Abstract
Saliva plays an important role in digestion, host defense, and lubrication. The parotid gland contributes a variety of secretory proteins—including amylase, proline-rich proteins, and parotid secretory protein (PSP)—to these functions. The regulated secretion of salivary proteins ensures the availability of the correct mix of salivary proteins when needed. In addition, the major salivary glands are targets for gene therapy protocols aimed at targeting therapeutic proteins either to the oral cavity or to circulation. To be successful, such protocols must be based on a solid understanding of protein trafficking in salivary gland cells. In this paper, model systems available to study the secretion of salivary proteins are reviewed. Parotid secretory proteins are stored in large dense-core secretory granules that undergo stimulated secretion in response to extracellular stimulation. Secretory proteins that are not stored in large secretory granules are secreted by either the minor regulated secretory pathway, constitutive secretory pathways (apical or basolateral), or the constitutive-like secretory pathway. It is proposed that the maturing secretory granules act as a distribution center for secretory proteins in salivary acinar cells. Protein distribution or sorting is thought to involve their selective retention during secretory granule maturation. Unlike regulated secretory proteins in other cell types, salivary proteins do not exhibit calcium-induced aggregation. Instead, sulfated proteoglycans play a role in the storage of secretory proteins in parotid acinar cells. This work suggests that unique sorting and retention mechanisms are responsible for the distribution of secretory proteins to different secretory pathways from the maturing secretory granules in parotid acinar cells.
Keywords: secretory granules, regulated secretion, amylase, parotid, saliva
(I) INTRODUCTION
Saliva plays an important role in digestion, host defense, and lubrication. Impaired secretion of saliva is seen in the autoimmune disease Sjögren’s syndrome, as a side-effect of many medications, or after radiation treatments for head and neck cancer. The attendant dry mouth can lead to difficulty in swallowing, oral infection, and severe tooth decay (Liem et al., 1996; Jonsson et al., 2000). The parotid glands contribute a watery, proteinaceous secretion to whole saliva. The major protein components of parotid saliva are amylase, the leucine-rich parotid secretory protein (PSP), and proline-rich proteins (PRPs), in addition to multiple minor components (Wallach et al., 1975). At least 50 components of parotid saliva are resolved by 2-D gel electrophoresis (Yao et al., 2003). While these may include different variants of the same gene products, 35 distinct salivary proteins have been detected by two-dimensional liquid chromatography of whole human saliva (Wilmarth et al., 2004). Importantly, the major parotid secretory proteins and most of the minor protein components are stored in secretory granules in the parotid gland. The goal of this review is to summarize our understanding of the unique storage and secretion pathways for secretory proteins of the parotid gland, with special emphasis on recent advances.
(II) MODEL SYSTEMS FOR THE STUDY OF SALIVARY SECRETION
Secretion of salivary proteins is measured by collection of unstimulated or stimulated saliva. The use of collection devices (e.g., Carlson-Crittenden cups) allows the saliva to be collected directly from the ducts of the major salivary glands. Such studies yield information on the flow and composition of saliva under different physiological or experimental conditions. In addition to apical secretion into the oral cavity, the quantification of salivary proteins in the circulation reveals the extent of basolateral secretion from the salivary glands (Kagami et al., 1996; Baum et al., 1999). Molecular manipulation of protein secretion in whole animals has been achieved by the use of transgenic animals (Samuelson, 1996; Golovan et al., 2001) or by gene transfer to salivary glands in vivo (Kagami et al., 1996; Goldfine et al., 1997). As an alternative to whole-animal studies, several different model systems have been used for the study of protein secretion in salivary cells in vitro.
(A) Parotid Tissue
Parotid tissue slices and freshly isolated parotid cells have been used extensively to study protein trafficking in parotid acinar cells. Careful quantitative analyses of protein traffic by pulse-chase techniques have provided much insight into the relative distributions of secretory proteins in the cellular pathways of the acinar cells (von Zastrow and Castle, 1987; Venkatesh and Gorr, 2002). In general, these studies are limited to a few hours, due to the rapid de-differentiation of parotid acinar cells in culture. As an example, isolated primary parotid acinar cells maintain some secretory granules and other organelles of the secretory pathway for up to 6 mos in culture. However, the amount of amylase decreased to less than 1% of the starting level after only 3 days in culture, and it is not clear if the cells retain the ability to undergo stimulated secretion (Yeh et al., 1991). Prasad and coworkers (1992) also reported the long-term culture of primary parotid acinar cells, but it is not clear if the cells supported stimulated secretion during the extended culture conditions.
