many bacteria (4).
In nature, iron is present in an insoluble form, which is unavailable to microorganisms (16). Animal pathogens encounter an additional difficulty in obtaining iron because iron is present not in a free form but as complexes with iron-binding proteins in a mammalian host (1) as well as bound by the siderophore-mediated iron transport systems produced by normal-flora microbes. As a consequence, the synthesis of many toxins and virulence determinants is regulated by the intracellular iron concentration of the bacterial cells (10, 14). The correlation between the virulence of V. vulnificus and iron availability (19) suggests that iron is particularly important in the pathogenesis of V. vulnificus infections. The gene encoding Fur of V. vulnificus has been cloned (11), and it was later discovered that the synthesis of two outer membrane proteins—HupA, a heme uptake receptor, and VuuA, a ferric vulnibactin receptor—was regulated by iron concentration via the action of the Fur protein (12, 18).
In this study, the decreased level of Fur protein in rpoS mutant V. vulnificus was confirmed at a transcriptional level by using the fur::luxAB fusion, and the physiological connection between these two control systems in response to iron was investigated.
Reduced production of the Fur protein in an rpoS knockout mutant of V. vulnificus.
Both the wild type and the rpoS mutant of V. vulnificus were cultivated in Luria-Bertani broth (1% tryptone, 0.5% yeast extract) supplemented with 2.5% (wt/vol) NaCl (LBS) to the stationary phase. Cell extracts were prepared in a 2-dimensional gel electrophoresis (2DGE) sample buffer (5 M urea, 2 M thiourea, 0.1% carrier ampholytes, 2% [wt/vol] sulfobetaine, 2 mM tributylphosphine), and then used for overnight rehydration of either pH 3 to 10 or pH 4 to 7 immobilized pH gradient (IPG) gel strips (13 cm; Amersham Pharmacia Biotech). A three-phase program was used for the isoelectric focusing: the first phase was set at 1,000 V for 1 h, the second phase was at 2,000 V for 2 h, and the third phase was a linear gradient from 2,000 to 8,000 V over 14 h. The second-dimension separation was carried out at room temperature on sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gels (16 by 20 cm) without stacking gels. After electrophoresis at 60 mA gel−1 for 6 h, the proteins were visualized by silver staining.
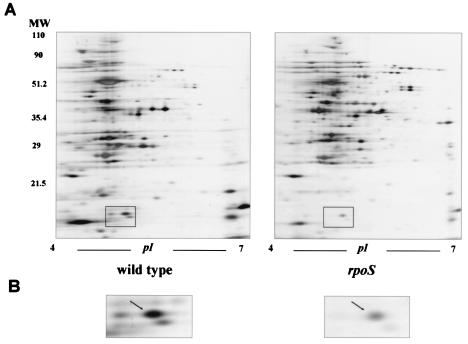
According to 2DGE analysis using a pH 3 to 10 IPG strip, the majority of V. vulnificus proteins clustered between pH 4.0 and 7.0 and had molecular weights between 30,000 and 100,000 (data not shown). In another separation using a pH 4 to 7 IPG strip, the protein spot patterns of the two strains, i.e., the wild type and the rpoS knockout mutant, were compared by using the PDQuest program (Fig. 1). A high degree of conservation was observed between these two strains within the majority of the encoded amino acid sequences. Protein spots showing differential synthesis between the two strains were digested with trypsin (6, 17). Mass-spectrometric analyses were performed with a PerSeptive Biosystems (Framingham, Mass.) matrix-assisted laser desorption ionization-time of flight (MALDI/TOF) Voyager DE-RP mass spectrometer operated in delayed extraction and reflector mode. Peptide mixtures were analyzed by using a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile-0.1% trifluoroacetic acid (5). The PEPTIDENT program of ExPASY was used for database searching. A protein spot (17 kDa, pI = 5.4) down-regulated in the rpoS mutant of V. vulnificus showed homology to the Fur proteins of gram-negative bacteria, including that of Haemophilus ducreyi (accession number P71333) (2) (Fig. 1). The Fur protein spot was clearly larger in extracts from the wild type than in extracts from the rpoS mutant (Fig. 1B). However, a low level of Fur was still detected in the mutant V. vulnificus, indicating that significant amounts of Fur were present in this strain at the stationary phase, from which cellular extracts were prepared for 2DGE.
