Abstract
In Ralstonia sp. strain U2, the nag catabolic genes, which encode the enzymes for the pathway that catabolizes naphthalene via the alternative ring cleavage gentisate pathway, are transcribed as an operon under the same promoter. nagR, which encodes a LysR-type transcriptional regulator, is divergently transcribed compared to the nag catabolic genes. A 4-bp frameshift deletion in nagR demonstrated that NagR is required for expression of the nag operon. The transcriptional start of the nag operon was mapped, and a putative −10, −35 σ70-type promoter binding site was identified. Further upstream, a site proximal to the promoter was identified as a site that has bases which have been found to be conserved in the activator-binding motif of other naphthalene pathways. Transcriptional fusion studies demonstrated that NagR regulates the expression of the nag operon positively in the presence of salicylate and to a lesser extent in the presence of 2-nitrobenzoate. Mutation of the LysR-type activator-binding motif in the nag promoter-proximal region resulted in a loss of inducibility of a lacZ reporter gene transcriptionally fused to nagAa, the first gene of the operon. However, other mutations in the region increased the effectiveness of salicylate as an inducer.
The nag genes of Ralstonia sp. strain U2 code for the enzymes of the alternative pathway for the catabolism of naphthalene, which converts naphthalene to fumarate and pyruvate via salicylate (2-hydroxybenzoate) and gentisate (2,5-dihydroxybenzoate), and they are organized in a continuous sequence of adjacent genes (8, 28, 29). This is in contrast to the classical naphthalene pathway enzymes, which are encoded on two separate operons, the upper-pathway operon (genes nahAa to nahF) for the conversion of naphthalene to salicylate and the lower-pathway (or meta-pathway) operon (genes nahG to nahM) for the conversion of salicylate to acetyl coenzyme A and pyruvate via catechol (5, 27).
Transcriptional control of the classical naphthalene pathway is regulated by NahR, a regulator protein belonging to the LysR-type family of transcriptional regulators (23). NahR is responsible for the regulation of both nah operons (21, 22), and the gene that encodes it is located upstream of and is transcribed divergently from nahG, the first gene of the meta-pathway operon (27). This gene arrangement has been found in several different classical naphthalene genes cloned from different bacteria (3, 4, 19, 21; GenBank accession number AF491037). The nag pathway in Ralstonia sp. strain U2 also contains a putative regulator gene, nagR, which has high sequence similarity to nahR. In contrast to nahR, this gene is located upstream of and is divergently transcribed from nagAa (28).
In the nah system, NahR is expressed constitutively at low levels (20) and binds to promoter-proximal target DNA, regardless of the presence of salicylate. Transcription is activated when the inducer, salicylate (1, 27), binds to NahR and activates both the nah upper- and lower-pathway operons by relieving DNA bending at the promoter sites (10, 24). Binding of NahR to its target DNA also appears to autoregulate its own expression by repressing transcription of nahR that is transcribed divergently from nahG (24). The DNA to which the regulator protein binds is conserved in various naphthalene degradation pathways in Pseudomonas (4, 19, 22) (GenBank accession number AF491307), and a similar motif is found upstream of the salicylate hydroxylase gene (salA) in Acinetobacter sp. strain ADP1, which is also regulated by a NahR homolog, SalR (13).
In this study we investigated regulation of the nag genes in Ralstonia sp. strain U2. We found that nagR is the regulatory gene that controls nag gene expression, and we demonstrated the operonic structure of the nag genes and determined the inducers. The location of the NagR-binding motif was also probed by examining the effects of some designed mutations on expression.
MATERIALS AND METHODS
Strains and plasmids.
The plasmids and bacterial strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype and/or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| Ralstonia sp. strain U2 | Wild-type naphthalene degrader containing plasmid pWWU2 | 7 |
| P. putida PaW340 | Trp− Strr plasmid-free derivative of P. putida mt-2 | 11 |
| Plasmids | ||
| pUC19 | Vector; Apr; multiple cloning site in lacZα | 27 |
| pKOK6.1 | Apr; promoterless lacZ | 15 |
| pRK415 | Tcr; mobilizable broad-host-range vector; origin of replication (oriV) and origin of transfer (oriT) of RK2 | 14 |
| pRK415-ZP | pRK415 with lacZ-Km cassette from pKOK6.1 inserted into PstI site, with the start of lacZ adjacent to the SalI site of pRK415 | This study |