(B) Salivary Cell Lines
Established cell lines offer the maximum convenience and flexibility in experimental design. The ability to expand cells, transfect cells, and conduct long-term experiments opens several avenues of investigation that are not readily available in primary cultures. On the other hand, care must be taken in the interpretation of results, due to the altered physiology of most cell lines in vitro, when compared with in vivo tissue conditions (Royce et al., 1993).
Several salivary gland cell lines have been described (Patton and Wellner, 1993). In general, these cell lines are not as highly differentiated as the native tissue, but they do provide insight into aspects of salivary physiology when used appropriately. As an example, the human submandibular gland (HSG) cell line is not highly differentiated under standard culture conditions, but the cells differentiate to a more acinar-like cell type when cultured on matrigel (Royce et al., 1993; Hoffman et al., 1998; Zheng et al., 1998; Jung et al., 2000). The differentiated cells grow in acinar-like structures and support the induction of the amylase promoter by growth factors, as measured by the expression of a reporter protein. However, the cells do not express detectable levels of endogenous amylase or PSP under these conditions (Geetha and Gorr, unpublished).
To test if HSG cells support stimulated secretion of salivary proteins, we over-expressed amylase and PSP in these cells. It was thought that high levels of secretory granule cargo proteins may be required to induce protein storage and stimulated secretion (Keeler et al., 2004). PSP secretion exhibited moderate stimulation in response to secretagogues, whereas amylase did not exhibit significant stimulated secretion (Geetha and Gorr, manuscript in preparation). The lack of stimulated amylase secretion suggested that HSG cells do not support a fully functional regulated secretory pathway. The epithelial secretory protein, secreted alkaline phosphatase (SEAP), a form of human placental alkaline phosphatase that lacks the C-terminal sequence for attachment of a GPI anchor (Brown et al., 1989), exhibited stimulated secretion in HSG cells (Geetha et al., unpublished observation). It is not known why this protein was secreted more effectively by stimulated secretion than amylase. However, the protein is also sorted to secretory granules in pancreatic exocrine AR42J cells (Gorr and Moore, 1999), but not in endocrine cells (Gorr, 1996). Thus, it is possible that the structure of SEAP fortuitously allows for its sorting to exocrine secretory granules.
Similarly, the immortalized rat parotid acinar cell line PAR C5 exhibits only a few secretory granules (Quissell et al., 1998), but moderate stimulated secretion of amylase and PSP is observed when these proteins are over-expressed in transfected cells (Geetha and Gorr, manuscript in preparation). In addition, the PAR C5 cells form monolayers when cultured on membrane supports and exhibit apical secretion of SEAP, a marker for the apical secretory pathway in other epithelial cells (Kuhn et al., 2000). The low level of stimulated secretion observed in both these cell lines makes them difficult to use for experiments aimed at quantifying and modulating the regulated secretory pathway.
(III) SECRETORY PATHWAYS IN PAROTID ACINAR CELLS
Data obtained with a combination of the salivary model systems described above have provided insight into the complex secretory patterns of salivary glands and the multiple pathways for protein secretion. The identification of these pathways, and the protein components secreted by each, has been the subject of intense study, as outlined below and summarized in the Fig.
Figure.