FIG. 1.
(A) 2D protein patterns of wild-type V. vulnificus AR and of its isogenic rpoS knockout mutant. The box indicates a spot down-regulated in the rpoS mutant and identified as Fur by subsequent MALDI/TOF analysis. (B) Enlarged views of the areas on the 2D gels representing Fur.
Decreased expression of the fur::luxAB fusion in rpoS knockout V. vulnificus.
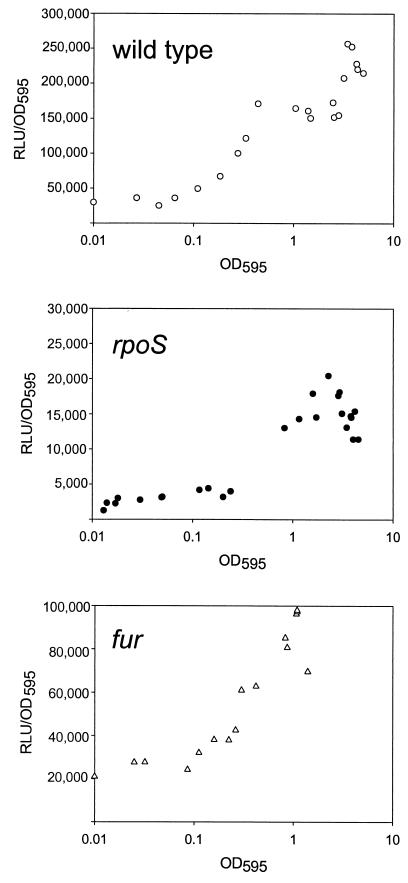
Based on the sequences published by Litwin and Calderwood (11) (GenBank database accession number, AAL67892), the oligonucleotides furp-F and furp-R (Table 1) were made and then used to amplify a 426-bp DNA fragment encoding the first 12 amino acids of the Fur protein and the 382-bp fur promoter region. A KpnI site and a BamHI site, located at either end of the resultant fur DNA, were used to clone this DNA into the corresponding sites of pHK0011, a luxAB reporter vector (3). The resultant fur::luxAB fusion, pHL01, was mobilized either to the wild-type V. vulnificus AR or to the rpoS mutant strain. The levels of expression of this fusion in both strains were monitored throughout the growth stage. Overnight cultures were diluted 100-fold with fresh LBS containing tetracycline (3 μg ml−1) and grown with shaking at 30°C. Luciferase activities were determined with a luminometer by adding n-decyl aldehyde solution (0.006% in 20 mM Tween 80) to the cultures and expressed as specific bioluminescence by dividing the number of relative light units by the cell density (optical density at 595 nm [OD595]) of the cultures. In wild-type V. vulnificus, the expression of fur::luxAB increased about ninefold as the cells entered the stationary phase (Fig. 2A). As expected from its proteomic pattern, the expression of fur::luxAB was significantly lower in rpoS knockout V. vulnificus; and less than 10% of the wild-type expression was observed in the rpoS mutant at all growth stages (Fig. 2B). Despite the lower expression, an increase of fur::luxAB expression at the stationary phase was also observed in the rpoS mutant.