| pWWF24 | 8.3-kb XhoI fragment from strain U2 carrying the naphthalene dioxygenase region cloned in the XhoI site of pBluescript II SK(+) | 8 |
| pWWF116 | pWWF24 with a 4-bp deletion at the NsiI site within nagR | This study |
| pWWF119 | pGEM-T Easy with RACE fragment created by using Pnag as the template | This study |
| pWWF120F | 1.6-kb wild-type fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites | This study |
| pWWF120R | Like pWWF120F, but with restriction sites reversed, creating a 1.6-kb fragment in the opposite direction | This study |
| pWWF121 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having bases −68 to −70 of Pnag deleted | This study |
| pWWF122 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having bases −60 to −62 of Pnag deleted | This study |
| pWWF123 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having bases −64 to −66 of Pnag deleted | This study |
| pWWF124 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having bases −60 to −71 of Pnag deleted | This study |
| pWWF125 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having a C-to-T substitution at base −70 of Pnag | This study |
| pWWF126 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having a G-to-A substitution at base −61 of Pnag | This study |
| pWWF128 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having C-to-T and G-to-A substitutions at bases −51 to −53 of Pnag | This study |
| pWWF129 | 1.6-kb fragment in pUC18 containing nagR and 347 bp of nagAa PCR amplified at designed XbaI and KpnI restriction sites and having C-to-T and G-to-A substitutions at bases −77 to −79 of Pnag | This study |
| pWWF120F-Z | 1.6-kb XbaI/KpnI fragment from pWWF120 inserted into pRK415-ZP | This study |
| pWWF120R-Z | 1.6-kb KpnI/XbaI fragment from pWWF120 inserted into pRK415-ZP | This study |
| pWWF121-Z | 1.6-kb XbaI/KpnI fragment from pWWF121 inserted into pRK415-ZP | This study |
| pWWF122-Z | 1.6-kb XbaI/KpnI fragment from pWWF122 inserted into pRK415-ZP | This study |
| pWWF123-Z | 1.6-kb XbaI/KpnI fragment from pWWF123 inserted into pRK415-ZP | This study |
| pWWF124-Z | 1.6-kb XbaI/KpnI fragment from pWWF124 inserted into pRK415-ZP | This study |
| pWWF125-Z | 1.6-kb XbaI/KpnI fragment from pWWF125 inserted into pRK415-ZP | This study |
| pWWF126-Z | 1.6-kb XbaI/KpnI fragment from pWWF126 inserted into pRK415-ZP | This study |
| pWWF128-Z | 1.6-kb XbaI/KpnI fragment from pWWF128 inserted into pRK415-ZP | This study |
| pWWF129-Z | 1.6-kb XbaI/KpnI fragment from pWWF129 inserted into pRK415-ZP | This study |
Chemicals and media.
Aromatic substrates were obtained from Sigma-Aldrich Co. Luria-Bertani (LB) medium (20) was used to cultivate bacteria unless noted otherwise. For growth on minimal medium, single carbon sources were added to the minimal salts medium (2) at the following concentrations: salicylate, 2.5 mM; and succinate, 10 mM. Aromatic compounds for induction experiments were added to a final concentration of 1 mM. Where appropriate, ampicillin was added at a concentration of 100 μg/ml, kanamycin and spectinomycin were added at a concentration of 50 μg/ml, and tetracycline was added at a concentration of 25 μg/ml.
DNA manipulation.
Unless indicated otherwise, standard methods for DNA manipulation were used (20). Plasmid DNA was prepared from Escherichia coli strains by using CONCERT rapid plasmid miniprep systems (Gibco BRL). DNA fragments were recovered from agarose gels by using Qiaquick columns (Qiagen).
RT-PCR.
Cells were grown on minimal medium containing salicylate or succinate to a density of about 108 cells/ml. Total RNA was prepared from 10 ml of the culture with RNeasy Mini columns (Qiagen), with elution in 50 μl of water. The RNA was treated with DNase I to remove any genomic DNA contamination by incubation with 1 U of RNase-free DNase (Promega) and 1 U of RNasin (Promega) in 40 mM Tris-HCl (pH 7.9) containing 10 mM NaCl, 10 mM CaCl2, and 6 mM MgSO4 for 30 min at 37°C. The RNA was cleaned by passage through an RNeasy Mini column prior to use in a reverse transcriptase PCR (RT-PCR). The RT-PCR was carried out by using SuperScript II RT (Invitrogen Life Technologies, Carlsbad, Calif.).
The intergenic regions between the nag genes were amplified by using primer pairs (Table 2). PCR were performed with 50-μl mixtures containing 0.5 μg of total RNA, 50 pmol of each primer, each deoxynucleoside triphosphate at a concentration of 50 μM, 1 mM MgSO4, 5 U of avian myeloblastosis virus RT, and 5 U of Tfl DNA polymerase in the reaction buffer supplied by the manufacturer. After reverse transcription at 48°C for 1 h, the reaction mixtures were heated to 94°C for 2 min and subjected to 40 cycles of 30 s at 94°C, 1 min at 55°C, and 2 min at 68°C. Negative control reactions to eliminate the possibility that residual DNA was amplified were performed in the same way, except that the RT was omitted from the reaction mixtures.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ → 3′) | Relevant information |
