Abstract
Mechanical forces arising from strain, pressure, and fluid shear stress are sensed by cells through an unidentified mechanoreceptor(s) coupled to intracellular signaling pathways. In vascular endothelial cells, fluid shear stress is transduced via pathway(s) involving heterotrimeric guanine nucleotide-binding proteins (G proteins) by molecular mechanisms that are unknown. In the present study, we investigated the activation of purified G proteins reconstituted into phospholipid vesicles. Vesicles containing G proteins were loaded with [γ-32P]GTP and subjected to physiological levels of fluid shear stress in a cone-and-plate viscometer. Steady-state GTP hydrolysis was measured as an index of G protein function. Shear stress (0–30 dynes/cm2) activated G proteins in dose-dependent manner (0.48–4.6 pmol/min per μg of protein). Liposomes containing lysophosphatidylcholine (30 mol %) or treated with benzyl alcohol (40 mM), conditions that increase bilayer fluidity, exhibited 3- to 5-fold enhancement of basal GTPase activity. Conversely, incorporation of cholesterol (24 mol %) into liposomes reduced the activation of G proteins by shear. These results demonstrate the ability of the phospholipid bilayer to mediate the shear stress-induced activation of membrane-bound G proteins in the absence of protein receptors and that bilayer physical properties modulate this response.
Mechanotransduction is a phenomenon that enables cells to perceive and respond to stimuli such as strain, pressure, and fluid shear stress (1). The molecular mechanisms by which cells sense and transduce external forces into intracellular biochemical events are not well understood. Such mechanisms require virtually instantaneous activation to provide rapid accommodation to an altered environment (2). In sensory transduction systems such as photoreception, taste, and olfaction, signaling pathways involve G protein activation and are linked to seven-transmembrane-domain receptors (3). Many shear stress-induced responses are also mediated by G proteins (1), with both pertussis toxin-sensitive (Gi) and -insensitive (Gq) subunits rapidly activated by fluid shear in human vascular endothelial cells (4). However, the mechanism(s) of G protein activation by shear stress is unknown.
We hypothesize that shear stress is transduced via the lipid bilayer to directly activate heterotrimeric G proteins on the cytosolic face of the plasma membrane. Several lines of evidence suggest the plausibility of such a mechanism. First, shear stress can directly affect membrane physical properties such as membrane permeability (5). Second, membrane physical properties can regulate the function of membrane proteins (6). Finally, G proteins are covalently modified with a lipid tail (7, 8) ensuring their association with the membrane. These lipid modifications contribute to the interaction of G protein subunits by restricting their relative mobility to the two-dimensional plane of the lipid bilayer (9). Direct lipid–lipid interactions also play a role in subunit association and nucleotide exchange (10). Despite its potential importance in signal transduction, the role of the phospholipid bilayer has not been extensively investigated.
We sought to test the hypothesis that shear stress activates G proteins via the lipid bilayer by reconstituting purified G proteins into phospholipid vesicles of defined composition, loading the reconstituted vesicles with GTP, and subjecting the vesicles to a shear field. We find that shear stress activates GTP hydrolysis by G proteins in a vectorial (transbilayer) manner. In addition, we observe a modulation of steady-state and shear-activated GTP hydrolysis by membrane lipid composition.
EXPERIMENTAL PROCEDURES
Materials.
Phosphatidylethanolamine (PE) and phosphatidylserine (PS) were purchased from Avanti Polar Lipids. Lysophosphatidylcholine (LPC), benzyl alcohol, trypsin, soybean trypsin inhibitor, dithiothreitol, sodium cholate, Sephadex G-50, and phenylmethylsulfonyl fluoride were from Sigma. Rabbit polyclonal antibodies specific to Gα subunits and common Gβ subunit, mastoparan, leupeptin, aprotinin, pepstatin A, and cholesterol were obtained from Calbiochem. Column matrices for protein purification DEAE-Sepharose, Ultrogel AcA 34, and phenyl-Sepharose were purchased from Pharmacia Biotechnology. GTP, GDP, guanosine 5′-O-(3-thiotriphosphate (GTP[γ-S]), and Thesit (C12E9, nonaethylene glycol monododecyl ether) were from Boehringer Mannheim, and aminolink reagents kit for antibody immobilization was from Pierce. [γ-32P]GTP (3,000 Ci/mmol; 1 Ci = 37 GBq) was from Amersham and 35S-labeled GTP[γ-S] (GTP[γ-35S]; ≈1,100–1,300 Ci/mmol) was from DuPont NEN. All other chemicals were of analytical grade and obtained from Sigma.