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristic(s) or nucleotide sequence | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Laboratory collection |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Muλpir, OriT of RP4 Kmr | Laboratory collection |
| V. vulnificus | ||
| ATCC 29307 | Clinical isolate | 7 |
| AR | ATCC 29307, Rifr | Park et al., unpublished |
| KPR101 | AR, ΔrpoS | Park et al., unpublished |
| MO6-24/O | Clinical isolate | 19 |
| MR | MO6-24/O, Rifr | This study |
| HLM101 | MO6-24/O, Δfur | This study |
| Plasmids | ||
| pRK415 | Broad-host-range plasmid | 8 |
| pHK0011 | pRK415, a promoterless luxAB, Tcr | 3 |
| PHL01 | pHK0011, fur::luxAB | This study |
| PHL02 | pHK0011, hupA::luxAB | This study |
| pBluescript SK(+) | pUC19 | New England Biolabs |
| pSKfurup | pBluescriptSK(+), 0.4-kb fur upstream DNA | This study |
| PSKfurup/down | pBluescriptSK(+), 0.9-kb Δfur | This study |
| pDM4 | oriR6K Mob RP4 Cmr | 15 |
| pDMΔfur | pDM4, 0.9 kb Δfur | This study |
| Used for construction of HLM101 | ||
| Primers | ||
| furp-F | 5′-CCCCGGTACCACTCCCGCCATACTGAG-3′ | This study |
| furp-R | 5′-ACCAGGATCCTTTAGCCGTTGGTTATTGTCT-3′ | This study |
| furup-F | 5′-TAAAGAGCTCATACTCCCGCCATACTGA G-3′ | This study |
| furup-R | 5′-ACCAGGATCCTTTAGCGCTTGGTTATTGTCT-3′ | This study |
| furdown-F | 5′-CATGGGATCCTAGTTTGTCTCGACTGCGG-3′ | This study |
| furdown-R | 5′-AAGCGGTACCTGAAAAAGGCTATCAGTTTGA-3′ | This study |
| hupAp-F | 5′-CGCGGGTACCAAGGTTATGGGGCTTTGCA-3′ | This study |
| hupAp-R | 5′-GGGCTCTAGAAGGAACGTTCTGTTGTACAT-3′ | This study |
FIG. 2.
Expression patterns of fur::luxAB (pHL01) in the wild type (AR), the rpoS knockout mutant (KPR101), and the fur knockout mutant (HLM101). V. vulnificus fur::luxAB activities were monitored throughout the growth stages and plotted against cell OD595. Luciferase activities are expressed as normalized values. The results are from a single representative experiment from experiments performed in triplicate. RLU, relative light units.
The promoter region of the fur gene has been determined by primer extension (11), demonstrating a single transcript with a well-conserved −10 region but without any −35-like sequences. The observation that the expression of fur::luxAB increased as cells entered the stationary phase suggested that the fur gene may be transcribed by RNA polymerase containing sigma S under certain conditions. However, this growth-phase-dependent increase in the fur::luxAB expression was also observed in V. vulnificus without a functional RpoS. Thus, it is premature to speculate that RpoS is directly involved in the expression of the fur gene as a component of RNA polymerase at the present level of knowledge. However, RpoS was involved in the overall expression of the fur gene at all growth stages in an unidentified way. To understand the exact function of RpoS in fur expression, extensive analysis of the use of the fur promoter region should be performed in different strains of V. vulnificus during the exponential and stationary phases.
Effect of iron on the fur::luxAB expression.
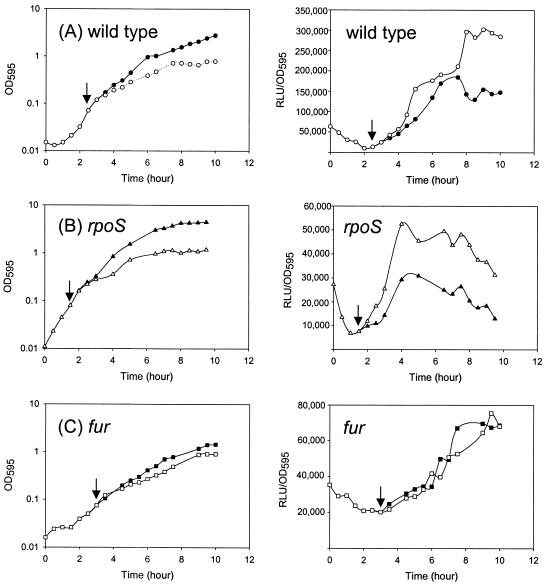
The effects of iron availability on the expression of the fur gene were examined by adding 2,2′-dipyridyl, an iron chelator, to cultures at the early exponential stage (OD595 of ∼0.1). Upon the addition of 2,2′-dipyridyl, the cellular growth of both strains, the wild type and the rpoS mutant, was retarded (Fig. 3A and B). Five hours after the addition of the iron chelator, the activity of fur::luxAB in the wild type increased about twofold versus that in untreated V. vulnificus. Several concentrations of 2,2′-dipyridyl ranging from 0.01 to 1 mM were tested. It was found that the maximal expression of fur::luxAB occurred at 0.6 mM (data not shown). However, we chose to use 0.2 mM 2,2′-dipyridyl in the following experiments, because at this level, fur::luxAB expression was significantly induced and the toxic effect of 2,2′-dipyridyl of cellular growth was minimized (data not shown). Regardless of the presence of iron chelators, the expression of fur::luxAB was induced in the wild type as the cells entered the stationary phase. However, in the case of the rpoS mutant, the overall activity of fur::luxAB decreased in both the presence and absence of 2,2′-dipyridyl. Despite the lower level of expression of fur::luxAB, the induction of its expression by the iron chelator was observed in the rpoS mutant strain.