|---|---|---|
| Primers used in RT-PCRa | ||
| nagRT-1 | ATCAGCGGCCCCCTCGGAACGGCTTATCT | 2.802 to 4.970 kb |
| nagRT-2 | GTGCGCACGATCACGTCTCGGTAATAGC | |
| nagRT-3 | CGCCACGGCAACGCCAAGGACTTCTTCT | 3.596 to 5.786 kb |
| nagRT-4 | GAGCCAAAGCCCCAGCCGTGGTAACTGC | |
| nagRT-5 | TGCAGAGGCCCCCACGCTTGTCGATTATCT | 5.916 to 7.915 kb |
| nagRT-6 | CCGCCGCCGGCCGCATAGGAAG | |
| nagRT-7 | CGGCGGCGGCGGCTCTTGCTACAT | 7.907 to 10.230 kb |
| nagRT-8 | GTGGCCCAGGCCCTGATCACCGGTTAGAAA | |
| nagRT-9 | GCCTGCGGCCAAGCGCCTCAATCATCT | 10.359 to 12.977 kb |
| nagRT-10 | TCGCTCCCTGGCTGCCGGGTAATACAGTCC | |
| nagRT-11 | CGGGTGAATGCCGGACTGTATTAC | 12.936 to 14.966 kb |
| nagRT-12 | GCCGGGCTTGAGCATCAGGGCGTAGAAG | |
| nagRT-13 | GAAACGGGTGACGACGCCGAGAACATCC | 14.907 to 15.827 kb |
| nagRT-14 | CAGGTCGCCGGGTTGCAAGTGGTAGTAGGT | |
| nagRT-15 | GCGGCCGCTACAGCTTTGGCGACACA | 16.401 to 18.452 kb |
| nagRT-16 | CGGGGTAGTCTGCACCGAACTTTTGCTCTG | |
| Primers used for overlap extension mutagenesisb | ||
| 121-3 | CATTATTGCTGGTGATTTTAACTATCA | Delete positions −68 to −70 |
| 121-4 | AATCACCAGCAATAATGGTGAGGCATCATG | |
| 122-3 | CATTATTCATGCTGGTTTTAACTATC | Delete positions −60 to −62 |
| 122-4 | GTTAAAACCAGCATGAATAATGGTGAG | |
| 123-3 | CATTATTCATGGTGATTTTAACTATC | Delete positions −64 to −66 |
| 123-4 | GTTAAAATCACCATGAATAATGGTGAGGCA | |
| 124-3 | CATTATTTTTAACTATCAGACTTGA | Delete positions −60 to −71 |
| 124-4 | GTCTGATAGTTAAAAATAATGGTGAGGCA | |
| 125-3 | CATTATTTATGCTGGTGATTTTAACTAT | C-to-T substitution at position −70 |
| 125-4 | GTTAAAATCACCAGCATAAATAATGGTGAGGCA | |
| 126-3 | CATTATTCATGCTGGTAATTTTAACTATCA | G-to-A substitution at position −61 |
| 126-4 | GTTAAAATTACCAGCATGAATAATGGTGAG | |
| 128-3 | GTGATTTTAATGATCAGACTTGATCTATAGCG | CTA-to-TGC substitution at position −51 to −53 |
| 128-4 | TCAAGTCTGATCATTAAAATCACCAGCATG | |
| 129-3 | GATGCCTCGTTATTATTCATGCTGGTGA | ACC-to-GTT substitution at position −77 to −79 |
| 129-4 | GAATAATAACGAGGCATCATGGATCTGCGCGA |
Mapping the nagAa transcription start site.
The start of transcription of the nagAa gene was mapped by using a kit for rapid amplification of cDNA ends (RACE) as recommended by the manufacturer (5′ RACE System; Invitrogen Life Technologies).
Total RNA was prepared from Ralstonia sp. strain U2 by using a Qiagen RNeasy Mini kit. DNA contamination was removed by digestion with 10 U of RQ1 RNase-free DNase (Promega) for 30 min at 37°C. Total RNA (2 μg) in a 20-μl reaction sample was reverse transcribed by using the manufacturer's instructions and a nagAa-specific primer, RACEAa-1 (5′-GGGCGGACGCATTCGGGCGTGAAC-3′), which anneals 428 bp downstream of the nagAa translational start site. A homopolymeric tail was added to the 3′ end of the synthesized cDNA (corresponding to the 5′ end of nagAa mRNA that was reverse transcribed in the reaction described above) by using terminal transferase and dCTP by incubation at 37°C for 10 min as described in the RACE kit protocol.
The dC-tailed cDNA was PCR amplified by using the abridged anchor primer provided with the kit (5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG-3′) and another nagAa-specific primer, RACEnagAa-2 (5′-CAGGGCTGAACTCAAGCGGTTTGGCCAG-3′), which anneals 385 bp downstream of the nagAa translational start site. The PCR product obtained was then PCR amplified with primer RACEnagAa-2 and the abridged universal amplification primer provided with the kit (5′-GGCCACGCGTCGACTAGTAC-3′) in order to eliminate any nonspecific PCR products from the first reaction. The amplified products were purified by using a Qiaquick PCR clean-up column (Qiagen) and were cloned into the pGEM-T Easy vector (Promega). Sequences of the cloned 5′ RACE inserts were determined by MWG-Biotech AG (Ebersberg, Germany).
Preparation of cell extracts.
Cells were harvested by centrifugation, washed with 100 mM phosphate buffer (pH 7.4), and stored as pellets at −20°C. Cell extracts were prepared by suspending frozen pellets in ice-cold 100 mM phosphate buffer (pH 7.4), disrupting them with a French pressure cell (SLM Instruments, Inc., Urbana, Ill.), and centrifuging them at 120,000 × g for 30 min at 4°C. Each supernatant was stored frozen as 1-ml aliquots at 20°C. The total protein concentrations of the cell extracts were determined by using a Microprotein PR kit (Sigma Diagnostics, Inc.).
Construction of nagR-nagAa::lacZ fusions.
The promoterless lacZ-Kmr cassette from pKOK6.1 (15) was inserted into the multiple cloning site of pRK415 by digestion with PstI (which excised the cassette on a single fragment), creating pRK415-ZP. The orientation of the cassette was such that the KpnI site and an XbaI site were upstream of the lacZ translational start.