G Protein Purification.
Heterotrimeric G proteins were purified from bovine brain by using previously described procedures by Sternweis and Robishaw (11). G protein purification was achieved by sequential ion-exchange, gel-filtration, and hydrophobic column chromatography. G proteins were monitored by SDS/PAGE/immunoblotting and by their ability to bind GTP[γ-S] irreversibly. All steps of the purification procedure were carried out at 4°C, and protease inhibitors (2 μg/ml each leupeptin, pepstatin A, aprotinin, and phenylmethylsulfonyl fluoride) were included in all buffers.
G protein α subunits Gq and Gi3 were affinity purified by adopting the procedures described by Pang and Sternweis (12) for α subunit purification using immobilized βγ subunits. Polyclonal antibodies specific for α subunits were amino-linked to agarose beads by using a aminolink immobilization kit (Pierce). Heterotrimeric G proteins purified from bovine brain were added to antibody immobilized on agarose beads (250 μl) in 20 mM sodium Hepes, pH 7.4/1 mM EDTA/3 mM dithiothreitol/0.5% Thesit/150 mM NaCl/5 μM GDP, mixed overnight at 4°C, and packed into a small column. Immunoaffinity column with bound proteins was washed extensively with the same buffer. Proteins specifically bound to agarose beads were eluted with acidic pH buffer (100 mM glycine⋅hydrochloride, pH 2.8) directly into tubes containing 1 M Tris base and dialyzed against 20 mM sodium Hepes, pH 7.4/1 mM EDTA/3 mM dithiothreitol/0.5% Thesit/150 mM NaCl/5 μM GDP with protease inhibitors. Proteins in the eluted fractions were monitored by SDS/PAGE, and Western blots were probed with antibodies specific for G protein α and β subunits.
G Protein Reconstitution.
The nearly homogenous G protein preparation was reconstituted into liposomes by using detergent-mediated methods described by Cerione and Ross (13). The reconstitution of purified G proteins into phospholipid vesicles was typically performed as follows: 0.5 vol of bovine brain PE (0.6 mg/ml) and PS (0.4 mg/ml), 0.4% sodium deoxycholate, and 0.04% sodium cholate in reconstitution buffer (20 mM sodium Hepes, pH 8.0/3 mM MgCl2/1 mM EDTA/100 mM NaCl) is mixed with 0.1 vol of G proteins (3 nmol) in 10 mM sodium Hepes, pH 8.0/0.1 mM dithiothreitol/1 mM EDTA/0.1% Thesit on ice and extruded through polycarbonate membrane (0.1 μm). Vesicles were applied onto a Sephadex G-50 column, from which they were eluted in the void volume. Protein reconstitution efficiency was measured in each preparation and ranged from 37% to 41%.
In some experiments, reconstituted liposomes were digested with trypsin for 3 min at 37°C (trypsin, 100 units) to remove exofacial G proteins. After incubation, soybean trypsin inhibitor was added and the enzyme and inhibitor were immediately separated on a Sephadex G-50 column. Collected vesicles were assessed for GTP[γ-35S] binding to exofacial G proteins, which was low (0.3 pmol/μg of protein) compared with nonproteolyzed controls (9.9 pmol/μg of protein) during a 30-min incubation at 37°C. Integrity of the inner leaflet G proteins was assessed by making them available for GTP[γ-35S] binding by solubilization with detergent (Triton X-100).
GTP Loading and Shear Stress.
Liposomes reconstituted with G proteins were loaded with [γ-32P]GTP (100 μM) for 12 hr on ice through passive diffusion and passed through Sephadex G-50 to remove excess GTP. These vesicles (100-μl aliquots) were subjected to shear stress (0–30 dynes/cm2; 1 dyne = 10 μN) in a motor-driven cone-and-plate viscometer at 37°C (water jacketed) for 1 min, and the GTP hydrolysis was measured. Steady-state GTPase activity (GTP hydrolysis) was measured by using a charcoal adsorption method (14). Experiments with protein-free liposomes were performed simultaneously and the values (0.3 ± 0.08 pmol) were taken as background. Further, GTP hydrolysis during the vesicle loading (2.7 ± 1 pmol) was corrected for nonspecific hydrolysis. The shear stresses applied here are in the physiological range and similar to those experienced by vascular endothelial cells in vivo (15). The vesicles are, however, suspended, and they experience a pulsatile shear stress as they rotate in the flow field (16).