FIG. 3.
Effects of iron availability on the expression of fur::luxAB (pHL01) in the wild type, the rpoS mutant, and the fur mutant. Iron in the medium was depleted by adding 0.2 mM 2,2′-dipyridyl to one of two cultures at the times indicated by the arrows. Culture OD595 and luciferase activity were determined in the absence (closed symbols) and presence (open symbols) of 2,2′-dipyridyl. Luciferase activities (right panels) are expressed as normalized values. The results are from a single representative experiment from experiments performed in triplicate.
To examine the role of Fur in the iron control of fur expression, a fur knockout V. vulnificus was made by three series of recombinations. A PCR product containing a 420-bp upstream sequence of the Fur open reading frame (PCR product obtained with the primers furup-F and furup-R) (Table 1) was cloned into the SacI and BamHI sites of pBluescript SK(+) to produce pSKfurup. In addition, a 528-bp downstream sequence of the fur gene, the PCR product of the primers furdown-F and furdown-R (Table 1), was cloned into the BamHI and KpnI sites of pSkfurup, resulting in pSKfurup/down, which contains a DNA fragment with regions adjacent to fur but with fur deleted. This DNA was cloned into the suicide vector pDM4 (15), yielding pDMΔfur, which was then mobilized from Escherichia coli SM10λpir into a rifampin-resistant derivative of the wild-type strain V. vulnificus MO6-24/O. The resulting fur deletion mutant, HLM101, was then selected.
The fur::luxAB fusion was also transferred to the parental strain MO6-24/O, or HLM101, and its expression was monitored. The expression pattern of fur::luxAB in MO6-24/O was basically similar to that in the AR strain (data not shown). The cellular growth of the V. vulnificus fur mutant was slightly retarded in the presence of an iron chelator and impaired even under iron-supplemented conditions (i.e., in the absence of 2,2′-dipyridyl). The expression level of fur::luxAB in the fur mutant strain was reduced to 50% of that in the wild-type strain (Fig. 2C). The growth stage-dependent induction of fur::luxAB expression was, however, maintained in the fur mutant strain. The fur::luxAB activities in the fur mutant strain remained consistently a half or a third of that of the wild type regardless of the presence of an iron chelator in the medium. Of note, no distinct induction of the expression of fur::luxAB was observed after the addition of 2,2′-dipyridyl to the fur mutant strain (Fig. 3C).