A region of DNA which included all of nagR and the first 347 bp of nagAa was PCR amplified by using Proofstart DNA polymerase (Qiagen) according to the manufacturer's instructions together with primers Nag-kpn (5′-GGCAGTTCGGTACCGCTGCCTACGCACAAGAC-3′) and Nag-xba (5′-AGGCGCCTCTAGACATGGGTGGCTTCGTC-3′), which contain engineered KpnI and XbaI restriction endonuclease sites (underlined), respectively. For construction of nagR-nagAa::lacZ fusions in which the region upstream of the nag promoter contained a mutation in the lysR-type activator-binding motif, complementary overlapping primers were designed that incorporated mutations in the region (Table 2). These primers were used to amplify a region that included all of nagR and the first 347 bp of nagAa by overlap extension PCR (9); Nag-kpn and Nag-xba were used as the flanking primers. The amplified fragment was incubated at 72°C for 10 min in the presence of Taq DNA polymerase, polymerase buffer, and 0.5 mM dATP and was ligated into the pGEM-T Easy TA cloning vector (Promega Inc.). Subsequently, the wild type and the mutated amplified fragments were subcloned from pGEM-T Easy into pRK415-ZP on a KpnI/XbaI fragment. This cloning created a nagR-nagAa::lacZ gene fusion in pRK413, in which the transcription of nagAa runs into lacZ. The amplified fragments cloned into pGEM-T Easy were sequenced to confirm that the mutation designed had been created and that no PCR errors occurred during amplification.
Triparental mating for transfer of pRK415 constructs into PaW340.
The donor, the recipient, and E. coli HB101 carrying pRK2103 as a helper plasmid were grown in LB medium until they reached an optical density at 600 nm of 0.6. Then 500-μl portions of the three cultures were mixed and centrifuged, and the pellets were washed in minimal medium. The pellets were finally resuspended in 50 μl of minimal medium and dispensed onto a sterile nylon membrane (Bio-Rad) laid on the surface of an LB medium plate. Following incubation overnight at 30°C, the cells were washed off the filter into 2 ml of minimal medium, and appropriate dilutions were spread onto selective medium. Donor-only and recipient-only controls were treated in the same way.
β-Galactosidase assays.
Cultures were grown overnight in 5 ml of minimal media containing 10 mM succinate with or without inducer. Cells were lysed with chloroform and sodium dodecyl sulfate, and β-galactosidase activities were determined as described by Miller (17).
DNA sequencing and sequence analysis methods.
Nucleotide sequences of both DNA strands were determined by MWG-Biotech Ltd. PCR primers were designed with the aid of the Lasergene software package (DNAStar, Inc., Madison, Wis.).
RESULTS
Inactivation of NagR.
To demonstrate the role of NagR as the regulatory protein, a 4-bp frameshift deletion in nagR was created by digesting pWWF24 (Table 1) at the unique NsiI site within nagR and filling in the overhanging ends with T4 DNA polymerase, creating pWWF116. pWWF24 harbors nagR and all of the nagAaGHAb genes coding for the salicylate 5-hydroxylase gene products which are responsible for catalytic conversion of salicylate to gentisate. E. coli(pWWF24) accumulates gentisate when it is cultured in LB media containing salicylate, which can be detected by the characteristic UV spectrum and by accumulation of a brown color due to autoxidation. No such accumulation of gentisate occurred in E. coli(pWWF116) under the same growth conditions. Moreover, spectrophotometric assays of cell extracts of E. coli DH5α(pWWF24) showed that there was NADH-linked conversion of salicylate to gentisate, whereas no conversion was detected when extracts of E. coli DH5α(pWWF116) were used (data not shown).
Operon structure of nag genes.
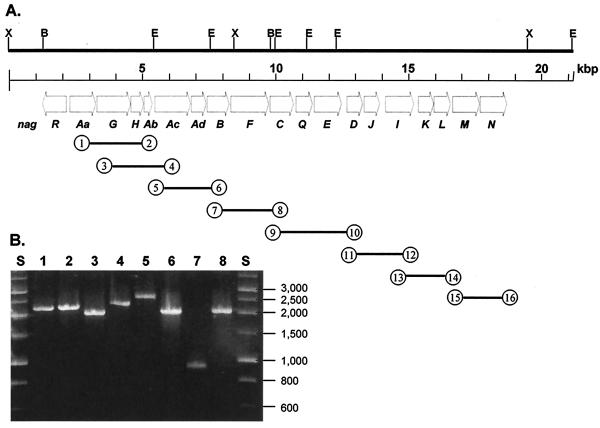
RT-PCR of RNA purified from salicylate-grown Ralstonia sp. strain U2 cells amplified products that extended across the boundaries between the nag genes (Fig. 1A). The amplified products were analyzed by agarose gel electrophoresis (Fig. 1B). The presence of amplified DNA fragments obtained with each primer pair suggests that all of the nag genes are transcribed on the same message. No amplification product was obtained when RT was omitted from the reaction mixture.
FIG. 1.