Assays.
GTPase activity was measured essentially as described by Brandt and Ross (14). Briefly, we used 100-μl vesicles with 100 μM GTP plus 0.1–0.2 μCi of [γ-32P]GTP in 20 mM sodium Hepes (pH 7.5) containing 100 mM NaCl, 0.2 mM ascorbic acid, and 1 mM EDTA. After appropriate stimulations the reaction was stopped by transferring an aliquot (50–75 μl) of the reaction mixture to 750–800 μl of Norit A charcoal suspension (5%, wt/vol) in 50 mM sodium phosphate buffer (pH 7.4) at 0°C. The tubes were mixed thoroughly and centrifuged for 20 min at 15,430 × g, and a 400-μl aliquot was withdrawn and recentrifuged at the same speed. After the second centrifugation, the amount of Pi released was determined by Cerenkov counting of the supernatant fraction in a liquid scintillation counter.
Binding of GTP[γ-35S] was assayed as described by Northup et al. (17). Samples to be assayed were diluted in 20 mM sodium Hepes, pH 8.0/1 mM EDTA/1 mM dithiothreitol/0.1% Thesit. The diluted samples were added to 50 μl of 50 mM sodium Hepes, pH 8.0/100 mM MgCl2/1 mM EDTA/200 mM NaCl/4 μM GTP[γ-S] containing 1–2 × 105 cpm of GTP[γ-35S]. After 1 hr of incubation at 30°C samples were diluted and filtered on BA85 nitrocellulose filters (0.45-μm pore size; Schleicher & Schuell) in a vacuum manifold. Nonspecific binding was determined in the presence of 500 μM unlabeled GTP[γ-S]. Filters were dried, dissolved in scintillation fluid, and assayed for radioactivity.
Proteins were separated by SDS/PAGE on 10% polyacrylamide gels by using the Laemmli system (18). For immunoblotting, proteins were transferred onto Immobilon-P (Millipore) and incubated with appropriate antibodies followed by 125I-labeled staphylococcal protein A. After extensive washing, the dried blots were subjected to autoradiography on Kodak XAR-5 film with intensifying screens.
The protein concentrations were determined by the method of Bradford (19) using a Bio-Rad protein determination kit and bovine serum albumin as a standard.
RESULTS AND DISCUSSION
Shear Stress Stimulates GTPase Activity of Reconstituted G Proteins.
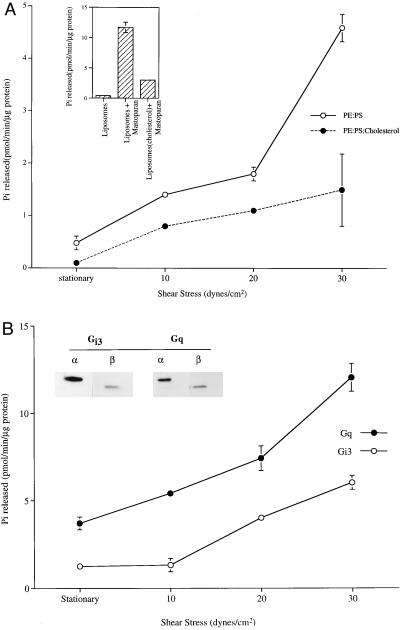
To examine the mechanism of shear-induced signal transduction, purified G proteins were reconstituted into liposomes composed of phospholipids PE and PS (20). These reconstituted vesicles were loaded with [γ-32P]GTP by passive diffusion and sheared in a motor-driven cone-and-plate rotating viscometer modified for suspended cells (15). There was a large increase in GTPase activity (4.6 pmol/min per μg of protein) with increased shear stress (30 dynes/cm2) from the basal level of activity (0.48 pmol/min per μg of protein) (Fig. 1A). Treatment with mastoparan, a wasp venom peptide that mimics the function of a receptor, stimulated GTPase activity (Fig. 1A Inset), verifying that the G proteins incorporated into the vesicles were functional.
Figure 1.