Therefore, we found that Fur directly or indirectly autoregulates its own expression in a rather unusual mode other than via RpoS-dependent fur expression. fur expression decreased significantly when V. vulnificus was deficient in Fur protein, though its effect was less than that of the rpoS knockout mutation. As expected, fur expression was induced by iron depletion in the wild type (Fig. 3), and its induction by iron depletion is retained in the rpoS mutant even though its overall expression in this strain is low under any conditions. Interestingly, the induction of fur expression under iron-depleted conditions was abolished in a fur mutant background (Fig. 3). In this case, Fur seems to serve as a transcriptional activator for its own expression under iron-depleted conditions, which is a different type of regulation from its well-characterized function as a repressor under iron-supplemented conditions. The definition of the nature of this autoregulation requires further investigation to determine whether Fur exerts an effect on its own expression by directly interacting with its own promoter or through interacting with other unidentified factors. A set of genes were previously shown to be positively regulated by Fur in E. coli. These included two ferritin genes (ftnA and bfr), a gene encoding superoxide dismutase, and some genes of the tricarboxylic acid cycle. This positive regulation by Fur was achieved indirectly, by repressing a small RNA, ryhB, in the presence of iron (13). Homology searches of the GenBank databases for the nucleotide sequence of the ryhB gene of E. coli (accession number AF480876) resulted in the identification of two candidate sequences of the ryhB gene of V. vulnificus. One of these was located in the noncoding region between the genes coding for DNA polymerase I and δ-aminolevulinic acid dehydratase on the chromosome I (accession number AE016800), whereas the other was located between the genes for a methyl-accepting chemotaxis protein and a periplasmic protein of the ABC-type phosphate transport system on chromosome II (accession number AE016813). Further study will be focused on the in vitro analysis of the fur promoter-Fur interaction as well as on the functional analysis of these ryhB-homologous genes.
Expression of the hupA::luxAB fusion in V. vulnificus.
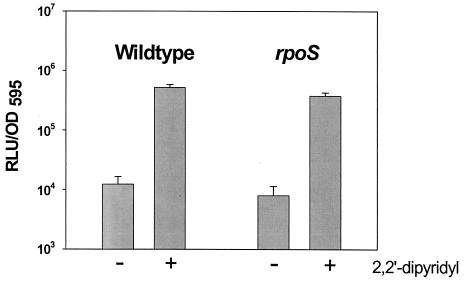
An important question that should be answered is that of the physiological significance of the connection between RpoS and Fur. Therefore, we examined whether RpoS is essential for the proper functioning of Fur with respect to iron control of the hupA gene. The hupA gene, encoding the heme uptake receptor in V. vulnificus, has been identified as a Fur-regulated gene, which is induced by the addition of an iron chelator (12). We constructed a transcriptional fusion between the promoter region of hupA and the luxAB gene and used this to examine the dependency of hupA expression on Fur and RpoS regulators. An upstream sequence of the hupA gene was cloned from the genomic DNA of V. vulnificus by using the primers hupAp-F and hupAp-R (Table 1) (12) (GenBank database accession number AF047484). A PCR-amplified hupA DNA product containing a 26-bp HupA coding region and a 254-bp promoter region was used to generate a hupA::luxAB fusion, pHL02. In wild-type V. vulnificus, the activities of hupA::luxAB were comparatively low in the absence of 2,2′-dipyridyl but were dramatically induced in the presence of the iron chelator (Fig. 4). The same pattern of induction of hupA::luxAB under iron-depleted conditions was also observed in the rpoS mutant strain. This result implies that even though less than 10% of Fur protein is present in the rpoS mutant cells relative to the wild type, this level of Fur protein is enough to properly repress the hupA gene under iron-supplemented conditions. In the fur mutant of V. vulnificus, the activities of hupA::luxAB were significantly increased (more than 100-fold) under iron-supplemented conditions (data not shown). Thus, the effect of iron on hupA expression is mediated by Fur, as was previously observed by using a different method (12).
FIG. 4.
Effect of iron on hupA::luxAB (pHL02) expression in the wild type (AR) and in the rpoS mutant. Iron in the medium was depleted by adding 0.2 mM 2,2′-dipyridyl. The hupA::luxAB activities were normalized by dividing the number of relative light units (RLU) by the OD595. The hupA::luxAB activities of four independent cultures at the exponential phase (OD595 = ∼0.5) were averaged and are indicated, with their standard deviations.