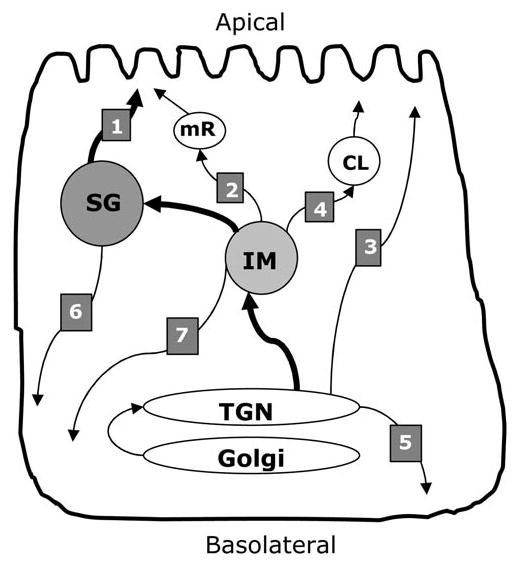
Secretory pathways in parotid acinar cells. The main organelles of the secretory pathway are labeled. TGN, trans-Golgi network; IM, immature secretory granule; SG, mature secretory granules; CL, constitutive-like secretory vesicle; mR, minor regulated pathway vesicle. The vesicular transport pathways discussed in the text are indicated by numbered arrows: (1) major regulated secretory pathway, (2) minor regulated secretory pathway, (3) apical constitutive secretory pathway, (4) constitutive-like secretory pathway, (5) basolateral constitutive secretory pathway, (6) basolateral secretion of secretory granules, and (7) alternate basolateral constitutive secretory pathway.
(A) Regulated Secretion
Parotid acinar cells exhibit two regulated secretory pathways that secrete proteins in response to extracellular stimulation (Castle, 1998). The major regulated secretory pathway involves large secretory granules that are exocytosed in response to muscarinic-cholinergic and adrenergic stimulation (Quissell, 1993) (Fig., pathway 1). This is the classic secretory granule pathway in exocrine cells (Palade, 1975), which accounts for 80–90% of total protein secretion from parotid acinar cells (D Castle and A Castle, 1998). The term ‘secretory granules’ is reserved for large dense-core vesicles that are utilized for protein storage in the major regulated secretory pathway. In addition, a minor regulated secretory pathway that originates in maturing secretory granules was identified more recently (Castle and Castle, 1996) (Fig., pathway 2). Distinct smaller transport vesicles of the minor regulated pathway are secreted in response to pilocarpine and low doses of isoproterenol, conditions that do not stimulate secretion of large secretory granules (D Castle and A Castle, 1998; Castle, 1998). The minor regulated pathway originates in maturing secretory granules and contains many of the same proteins stored in large secretory granules. Thus, the two regulated secretory pathways arise from the maturation of large secretory granules: One (the major pathway) depends on the retention of secretory proteins in developing large secretory granules, the other (minor pathway) is comprised of some of the proteins removed during maturation of secretory granules.
(B) Basal Secretion
Secretion that does not depend on strong extracellular stimulation of the acinar cells is termed ‘basal secretion’. This corresponds to the resting secretion of saliva between meals and includes the exocytosis from the minor regulated secretory pathway (in response to low levels of stimulation between meals) (Huang et al., 2001), as well as the constitutive and constitutive-like secretory pathways described below (in the absence of stimulation) (Fig., pathways 2,3,4).
(C) Constitutive Secretion
Parotid tissue exhibits a small continuous output of salivary proteins in the absence of stimulation. Pulse-chase analysis of unstimulated secretion from parotid tissue has identified three phases of unstimulated protein secretion (von Zastrow and Castle, 1987; D Castle and A Castle, 1998). The early phase constitutes proteins that appear to differ from granule proteins and hence may represent the constitutive secretory pathways in acinar cells (Fig., pathway 3) or constitutive secretion from other cell types in the tissue. No specific secretory marker proteins have been identified for the constitutive secretory pathways in acinar cells. However, it was recently reported that the AQP5 water channel is translocated to the apical cell surface independently of whether salivary protein secretion is stimulated or inhibited (Gresz et al., 2004). Therefore, this apical transport pathway may represent the constitutive secretory pathway in parotid acinar cells (Gresz et al., 2004).
In addition, it is likely that a constitutive secretory pathway exists for the delivery of basolateral plasma membrane proteins and extracellular matrix components (see below for a discussion of polarized secretion). Thus, constitutive secretion may be the only secretion from parotid acinar cells that does not originate in secretory granules, but instead originates in the trans-Golgi network (Fig., pathway 5).