Shear stress-stimulated GTPase activity of reconstituted G proteins. (A) G protein reconstituted liposomes loaded with [γ-32P]GTP were passed through Sephadex G-50 to remove external GTP. GTPase was stimulated when vesicles were subjected to shear stress (0–30 dynes/cm2) in a cone-and-plate viscometer (37°C, 1 min). This activity was attenuated by incorporation of cholesterol (24 mol %). (Inset) Mastoparan (900 μM) incubated with liposomes for 1 min (37°C) stimulated GTPase activity. (B) Affinity-purified Gαq and Gαi3, reconstituted into liposomes along with their respective βγ subunits (immunoblots for α and β are shown in Inset) were stimulated by shear stress. The values presented here are a mean ± SD of three measurements.
One possible mechanism for increased GTPase activity is that shear stress increased intravesicular transport of substrate (GTP) to the G proteins bound to the inner leaflet of the vesicles. This is unlikely, however, because the intravesicular concentration of GTP used (10 μM) is at near saturation for G proteins (21) and therefore any increased transport of substrate would have negligible effect on GTPase activity.
The increase in GTPase activity may also be due to a rise in temperature of the fluid under shear, because heat is generated by the shearing of viscous fluids. Non-vesicle-incorporated G proteins in suspension were subjected to shear stress and did not show an increase in GTPase activity (data not shown). In the present study, reconstituted liposomes were subjected to shear at 37°C a water-jacketed cone-and-plate viscometer, where the temperature was maintained by a circulating water bath. Finally, analytical calculations showed the maximal increase in temperature caused by shearing at 30 dynes/cm2 for 1 min was 0.006°C (not shown). Therefore, it is unlikely that heat produced by shear stress causes the increase in enzyme activity.
Heterotrimeric G proteins Gq and Gi3 were previously shown to be activated by shear stress in endothelial cells (4). Reconstituted affinity-purified Gαq and Gαi3 subunits along with their respective βγ subunits were stimulated by shear stress in a dose-dependent manner (Fig. 1B). The shear stress-stimulated GTPase activity is similar in the magnitude to that observed in G proteins coreconstituted with a receptor (22). These results show that shear stress can activate reconstituted G proteins in the absence of any protein receptor.
Effect of Membrane Composition on G Protein Function.
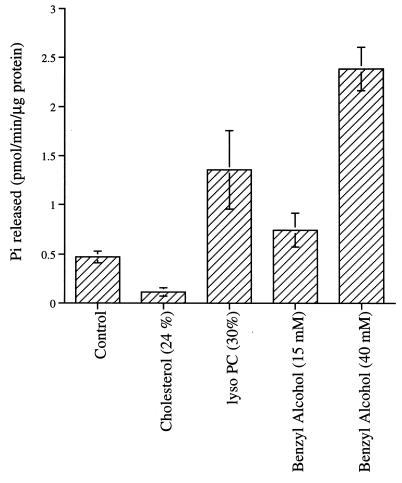
Shear stress may act by altering the physical properties of the bilayer membrane to modulate G protein function. Direct measurement of membrane physical properties of vesicles in a well defined shear field by spectroscopic methods is not currently feasible. However, membrane physical properties can be modulated by changing lipid composition or by exogenously added membrane-active agents. Here, G proteins were reconstituted into lipid vesicles containing LPC (23) or cholesterol in addition to the control PE/PS mix (Fig. 2). Incorporation of LPC (30 mol %) into liposomes increased the basal GTP hydrolysis almost 3-fold (from 0.47 to 1.35 pmol/min per μg of protein), whereas cholesterol (24 mol %) in the liposomes decreased the basal GTPase activity 4-fold to 0.113 pmol/min per μg of protein. Shear stress increased GTP hydrolysis of G proteins in cholesterol-containing vesicles, but only to about one-third the level of G proteins without cholesterol (Fig. 1A). Cholesterol also reduced the response of G proteins to mastoparan (Fig. 1A Inset). We also treated reconstituted G proteins (in the PE/PS vesicles) with the membrane-fluidizing agent benzyl alcohol (24). Incubation of vesicles with 40 mM benzyl alcohol increased the basal GTPase activity 5-fold to 2.37 pmol/min per μg of protein.
Figure 2.