Microorganisms adapt to various stresses by modulating the expressions of a series of genes in appropriate amounts and in a timely fashion. RpoS and Fur are key transcription factors which play roles in survival during the stationary phase and in adaptation to limited iron levels, respectively. Extensive investigations have been performed on the molecular mechanisms of these regulatory proteins. However, no relationship between these two regulons has been reported. As a result of an attempt to identify target genes regulated by RpoS in V. vulnificus, Fur was discovered to be a member of the RpoS regulon. The dependency of Fur production on RpoS was confirmed by two independent methods, proteome and gene fusion analyses. In particular, the expression of fur was found to be significantly impaired when V. vulnificus was deficient in RpoS, implying that RpoS is involved in Fur synthesis in an unidentified way. Attempting to uncover the molecular relationship between RpoS and Fur regulons becomes complicated, since the expression of rpoS is also controlled by iron concentration (K.-J. Park and K.-H. Lee, unpublished results). Thus, the RpoS-dependent expression of the fur gene we observed in this study may be a net expression of the reciprocal actions of RpoS and Fur. In addition, Fur and RpoS may control the expression of fur gene in different manners which depend on the physiological conditions of the bacterial culture. For example, RpoS and Fur may function cooperatively in the fur regulation of V. vulnificus in the stationary phase, whereas Fur may be the sole modulator of fur expression under iron-depleted conditions.
In this study, RpoS was identified, for the first time, as a major factor in the expression of fur in V. vulnificus. However, little is understood at present regarding the physiological significance of RpoS-dependent fur expression. Future investigations should focus on defining this connection between iron control (Fur regulon) and survival control (RpoS regulon).
Acknowledgments
This research was supported by a grant (MG02-0201-004-1-0-1) from the 21C Frontier Microbial Genomics and Applications Center Program of Ministry of Science and Technology, Republic of Korea.
REFERENCES
- 1.Aisen, P., and A. Leibman. 1972. Lactoferrin and transferrin: a comparative study. Biochem. Biophys. Acta 257:314-323. [DOI] [PubMed] [Google Scholar]
- 2.Carson, S. D., C. E. Thomas, and C. Elkins. 1996. Cloning and sequencing of a Haemophilus ducreyi fur homolog. Gene 176:125-129. [DOI] [PubMed] [Google Scholar]
- 3.Choi, H. K., N. Y. Park, D. Kim, H. J. Chung, S. Ryu, and S. H. Choi. 2002. Promoter analysis and regulatory characteristics of vvhBA encoding cytolytic hemolysin of Vibrio vulnificus. J. Biol. Chem. 277:47292-47299. [DOI] [PubMed] [Google Scholar]
- 4.Escolar. L., J. Perez-Matin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharahdaghi, F., M. Kirchner, J. Fernandez, and S. M. Mische. 1996. Peptide-mass profiles of polyvinylidene difluoride-bound proteins by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in the presence of nonionic detergents. Anal. Biochem. 233:94-99. [DOI] [PubMed] [Google Scholar]
- 6.Gharahdaghi, F., C. R. Weinberg, D. A. Meagher, B. S. Imai, and S. M. Mische. 1999. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20:601-605. [DOI] [PubMed] [Google Scholar]
- 7.Jeong, H. S., K. C. Jeong, H. K. Choi, K. Park, K. Lee, J. H. Rhee, and S. H. Choi. 2001. Differential expression of Vibrio vulnificus elastase gene in a growth phase-dependent manner by two different types of promoters. J. Biol. Chem. 276:13875-13880. [DOI] [PubMed] [Google Scholar]
- 8.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1998. Short communications improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 7:191-197. [DOI] [PubMed] [Google Scholar]
- 9.Kolter, R., D. A. Siegel, and A. Tormo. 1993. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 47:855-874. [DOI] [PubMed] [Google Scholar]
- 10.Konopka, K., A. Bindereif, and J. B. Neilands. 1982. Aerobactin-mediated utilization of transferrin iron. Biochemistry 21:6503-6508. [DOI] [PubMed] [Google Scholar]
- 11.Litwin, C. M., and S. M. Calderwood. 1993. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J. Bacteriol. 175:706-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litwin, C. M., and B. L. Byrne. 1998. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect. Immun. 66:3134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milton, D. L., A. Norqvist, and H. Wolf-Watz. 1992. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J. Bacteriol. 174:7235-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 17.Shevchenko, A., M. Willm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 18.Webster, A. C. D., and C. M. Litwin. 2000. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 68:526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]