(D) Constitutive-like Secretion
The constitutive-like secretory pathway is defined as protein secretion that originates in maturing secretory granules but does not require stimulation (Arvan et al., 1991; Beaudoin and Grondin, 1992; Arvan and Castle, 1998) (Fig., pathway 4). It represents the slower, second phase of basal protein secretion in pulse-chase experiments with parotid cells (von Zastrow and Castle, 1987). Transport vesicles in this pathway carry proteins that are not retained in large secretory granules during maturation, and thus contribute to the basal (resting) secretion of parotid acinar cells (Huang et al., 2001). As an example, amylase is prominent in both the regulated and constitutive-like secretory pathways, while parotid secretory protein (PSP) is abundant in the regulated secretory pathway and relatively depleted from the constitutive-like secretory pathway (von Zastrow and Castle, 1987). Thus, PSP is a more specific marker for large parotid secretory granules than the more-often-used amylase.
It has been proposed that the constitutive-like secretory pathway and the minor regulated pathway are active between meals when β-adrenergic stimulation is low (Huang et al., 2001). Both pathways are inhibited by brefeldin A, which supports the possible common origin of these pathways in maturing secretory granules. The finding that brefeldin A also inhibited the fusion of large secretory granules at the apical plasma membrane suggests that the minor regulated secretory pathway provides fusion sites for large secretory granules on the apical plasma membrane (Castle et al., 2002). Thus, secretion may be a two-stage process, where low doses of secretagogue ensure the priming of plasma membrane sites by the minor regulated secretory pathway, followed by massive exocytosis of large secretory granules when the dose of secretagogues rises.
(E) Polarized Secretion
Salivary proteins directed to the oral cavity by the regulated or constitutive-like secretory pathways undergo apical secretion. As indicated above, constitutive secretory pathways operate at the apical plasma membrane and perhaps at the basolateral cell surface. However, basolateral secretion into the circulation is poorly defined in parotid acinar cells. Basolateral secretion may involve a separate vesicle pathway that originates in the trans-Golgi network (constitutive secretion, Fig., pathway 5), or secretory vesicles from the granule-derived pathways may be re-routed from the apical to the basolateral cell surface (Fig., pathways 6 and 7). The finding that proteins stored in secretory granules can also be delivered to the basolateral cell surface, where they undergo stimulated secretion into the circulation (Baum et al., 1999), suggests that re-routing of apical secretory granules could play a role in basolateral secretion (Fig., pathway 6). In contrast, increased basolateral protein secretion from salivary glands was also observed in the presence of the weak base hydroxychloroquine in vivo (Hoque et al., 2001). Since weak bases have been shown to disrupt granule storage in parotid acinar cells (von Zastrow et al., 1989), and the increased secretion in vivo was independent of secretagogue, these findings suggest that a non-granule secretory pathway can be routed to the basolateral cell surface (Fig., pathway 7). Thus, while the focus of research on salivary gland secretion has been on apical secretion to the oral cavity, much remains to be learned about the basolateral secretory pathways in acinar cells.
(E) Protein Delivery to Circulation
The parotid and submandibular glands are under consideration as targets for gene therapy protocols aimed at expressing secretory proteins. While expression of therapeutic proteins in the oral cavity is a natural goal for such experiments, the glands are also being considered for protein expression into the circulation. Salivary glands offer the important advantage of easy access for transfection or infection via the salivary ducts, an external route of DNA administration that may cause less inflammation than systemic delivery of DNA vectors. The high capacity for protein synthesis and secretion from salivary glands offers added advantages for gene therapy. As described above, the major route of protein delivery is to the oral cavity. However, physiologically significant amounts of secretory proteins, including insulin and hGH, can also be delivered to the circulation, apparently by the basolateral secretory pathways (Goldfine et al., 1997; Baum et al., 1999, 2004). Protocols for enhanced basolateral secretion and stimulated basolateral secretion have been described (Hoque et al., 2001).
(IV) SECRETION OF SALIVARY PROTEINS FROM NON-SALIVARY CELL TYPES
The lack of salivary cell lines that store secretory proteins in granules has been a significant impediment to progress in the field. Thus, many of the experiments performed in endocrine cells to define sorting mechanisms and signals are not possible in primary parotid cells or tissue culture. Regulated secretory proteins are typically sorted to the regulated secretory pathway in foreign secretory cells (Burgess et al., 1985; Natori et al., 1998). Thus, the study of salivary protein secretion in non-salivary cells may provide information on protein-specific processes, although caution is warranted due to differences between cell types (Gorr et al., 2001).