(A) Physical map of the nag genes and the locations of RT-PCR amplicons, obtained by using mRNA purified from salicylate-grown Ralstonia sp. strain U2 as the template. The numbers in circles indicate the primers used for each amplification and correspond to the nagRT primers described in Table 2. Important restriction sites are indicated as follows: X, XhoI; B, BamHI; E, EcoRI. (B) Agarose gel electrophoresis of RT-PCR products amplified from Ralstonia sp. strain U2 grown on salicylate. The sizes of molecular size markers in lanes S (HyperLadder I; Bioline, London, United Kingdom) are indicated on the right (in base pairs). Lane 1, nagRT-1 plus nagRT-2 (expected size, 2.168 kb); lane 2, nagRT-3 plus nagRT-4 (2.190 kb); lane 3, nagRT-5 plus nagRT-6 (1.999 kb); lane 4, nagRT-7 plus nagRT-8 (2.323 kb); lane 5, nagRT-9 plus nagRT-10 (2.582 kb); lane 6, nagRT-11 plus nagRT-12 (2.030 kb); lane 7, nagRT-13 plus nagRT-14 (0.920 kb); lane 8, nagRT-15 plus nagRT-16 (2.051 kb). No detectable products were obtained in control reactions with each pair of primers from which RT had been omitted or in reactions carried out with succinate-grown cells (data not shown).
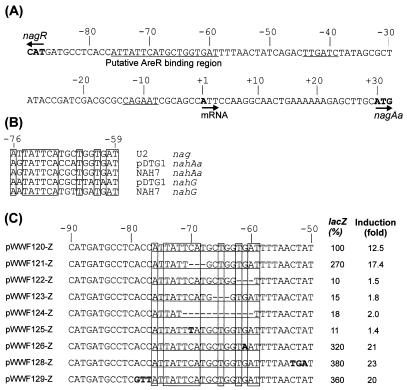
Mapping the transcriptional start site of nagAa. In order to map the site of initiation of transcription of nagAa, 5′ RACE PCR experiments were carried out with RNA extracted from Ralstonia sp. strain U2 grown on salicylate. The nucleotide sequence of the 5′ RACE PCR product showed that transcription starts at the A located 28 bases upstream of the nagAa translational start site (data not shown). Upstream of this transcriptional start site is a putative −10 sequence, CAGAAT, which is separated by 25 bp from a putative −35 sequence, TTGATC (Fig. 2A); these sequences are the best candidates for sequences that resemble the −10 and −35 promoter consensus sequences, TATAAT and TTGACA, respectively (6).
FIG. 2.
(A) Schematic representation of the organization of the nag promoter region. The arrows indicate the starts of transcription and translation. The transcriptional initiation nucleotide (+1) and the putative −35 and −10 motifs are underlined, as is the putative NagR-binding motif. (B) Alignment of the conserved regions of the upstream regions of operons controlled by NagR and NahR regulators. Conserved residues are enclosed in boxes. The sequence of pDTG1 was obtained from GenBank (accession number AF491307). (C) Plasmids and relevant sequences of the mutations in and around the putative binding domain for NagR. Deletions are indicated by dashes, and substitutions are indicated by boldface type. The wild-type sequence is the sequence of pWWF120-Z. The lacZ activity is expressed as a percentage of the salicylate-induced activity of the wild type (pWWF120-Z), and the induction value is the ratio of salicylate-induced activity to uninduced activity.
Upstream of this nagAa promoter, between bases −59 and −76, is the sequence ATTATTCATGCTGGTGA (Fig. 2A and B). This sequence contains the symmetrical dyad motif TTCAN6TGAT, identified by footprinting as the core of the NahR interaction with both of the naphthalene promoters on plasmid NAH7 (10, 25). Alignment of the 18-bp sequence with the similar sequences upstream of both the naphthalene catabolic operons on plasmids NAH7 and pDTG1 showed that there are 11 conserved bases in all five sequences. This conservation is in the context of very little other homology between the upstream regions of the nag and nah operons (8).
Determination of coinducers by using a nagR-nagAa::lacZ fusion.
We compared the levels of expression of the nagR-nagAa::lacZ fusion constructed in pWWF120F-Z. Attempts to select transconjugants of pWW120F-Z in Ralstonia sp. strain U2 were unsuccessful due to the high levels of intrinsic antibiotic resistance, and therefore, the construct was mated into Pseudomonas putida PaW340 (Table 1). PaW340(pWWF120F-Z) was grown in the presence of a range of aromatic compounds and assayed for β-galactosidase expression (Table 3). Only salicylate induced expression, and neither naphthalene nor gentisate resulted in detectable induction of nagAa. Of the other substituted aromatic compounds tested as potential gratuitous inducers, only 2-nitrobenzoate was marginally able to induce nagAa expression. 2-Aminobenzoate, a gratuitous inducer of the classical nah operons (1), did not function for the nag system.
TABLE 3.