Effect of membrane fluidity modulators on G protein GTPase activity. The histogram shows basal GTPase activity (37°C, 1 min) of G proteins reconstituted with control (PE/PS; 6:4, mol/mol), cholesterol (PE/PS/cholesterol; 4.5:3:2.5, mol/mol), and LPC (PE/PS/LPC; 4.2:2.8:3, mol/mol). Benzyl alcohol was added to control liposomes (PE/PS, 6:4). The values presented here are mean ± SD of three measurements.
Thus we find that the GTP hydrolysis rate of reconstituted G protein is sensitive to the physical properties of its membrane environment. Benzyl alcohol and LPC increased the basal GTPase activity of G proteins, whereas cholesterol decreased basal GTPase activity as well as shear- and mastoparan-induced increases in GTPase. Incorporation of cholesterol or LPC into the lipid bilayer did not affect the efficiency of G protein reconstitution significantly. The protein content in different reconstituted preparations were 1.24 nmol of protein per μmol of lipid for PE/PS, 1.1 nmol for PE/PS/LPC, and 0.87 nmol in the case of PE/PS/cholesterol, and the reconstitution efficiencies were 41%, 39%, and 36%, respectively.
Shear Stress Induces Transbilayer GTPase Activity.
Implicit in the shear stress activation of G proteins by the membrane bilayer is the assumption that a physical force on the external membrane is sensed by G proteins on the inside of the membrane. In reconstituted vesicles it is likely that G proteins are present on both the inner and outer leaflet of the liposome. The hypothesis that phospholipid bilayer can function as receptor to transduce the mechanical signal to G proteins was validated by subjecting the reconstituted vesicles to shear after proteolysis of peripheral G proteins. Though external [γ-32P]GTP was removed by gel filtration prior to the application of shear stress, it was possible that external G proteins may have had access to GTP that diffused out of the liposome. To address this possibility, reconstituted vesicles were treated with trypsin (25) to cleave the outer leaflet G proteins. This treatment digested external G proteins as measured by GTP[γ-35S] binding but did not affect GTP hydrolysis in response to shear (Fig. 3), confirming that the shear stress activation observed was vectorial (transbilayer) and because of G proteins on the inner leaflet.
Figure 3.
Brief proteolytic digestion of peripheral G proteins did not affect shear stress-stimulated GTPase activity in reconstituted G proteins. Proteolytic digestion was assessed by GTP[γ-S] binding efficiency (Inset). Integrity of inner leaflet G proteins in trypsinized vesicles was monitored by disrupting with Triton X-100, to make G proteins available for GTP[γ-S] binding.
There was no measurable effect on the size distribution of liposomes when they were exposed to shear stress (26). Transbilayer diffusion of extrinsic membrane proteins is an extremely slow process (27) in the absence of large-scale membrane disruption.
Regulation of Signal Transduction by the Physical Properties of the Lipid Bilayer.
The data presented here clearly demonstrate that the lipid bilayer can directly affect the activity of purified G proteins. However, the molecular mechanism underlying this phenomenon is not clear. A key feature of G protein-mediated signal transduction is GTP hydrolysis by the Gα subunit. This process involves a conformational change in the heterotrimeric G protein that facilitates the exchange of bound GDP for GTP by the α subunit, dissociation of the α and β/γ subunits, and hydrolysis of GTP to GDP followed by reassociation of α and β/γ to complete the cycle (28). The increased rate of GTP hydrolysis in reconstituted G proteins stimulated by shear must result from an increase in the rate of one of these steps in the catalytic cycle. How might a shear force imposed on the outside of a lipid vesicle result in a conformational change in a G protein located on the inside of the same vesicle? The results presented here suggest that this phenomenon is related to the effect of the lipid bilayer on protein function. In the absence of any shear force, agents that modify the physical properties of the lipid bilayer alter the steady-state level of GTP hydrolysis of the reconstituted G proteins (Fig. 2). What physical features of the membrane can affect protein function? One parameter that is often cited as being important is membrane “fluidity,” or the inversely related “microviscosity.” These terms are often used to describe solvents and can intuitively be understood to affect molecular motions of the solutes they contain. These simple analogies begin to break down when one considers the microenvironments in a lipid bilayer, which might be probed by different spectroscopic methods. For instance, in a three-dimensional fluid, rotational molecular motions would be expected to have a dependence on viscosity similar to that of diffusional motions, but in a lipid bilayer the rotational motion of a molecule on its long axis clearly has a different meaning than the lateral diffusion rate of a molecule within the plane of the bilayer. Still, the composite parameter of “fluidity” is useful for understanding general aspects of membrane phenomenon.