The sorting and storage of amylase, PRP, and PSP have been analyzed in endocrine cell lines (Castle et al., 1992, 1997; Cowley et al., 2002). Storage of PSP parallels that of the endocrine secretory protein chromogranin A in both pituitary GH4C1 cells and adrenal PC 12 cells (Cowley et al., 2002). This would suggest that the same sorting mechanisms are used by both cell types. However, we have found that chromogranins use different sorting mechanisms in these two cell lines (Gorr et al., 1999; Cowley et al., 2000). Therefore, the sorting mechanism for PSP cannot be deduced from these experiments. GH4C1 cells use calcium-induced aggregation for sorting chromogranin A (Cowley et al., 2000), but not sulfated proteoglycans (Gorr, 1996). In contrast, parotid acinar cells use sulfated proteoglycans for sorting PSP (Venkatesh and Gorr, 2002), but not calcium-induced aggregation (Venkatesh et al., 2004). Which, if any, of these mechanisms are responsible for sorting PSP in endocrine cells remains to be established. However, it is likely that PSP contains protein-specific sorting signals that are recognized in different cell types (Gorr et al., 2001). Unlike PSP, transfected amylase and PRP both are initially sorted to secretory granules in pituitary AtT-20 cells, but the proteins are poorly retained in maturing secretory granules, and instead are secreted by the constitutive-like secretory pathway (Castle et al., 1997).
Sorting signals, i.e., specific sequences that are necessary for sorting of a protein to secretory granules, were thought to be located in regulated secretory proteins. However, linear conserved sorting sequences, similar to the KDEL sequence involved in protein retention in the endoplasmic reticulum (Pelham, 1990), have not been found in regulated secretory proteins. Thus, it is now assumed that structural signals, perhaps similar to the signal peptides in secreted proteins (Blobel, 2000), are located in regulated secretory proteins. Indeed, an N-terminal potential sorting signal has been deduced in several regulated secretory proteins, including PRP (Gorr and Darling, 1995). This signal appeared to play a role in sorting PRP in endocrine cells (Castle et al., 1992). However, more recent studies suggest that a PRP-specific sorting signal is responsible for storage of this protein in secretory granules (Stahl et al., 1996). In fact, the interaction of acidic PRP and basic PRP may play a role in their storage in AtT-20 cells (A Castle and J Castle, 1998). This suggests that sulfated proteoglycans can play protein-specific roles in sorting in AtT-20 cells, which do not use sulfated proteoglycans for sorting of the endogenous peptide hormone ACTH (Burgess and Kelly, 1984). Thus, it appears that while secretory proteins may contain protein-specific sorting signals, these signals can be utilized in different ways by different cell types. This emphasizes the need for caution in the interpretation of experiments where heterologous cell types are used for the study of protein sorting.
(V) EXOCRINE SECRETORY GRANULES
Exocrine cells, including parotid acinar cells, are equipped to store large amounts of secretory proteins in secretory granules. The high protein concentration and large volume of each granule and the large number of granules per cell ensure the significant storage capacity of these cells.
(A) Parotid Secretory Granules
Due to their density and large size, parotid acinar granules can be purified by simple differential centrifugation protocols (Arvan et al., 1984). The granule contents closely match the composition of parotid saliva, suggesting that all major components of saliva are stored in secretory granules. The major proteins include amylase, PSP, and proline-rich proteins. While the composition varies between individuals, salivary composition remains relatively stable under most physiologic conditions (Oberg et al., 1982). However, changes in salivary gene expression are seen in hormone-treated or chronically stimulated animals (Johnson et al., 1987), and the induction of proline-rich proteins by isoproterenol or tannin is well-documented (Robinovitch et al., 1977; Tu et al., 1993). Several salivary proteins play roles in host defense, including PSP, histatins, cystatins, peroxidase, and defensins. Compared with the exocrine pancreas, there is a relative lack of proteolytic enzymes. It is estimated that 80–90% of the secretory proteins in parotid acinar cells are stored in large secretory granules.
Parotid secretory granules generally appear as electron-dense structures in transmission electron microscopy, although a fascinating variety of ultrastructures has been detected in secretory granules from different bat species (Phillips et al., 1993). In humans, secretory granules are seen in the 18-week fetus and are prominent after 24 wks (Yaku, 1983), although it is not known if these are functional granules. In the fetal rat pancreas, secretory granules initially undergo only constitutive secretion, suggesting that the machinery for regulated exocytosis develops after the appearance of secretory granules (Arvan and Chang, 1987).