β-Galactosidase activities expressed from nagR-nagAa::lacZ fusions with different aromatic compounds
| Inducera | β-Galactosidase activity of PaW340(pWWF120F-Z) (nagR-nagAa::lacZ)
|
|
|---|---|---|
| Miller unitsb | % of salicylate-induced culture | |
| None | 61 (6.5) | 3.6 |
| Salicylate | 1,700 (43.2) | 100 |
| 4-Methylsalicylate | 65 (4.8) | 3.8 |
| 5-Methylsalicylate | 61 (3.2) | 3.8 |
| 4-Chlorosalicylate | 64 (1.6) | 3.5 |
| 5-Chlorosalicylate | 59 (1.6) | 4.1 |
| Benzoate | 70 (6.5) | 3.9 |
| Gentisate | 67 (9.7) | 3.1 |
| Naphthalene | 53 (3.2) | 3.9 |
| 2-Aminobenzoate | 66 (4.8) | 4.0 |
| 5-Aminobenzoate | 68 (6.5) | 3.8 |
| 2-Chlorobenzoate | 65 (3.2) | 3.7 |
| 2-Nitrobenzoate | 135 (8.1) | 7.9 |
| 3-Nitrobenzoate | 58 (2.4) | 3.5 |
| 4-Nitrobenzoate | 60 (6.5) | 3.2 |
| 2-Nitrotoluene | 54 (3.2) | 3.2 |
| 3-Nitrotoluene | 56 (2.4) | 3.3 |
| 4-Nitrotoluene | 58 (6.5) | 3.4 |
| 2,4-Dinitrotoluene | 62 (9.7) | 3.6 |
| 2,6-Dinitrotoluene | 57 (5.7) | 3.4 |
| Nitrobenzene | 58 (6.5) | 3.4 |
| Ethyl salicylate | 65 (3.2) | 3.8 |
Inducers were present at a concentration of 1 mM.
The values are averages for three independent trials, each conducted in duplicate. The values in parentheses are standard deviations.
Analysis of expression of nagR-nagAa::lacZ fusions containing mutations in the activator-binding motif.
To test whether the region from bp −78 to −59 upstream of the nag promoter (Fig. 2A and B) is required for activation of transcription, we compared the salicylate-induced and uninduced β-galactosidase activities in eight strains carrying the nagR-nagAa::lacZ fusion and designed mutations in or around the putative regulator binding site (Table 4; Fig. 2C). Four mutations, incorporated into plasmids pWWF122-Z, pWWF123-Z, pWWF124-Z, and pWWF125-Z, resulted in failure of the system to express β-galactosidase. In contrast, four other mutations, incorporated into pWWF121-Z, pWWF126-Z, pWWF128-Z, and pWWF129-Z, resulted in a threefold increase in activity compared to the wild-type activity, but none of these mutations was constitutively expressed and all of them remained salicylate inducible.
TABLE 4.
β-Galactosidase activities expressed from wild-type and mutated nagR-nagAa::lacZ fusions
| Plasmid harbored in PaW340a | β-Galactosidase activity (Miller units)b
|
|
|---|---|---|
| Salicylate induced | Uninduced | |
| pRK415-Z | 61 (4) | 59 (2.4) |
| pWWF120R-Z | 72 (14) | 63 (6) |
| pWWF120F-Z | 1,700 (50) | 136 (5) |
| pWWF121-Z | 4,530 (230) | 260 (10) |
| pWWF122-Z | 180 (13) | 120 (7) |
| pWWF123-Z | 250 (20) | 140 (5) |
| pWWF124-Z | 306 (24) | 150 (4.0) |
| pWWF125-Z | 192 (10.6) | 135 (6) |
| pWWF126-Z | 5,400 (170) | 260 (14) |
| pWWF128-Z | 6,500 (160) | 280 (18) |
| pWWF129-Z | 6,000 (400) | 300 (25) |
DISCUSSION
As previously proposed based on the nucleotide sequence (28), the complete nag genes in Ralstonia sp. strain U2 have now been shown to be expressed as a single operon. Particular care was taken in RT-PCR for the nagD-nagJ-nagI intergenic regions since it is in one of these regions that a separate promoter for the gentisate pathway might be expected; they are the regions where the similarity to the classical nah upper-pathway operon (the 3′ gene of which is nahD) ends and the gentisate pathway genes start, but both RT-PCR of the nagDJI intergenic regions and the failure to obtain a RACE amplification product with a nagI-specific primer indicate that all of the nag genes are cotranscribed as an operon. This includes the two downstream genes, nagMN, which are homologous to each other but do not appear to determine any enzyme activity in the catabolism of gentisate and for which no function has been described yet (28).
Elevated levels of expression of the pathway genes were measured only when the inducer was salicylate, as observed for other nah-like genes (24). The only other gratuitous inducer which we found was 2-nitrobenzoate, which is weakly able to induce the nag genes by a factor of two, although the level is 10-fold less than the level at which salicylate induces the operon. Much of the interest in the nag operon lies in its similarities and differences with the two other catabolic routes, (i) the classical naphthalene degradative pathway (nah) found on plasmids pDTG1 (also known as pWW60-1 [5]) and NAH7 and (ii) the nitroaromatic pathways involving an initial dioxygenase attack (see below).
In the well-established nah pathways naphthalene is converted to salicylate by the enzymes encoded by the upper-pathway operon, and the salicylate is converted via catechol and meta (extradiol) cleavage to central metabolites by the enzymes encoded by the lower- or meta-pathway operon (genes nahG to nahM). On both plasmid pDTG1 and plasmid NAH7 the two operons are separated, but the relative orientations are different, and both operons are regulated by a LysR-type regulator, NahR; the gene that encodes NahR is divergently transcribed from nahG, the upstream gene of the meta-pathway operon.