Studying the lipid dependence of membrane proteins in reconstituted systems is an explicit way of determining the role of the membrane in the action of these molecules. Alcohols (23) and general anesthetics (6) are thought to alter bilayer properties and modulate membrane protein activity (29), by “fluidizing” or disordering the bilayer. LPC is a detergent-like lipid that, at the concentrations used, increases disorder in the bilayer (23). Both of these treatments increased the basal activity of reconstituted G proteins. The effects of cholesterol on membrane bilayers include a decrease in translational and rotational mobility of bilayer components, an increase in lipid packing, and an overall increase in membrane order (30). Cholesterol reduced the basal activity of reconstituted G proteins as well as the degree of activation of G proteins by experimental treatments. Taken together, these experiments indicate that membrane-bound G proteins are activated by membrane fluidity modulators in the absence of a protein receptor and that alterations in the bilayer physical properties can modulate shear stress activation.
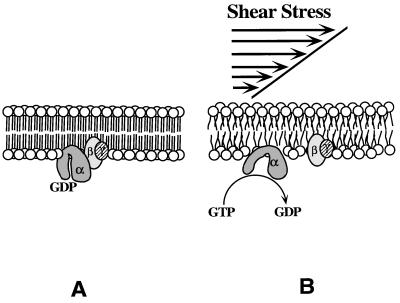
These data are consistent with a direct effect of shear stress on membrane physical properties. Previous studies have shown that fluid shear stress can increase membrane permeability in lipid vesicles (31) as well as intact cells (5). The molecular mechanism behind this increased permeability is not known but, in general, increased membrane permeability is related to increased membrane fluidity (32). These observations suggest that fluid shear stress alters the membrane microenvironment, such as microviscosity, leading to altered membrane protein activity. In theory, the molecular configuration and physical properties of a lipid bilayer can be modified by the application of an external force. Application of lateral pressure (FLP) to a lipid bilayer is expected to modify the area occupied by a molecule. The relationship between FLP and molecular area (AM) is the bilayer compressibility, which is a function of composition, lipid properties, and temperature (33). The ability of the phospholipid bilayer to activate Gα by shear stress strongly suggests that physical perturbations leading to changes in the microviscosity regulate the function of membrane-bound G proteins (Fig. 4). Under such conditions, a protein receptor may not be necessary to facilitate G protein activation. We speculate that shear stress disrupts lipid packing order of the phospholipid bilayer, thereby reducing the microviscosity surrounding membrane-bound G proteins. The decrease in membrane microviscosity would then increase the intramolecular dynamics and activity of membrane-bound enzymes (34).
Figure 4.
Schematic representation of protein receptor-independent activation of G proteins by fluid shear stress. Increase in the rotational and translational mobility in the lipid bilayer and the accompanying decrease in microviscosity activate membrane-bound G proteins by facilitating exchange of GDP for GTP. The intrinsic GTPase activity of the α subunit hydrolyzes GTP to GDP, releasing inorganic phosphate (Pi), and α-GDP recombines with βγ, ending the activation cycle.
The physicochemical properties of the reconstituted G protein vesicles used in this study are different from those of cellular plasma membranes. These differences include the phospholipid composition and protein concentration and the absence of a membrane skeleton. All of these factors are likely to contribute to differences in membrane fluidity. The results presented here are consistent with several other findings describing the effect of lipid environment on membrane protein function (35), and they make a link between shear stress and the lipid environment. The present study demonstrates that a phospholipid bilayer is capable of acting as a mechanoreceptor.
Acknowledgments
We thank Prof. Paul A. Insel (Depart. of Pharmacology, Univ. of California at San Diego) and Prof. G. R. Boss (Dept. of Medicine, Univ. of California at San Diego) for critically reading the manuscript and their helpful suggestions. This research was supported by a grant from the National Institutes of Health (HL-40696).