(VI) SECRETORY GRANULE BIOGENESIS
Secretory granules arise from the trans-Golgi network (TGN), either by vesicular budding or by maturation of the trans-Golgi network. In vesicular budding, secretory granule proteins are concentrated in the TGN and included in condensing vacuoles that bud off from the TGN. Formation of the initial immature secretory granules is not highly specific, since lysosomal and other non-resident proteins are found in immature granules. However, subsequent maturation results in secretory granules that are highly enriched in salivary proteins. Thus, maturation involves condensation of the granule protein contents and the removal of non-granule proteins and excess membrane. Both mature and immature secretory granules can undergo exocytosis in response to extracellular stimulation, but the relative amounts of secretory proteins differ between the two forms. As an alternative to the vesicle-budding hypothesis, it has been proposed that secretory granules are left behind when the TGN membrane and proteins are returned to earlier Golgi stacks by retrograde transport. In this view, the maturation of secretory granules would be the final step in successive compartmental maturations in the secretory pathway, but with an important difference: The proteins that are removed from the maturing secretory granules are not returned to the Golgi apparatus by retrograde transport. Instead, the maturation vesicles are transported to the cell surface for exocytosis (constitutive-like secretory pathway). The formation of secretory granules by budding of immature granules from the TGN is reminiscent of the vesicular transport model for Golgi maturation, while the biogenesis of granules by maturation of the TGN is similar to cisternal maturation of the Golgi complex (Rothman and Wieland, 1996; Elsner et al., 2003). In both models, granule maturation requires that secretory proteins are efficiently retained in the granules during maturation (sorting by retention; Shennan, 1996).
Protein transport vesicles are typically coated by lattice-like protein coats containing COP I, COP II, or clathrin, depending on the organellar origin (Brodsky et al., 2001; Duden, 2003). However, secretory granules do not appear to be completely coated by coat proteins but, instead, exhibit patches of clathrin coats. This suggests that clathrin is not involved in the formation of secretory granules, but mediates membrane removal during maturation (Brodsky et al., 2001). One function of these clathrin coats is the removal of lysosomal proteases from immature parotid secretory granules by a mannose-6-phosphate receptor-mediated process (Klumperman et al., 1998). Thus, the formation of secretory granules and their subsequent maturation may be a continuous process that follows from the maturation of the Golgi compartments.
It has been proposed that specific proteins are required for secretory granule biogenesis and regulated secretion (Day et al., 1995). In endocrine cells, chromogranin A (Kim et al., 2001) and/or chromogranin B (Huh et al., 2003) may act as on/off switches for secretory granule biogenesis, although questions remain about the role of these proteins in some cell types (Day and Gorr, 2003). Chromogranins are stored in acinar cells of the horse but not in rat parotid glands (Sato et al., 2002). Thus, it is unlikely that chromogranin A acts as a universal on/off switch for secretory granules in the parotid gland. While it cannot be excluded that functionally equivalent proteins exist in cell types that do not express chromogranins, such proteins have not been detected.
As an alternative to specific on/off switches for secretory granule biogenesis, it is possible that a “cargo effect” is involved in granule biogenesis (Beuret et al., 2004). According to this model, high levels of secretory proteins in the trans-Golgi network induce the formation of protein complexes that become membrane-enclosed, thus forming the secretory granules. While the simplicity of this model is appealing, the interactions of the granule core with specific membrane proteins remain to be established (Keeler et al., 2004).
(VII) SECRETORY GRANULES AS A CENTER FOR PROTEIN DISTRIBUTION
The presence of multiple secretory pathways in salivary acinar cells demands that specific mechanisms exist for distribution (sorting) of secretory proteins to the correct pathway. Large dense-core secretory granules appear to be at the center of these sorting mechanisms, since both the major and minor regulated secretory pathways, as well as the constitutive-like secretory pathway, originate in this organelle. Several factors suggest that retention (Shennan, 1996) or exclusion (Kuliawat et al., 1997) of secretory proteins in maturing secretory granules plays a key role in protein-sorting. The bulk of the secretory proteins are stored in secretory granules and released by regulated exocytosis. Since similar proteins are released by granule-derived regulated and constitutive-like secretory pathways, albeit in different relative amounts, it appears that most secretory proteins enter immature granules and exit at different rates, based on their retention in mature granules.