The organization of the nag genes has similarities but also significant differences. The nag genes that encode enzymes which convert naphthalene to salicylate are homologous to and in the same order as the genes of the nah upper-pathway operon, demonstrating that the genes have a common ancestral origin (Fig. 1). The genes differ (i) by the insertion between nagAa and nagAb of nagGH encoding the catalytic subunits of salicylate 5-hydroxylase, which converts salicylate to gentisate, and (ii) by their fusion to create a single operon with the genes for the gentisate pathway. In this study we found that both nag and nah genes are regulated in the same way, by homologous LysR-type regulatory proteins, but that the genes are located in entirely different relative positions. Whereas the entire intergenic region between nahR and nahG is relatively conserved for classical nah-type genes (19), the corresponding region in the nag system exhibits sequence similarity with the classical system only in the region of the putative LysR-type activator-binding motif (8). A few systematic mutations in this motif in the nag region resulted in varied responses when induction was with salicylate. Four mutants with mutations in the activator-binding motif, namely, pWWF122-Z and pWWF123-Z (both 3-bp deletions), pWWF124-Z (12-bp deletion), and pWWF125-Z (a single substitution), exhibited major decreases in gene expression, reducing the level of induction to less than twofold, compared with about 12-fold reduction for the wild-type (Fig. 2C; Table 4); all of the mutations involve bases which are conserved in the nah and nag systems (Fig. 2B). The likely effect of these changes is either that NagR is unable to bind to the mutant motif or that binding still occurs but the mutations render NagR unable to relieve the DNA bending which results in activation of transcription. In a corresponding study with the nah genes of plasmid NAH7, performed with only substitution mutants, Schell and Poser (24) found that there were only two bases in the putative NahR-binding site which, when changed, produced an equivalent uninducible phenotype, corresponding to bases −72 and −73 (Fig. 2B).
Surprisingly four of our mutations significantly increased the specific activity of induced cells without resulting in a major increase in the activity of uninduced cells, thus making salicylate a more effective inducer. The mutation in pWWF121-Z deleted three bases of the proposed NagR-binding motif (bases −68 to −70) and changed the dyad motif (10) from TTCAN6TGAT to TTATN6TGAT. This removed a guanine, thought to be an obligatory element of the dyad (10), from the 5′ end of the motif, and yet it made salicylate a more potent inducer. Similarly, a G-to-A substitution mutation in pWWF126-Z at position −61 also increased the effectiveness of salicylate as an inducer, and it removed the guanine from the 3′ end of the dyad motif. However, this base is not an absolutely conserved base since in the pDTG1 nahG promoter-proximal region it is an A, not a G (Fig. 2B), so it is perhaps not surprising that the change from one base to the other merely modulated activity rather than destroying it. More surprising were the results obtained with mutants pWWF128-Z and pWWF129-Z, which were included as controls and were expected to behave like the wild type, pWWF121-Z. In these mutants the substitutions were outside the conserved binding motif, but they caused increased levels of gene expression in induced cultures and, to a lesser extent, in uninduced cultures. The expression was not constitutive, and binding of and transcription initiation by the regulator protein NagR still had to occur, but these processes occurred more effectively than they occurred with the wild type. This suggests that the base changes which we introduced at these positions produced additional or alternative promoter or binding sites or that the composition of the DNA on either side of the NagR binding site had an important modulating effect on the initiation of transcription.
So far, we have had little success in trying to purify NagR and have been unable to carry out either footprinting assays or, on a less discriminating level, gel shift assays. Further productive examination of this region requires obtaining purified protein.
We investigated the inducing role of nitro-substituted aromatic compounds because of the clear similarity between the upstream end of the nag operon and the genes encoding the oxygenases involved in the first attack upon nitrobenzene (nbz) (16), 2-nitrotoluene (ntd) (18), and 2,4-dinitrotoluene (dnt) (12). In all of these cases, the four genes (AaAbAcAd) encoding the nitroaromatic dioxygenase are homologs of naphthalene dioxygenase genes, but the sequences are more similar to the sequences of the nag genes than to the sequences of the nah genes (8, 12, 29). Additionally, between the Aa and Ab genes they all contain residual sequences homologous to nagGH, the genes encoding salicylate 5-hydroxylase (8, 29). This fact has been interpreted as showing that the nitroaromatic dioxygenases evolved by recruitment of nag-like naphthalene dioxygenase genes carrying the nagGH insert but subsequently acquired inactivating mutations in the nagGH DNA but the nagGH DNA was not completely deleted (12, 18, 29). Further evidence of the close relationship of the nitroaromatic dioxygenase genes with the corresponding nag genes rather than the nah genes, which has particular relevance to this study, includes (i) the presence of a nagR homolog (>98% amino acid identity) upstream of and divergently transcribed from the dioxygenase genes in all the nitroaromatic pathways (12, 16, 18), (ii) the 99% identity of the nucleotide sequences of the intergenic regions between the Aa genes and the divergently transcribed R genes, and (iii) the fact that salicylate still is able to induce the nbz genes, although various nitroaromatic compounds can also induce their expression (R. E. Parales, personal communication). It is possible that the evolution of the nitroaromatic pathways from nag-like ancestral genes occurred because of a combination of the broad specificity of the dioxygenase and the ability of the induction mechanisms of the nag-like systems to adjust to nitroaromatic compounds as inducers. Our data give some minimal credence to this hypothesis because of the induction by 2-nitrobenzoate, but none of the nitro-substituted hydrocarbons tested had any effect as an inducer (Table 3); additional stronger evidence is required to explain why a nag-like system is the apparent ancestor of the nitroaromatic pathways.