ABBREVIATIONS
- G protein
GTP-binding protein
- GTP[γ-S]
guanosine 5′-O-(3-thiotriphosphate)
- Hepes
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- LPC
lysophosphatidylcholine
- Triton X-100
t-octylphenoxypolyethoxyethanol
References
- 1.Davies P F. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olesen S P, Clapham D E, Davies P F. Nature (London) 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd G M. Cell. 1991;67:845–851. doi: 10.1016/0092-8674(91)90358-6. [DOI] [PubMed] [Google Scholar]
- 4.Gudi S, Clark C B, Frangos J A. Circ Res. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- 5.Berthiaume F, Frangos J A. Biochim Biophys Acta. 1994;1191:209–218. doi: 10.1016/0005-2736(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell D C, Lawrence J T R, Litman B J. J Biol Chem. 1996;271:19033–19036. doi: 10.1074/jbc.271.32.19033. [DOI] [PubMed] [Google Scholar]
- 7.Wall M A, Coleman D E, Lee E, Iniguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 8.Wilson P T, Bourne H R. J Biol Chem. 1995;270:9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- 9.Bigay J, Faurobert E, Franco E, Chabre M. Biochemistry. 1994;33:14081–14090. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- 10.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 11.Sternweis P C, Robishaw J. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 12.Pang I-H, Sternweis P C. J Biol Chem. 1990;265:18707–18712. [PubMed] [Google Scholar]
- 13.Cerione R A, Ross E M. Methods Enzymol. 1991;195:329–342. doi: 10.1016/0076-6879(91)95178-m. [DOI] [PubMed] [Google Scholar]
- 14.Brandt D R, Ross E M. J Biol Chem. 1986;261:1656–1673. [PubMed] [Google Scholar]
- 15.Franke R P, Gräfe M, Schnittler H, Seiffge D, Mittermayer C, Drenckham D. Nature (London) 1984;307:648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 16.Pozrikidis C. In: Introduction to Theoretical and Computational Fluid Dynamics. Pozrikidis C, editor. New York: Oxford Univ. Press; 1997. pp. 264–273. [Google Scholar]
- 17.Northup J K, Smigel M D, Gilman A G. J Biol Chem. 1982;257:11416–11423. [PubMed] [Google Scholar]
- 18.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Rubalcava B, Rodbell M. J Biol Chem. 1973;248:3831–3837. [PubMed] [Google Scholar]
- 21.Bourne H R, Sanders D R, McCormick F. Nature (London) 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 22.Cerione R A, Staniszewski C, Benovic J L, Lefkowitz R J, Caron M G, Gierschik P, Somers R, Spiegel A M, Codina J, Birnbaumer L. J Biol Chem. 1985;260:1493–1500. [PubMed] [Google Scholar]
- 23.Yuan Y, Schoenwaelder S M, Salem H H, Jackson S P. J Biol Chem. 1996;271:27090–27098. doi: 10.1074/jbc.271.43.27090. [DOI] [PubMed] [Google Scholar]
- 24.Gordon L M, Sauerheber R D, Esgate J A, Dipple I, Marchmont R J, Houslay M D. J Biol Chem. 1980;255:4519–4527. [PubMed] [Google Scholar]
- 25.Fung B K K, Nash C R. J Biol Chem. 1983;258:10503–10510. [PubMed] [Google Scholar]
- 26.Chakravarty S R, Giorgio T D. Biochim Biophys Acta. 1992;1112:197–204. doi: 10.1016/0005-2736(92)90392-y. [DOI] [PubMed] [Google Scholar]
- 27.Edidin M. Annu Rev Biophys Bioeng. 1974;3:179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- 28.Hebureich E J, Hofmann H P. Biochim Biophys Acta. 1996;1286:285–322. doi: 10.1016/s0304-4157(96)00013-5. [DOI] [PubMed] [Google Scholar]
- 29.Franks N P, Leib W R. Nature (London) 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 30.Yeagle P L. FASEB J. 1989;3:1833–1842. [PubMed] [Google Scholar]
- 31.Giorgio T D, Yek S H. Biochim Biophys Acta. 1995;1239:39–44. doi: 10.1016/0005-2736(95)00137-r. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel R A, Phelps B M, Waggoner A, Terada L, Williamson P. Cell. 1980;20:321–328. doi: 10.1016/0092-8674(80)90618-2. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell D C, Litman B J. Biochemistry. 1994;33:12752–12756. doi: 10.1021/bi00209a004. [DOI] [PubMed] [Google Scholar]
- 34.Beece D, Eisenstein L, Frauerfelder H, Good D, Marden M C, Reinisch L, Reynolds A H, Sorensen L B, Yue K T. Biochemistry. 1980;19:5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- 35.Fan Z, Makielski J C. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]