(A) Protein Retention and Storage Mechanisms in Secretory Granules
If maturing secretory granules are the main distribution center for secretory proteins in salivary acinar cells, the mechanisms for protein retention in secretory granules will be key regulators of protein secretion. Secretory proteins are stored at high concentrations in secretory granules. However, the storage complex differs between parotid secretory granules and secretory granules from other exocrine and endocrine cell types. Understanding the parotid granules will be important for the targeting of proteins for gene therapy directed at the oral cavity or the circulation. Parotid secretory granules are osmotically active and undergo lysis in low-ionic-strength buffers (Arvan et al., 1984). Thus, the granule core appears to be loosely organized and does not contain aggregated secretory proteins. In contrast, the endocrine secretory granule cores can be highly organized, and some resist hypotonic conditions, suggesting that the contents are not osmotically active (Giannattasio et al., 1975). In pancreatic β-cells, the granule cores consist of crystallized insulin (Coore et al., 1969), while the cores of pituitary secretory granules can be isolated from purified secretory granules after membrane dissolution (Zanini et al., 1980). NMR experiments have suggested that a storage complex held by electrostatic interactions exists in adrenal chromaffin cells (Sharp and Richards, 1977a,b). This complex does not appear to be a rigid aggregate or a gel, but rather a protein complex where the protein polyelectrolytes are complexed with smaller counter-ions (ATP, catecholamines, calcium).
The storage complex in adrenal chromaffin granules depends on calcium. Lysis of secretory granules occurs in the presence of the divalent cation ionophore A23187, with EDTA as an external chelator. Lysis under these conditions is prevented by increased sucrose concentration of the medium, suggesting that the granules undergo osmotic lysis after calcium depletion (Sudhof, 1983). Similarly, exocrine pancreatic secretory granules require calcium for stability (Lebel et al., 1988). In this case, lysis occurred in the presence of A23187, but it did not require an external chelator, suggesting that granular calcium is more loosely bound in pancreatic exocrine granules than in adrenal chromaffin granules. Unlike the pancreatic exocrine granules, calcium is not required for stability of parotid secretory granules (Flashner and Schramm, 1977). Extensive depletion of calcium from isolated granules did not affect the release of amylase. This finding suggests that the storage complex in parotid secretory granules differs from those of endocrine secretory granules and the exocrine pancreas.
(B) Calcium-induced Protein Aggregation
The differences in granule stability seen in calcium-depletion experiments are reflected in the calcium-aggregation properties of secretory proteins from endocrine cells and exocrine pancreas, on the one hand, and the parotid gland, on the other hand. Endocrine secretory proteins and exocrine pancreatic secretory proteins aggregate in vitro under conditions that mimic the high calcium concentration and acidic pH of secretory granules and the trans-Golgi network (Orci et al., 1987; Gorr et al., 1989; Freedman and Scheele, 1993; Leblond et al., 1993). The role of aggregation in granule storage has been studied in particular detail for the endocrine secretory protein chromogranin A (Gorr et al., 1987, 1988, 1989; Yoo and Albanesi, 1990). This protein aggregates in the presence of 10–15 mM calcium and at a pH around 5.5 to 6.0, i.e., the conditions found in endocrine secretory granules. The C-terminal domain of chromogranin A is necessary for both calcium-induced aggregation and storage in some endocrine secretory granules (Cowley et al., 2000). Conversely, when calcium-induced aggregation is enhanced by helper proteins, chromogranin A exhibits increased storage in secretory granules of the endocrine cell line GH4C1 (Jain et al., 2000, 2002).
Exocrine pancreatic granule contents also aggregate in the presence of calcium and at low pH, suggesting that these proteins may also form a storage complex in pancreatic zymogen granules. Unlike endocrine proteins, the purified pancreatic proteins do not aggregate in vitro (Gorr and Tseng, 1995), suggesting that a mixture of pancreatic proteins is necessary for aggregation. Indeed, we have found that chymotrypsinogen forms protein-protein interactions with other regulated secretory proteins, including amylase (Gorr et al., 1992). Such interactions between secretory granule proteins are presumably required to form a storage complex in pancreatic zymogen granules.