Acknowledgments
This research was supported by funds from the Biotechnology and Biological Sciences Research Council (to R.M.J.).
REFERENCES
- 1.Barnsley, E. A. 1975. Induction of enzymes of naphthalene metabolism in Pseudomonas by salicylate and 2-aminobenzoate. J. Gen. Microbiol. 88:193-196. [DOI] [PubMed] [Google Scholar]
- 2.Bauchop, T., and S. R. Elsden. 1960. The growth of microorganisms in relation to energy supply. J. Gen. Microbiol. 23:457-469. [DOI] [PubMed] [Google Scholar]
- 3.Bosch, R., E. Garcia-Valdes, and E. R. Moore. 1999. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 236:149-157. [DOI] [PubMed] [Google Scholar]
- 4.Bosch, R., E. Garcia-Valdes, and E. R. Moore. 2000. Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene 245:65-74. [DOI] [PubMed] [Google Scholar]
- 5.Cane, P. A., and P. A. Williams. 1986. A restriction map of the catabolic plasmid pWW60-1 and the location of some of its catabolic genes. J. Gen. Microbiol. 132:2919-2929. [Google Scholar]
- 6.deHaseth, P. L., M. L. Zupancic, and M. T. Record, Jr. 1998. RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 180:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuenmayor, S. L., and V. Rodriguez-Lemoine. 1992. Characterization of polycyclic aromatic hydrocarbons degradative soil Pseudomonas. Acta Cient. Venez. 43:349-354. [PubMed] [Google Scholar]
- 8.Fuenmayor, S. L., M. Wild, A. L. Boyes, and P. A. Williams. 1998. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J. Bacteriol. 180:2522-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 10.Huang, J. Z., and M. A. Schell. 1991. In vivo interactions of the NahR transcriptional activator with its target sequences. Inducer-mediated changes resulting in transcription activation. J. Biol. Chem. 266:10830-10838. [PubMed] [Google Scholar]
- 11.Jeenes, D. J., and P. A. Williams. 1982. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J. Bacteriol. 150:188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, G. R., R. K. Jain, and J. C. Spain. 2002. Origins of the 2,4-dinitrotoluene pathway J. Bacteriol. 184:4219-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, R. M., V. Pagmantidis, and P. A. Williams. 2000. sal genes determining the catabolism of salicylate esters are part of a supraoperonic cluster of catabolic genes in Acinetobacter sp. strain ADP1. J. Bacteriol. 182:2018-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 15.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84:467-471. [DOI] [PubMed] [Google Scholar]
- 16.Lessner, D. J., G. R. Johnson, R. E. Parales, J. C. Spain, and D. T. Gibson. 2002. Molecular characterization and substrate specificity of nitrobenzene dioxygenase from Comamonas sp. strain JS765 Appl. Environ. Microbiol. 68:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Parales, J. V., A. Kumar, R. E. Parales, and D. T. Gibson. 1996. Cloning and sequencing of the genes encoding 2-nitrotoluene dioxygenase from Pseudomonas sp. JS42. Gene 181:57-61. [DOI] [PubMed] [Google Scholar]
- 19.Park, W., P. Padmanabhana, S. Padmanabhan, G. J. Zylstra, and E. L. Madsen. 2002. nahR, encoding a LysR-type transcriptional regulator, is highly conserved among naphthalene-degrading bacteria isolated from a coal tar waste-contaminated site and in extracted community DNA. Microbiology 148:2319-2329. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Schell, M. A. 1985. Transcriptional control of the nah and sal hydrocarbon-degradation operons by the nahR gene product. Gene 36:301-309. [DOI] [PubMed] [Google Scholar]
- 22.Schell, M. A. 1986. Homology between nucleotide sequences of promoter regions of nah and sal operons of NAH7 plasmid of Pseudomonas putida. Proc. Natl. Acad. Sci. USA 83:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 24.Schell, M. A., and E. F. Poser. 1989. Demonstration, characterization, and mutational analysis of NahR protein binding to nah and sal promoters. J. Bacteriol. 171:837-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schell, M. A., and P. E. Wender. 1986. Identification of the nahR gene product and nucleotide sequences required for its activation of the sal operon. J. Bacteriol. 166:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains—nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 27.Yen, K.-M., and I. C. Gunsalus. 1982. Plasmid gene organization: naphthalene/salicylate oxidation. Proc. Natl. Acad. Sci. USA 79:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, N.-Y., S. L. Fuenmayor, and P. A. Williams. 2001. nag genes of Ralstonia (formerly Pseudomonas) sp. strain U2 encoding enzymes for gentisate catabolism. J. Bacteriol. 183:700-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou, N. Y., J. Al-Dulayymi, M. S. Baird, and P. A. Williams. 2002. Salicylate 5-hydroxylase from Ralstonia sp. strain U2: a monooxygenase with close relationships to and shared electron transport proteins with naphthalene dioxygenase. J. Bacteriol. 184:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]