Parotid secretory granule proteins do not aggregate in vitro. Aggregation has been tested for granule content proteins at different pH conditions and in the presence or absence of calcium. While small amounts of PSP could be precipitated by centrifugation, amylase and most other granule proteins did not exhibit aggregation (Venkatesh et al., 2004). Protein concentration is considered a critical factor in protein aggregation. To mimic the conditions of the secretory granules, isolated parotid granules were permeabilized with Saponin under aggregating or non-aggregating conditions. In each case, the content proteins were readily released from the permeabilized granules (Venkatesh et al., 2004). In contrast, amylase was partially retained in permeabilized pancreatic zymogen granules. Thus, the aggregation properties of pancreatic and parotid secretory granule proteins are consistent with the results of the calcium-depletion experiments. The finding that pancreatic and parotid amylase exhibit different aggregation properties suggests that other secretory granule components play a role in their aggregation (Gorr et al., 1992; Gorr and Tseng, 1995).
(C) The Role of pH in Granule Function
Organelles of the distal secretory pathway exhibit an acidic pH. Vacuolar H+ ATPase is found in the membrane of secretory granules, endosomes, and lysosomes and combines with proton leakage and other ion pumps to regulate the luminal pH of these organelles (Grabe and Oster, 2001). In endocrine cells, the secretory granules exhibit an acidic pH in the range from 5.5 to 6.0 (Anderson and Orci, 1988). Immature exocrine secretory granules also exhibit an acidic pH, but the mature granules exhibit a pH just below neutral (Arvan et al., 1984; Orci et al., 1987). The acidic pH of immature parotid secretory granules may play a role in protein retention. When weak bases are added to parotid acinar cells, newly synthesized secretory proteins still enter secretory granules and can undergo stimulated secretion. However, the proteins are poorly retained in mature granules and are preferentially secreted by the constitutive-like secretory pathway (von Zastrow et al., 1989). This effect is particularly pronounced for PSP (p25) and acidic epididymal glycoprotein (p32), which are normally stored with high efficiency in secretory granules (von Zastrow and Castle, 1987). In contrast, weak bases have no effect on the storage of proteins that are already located in mature secretory granules (von Zastrow et al., 1989). These findings suggest that the weak base acts on the storage of secretory proteins by interfering with their selective retention in maturing secretory granules.
In addition to protein storage in secretory granules, it appears that polarized secretion of secretory proteins is also affected by the pH in the secretory pathway. When the endocrine-regulated secretory protein hGH is expressed in the rat submandibular gland, the protein is predominantly stored in secretory granules and secreted into the oral cavity (Mastrangeli et al., 1994). However, when the animals are treated with the weak base hydroxychloroquine, basolateral secretion into circulation is significantly increased (Hoque et al., 2001). This finding suggests that alkalinization of immature secretory granules causes re-routing of hGH to the basolateral cell surface. The finding that basolateral secretion can be enhanced by modification of secretory granule storage may have practical implications for the delivery of therapeutic proteins to circulation (Mastrangeli et al., 1994; Kagami et al., 1996; Goldfine et al., 1997).
If the pH of secretory granules modulates the storage of proteins in maturing granules, then proteins that affect granule pH will directly affect protein storage in secretory granules. Granule pH is regulated by a combination of V-H+ ATPase and proton leakage. The latter, in turn, may be affected by the combination of acidic and basic proteins that are stored in secretory granules. Thus, changes in the relative amounts of basic and acidic proteins may affect the storage of secretory proteins in secretory granules. As an example, rats treated with the β-adrenergic agonist isoproterenol exhibit more translucent parotid granules than untreated animals (Bloom et al., 1979), suggesting that the granule contents are less condensed in treated rats than in untreated rats. This is supported by measurements of the mean internal space in isolated parotid secretory granules. Thus, granules from isoproterenol-treated rats exhibit twice the internal space (expressed as μL/mg protein) as granules from untreated rats (Arvan et al., 1984; Arvan and Castle, 1986). Isoproterenol treatment induces the expression of basic and acidic proline-rich proteins (PRPs) (Robinovitch et al., 1977), including sulfated proteoglycans (Blair et al., 1991). However, basic PRPs account for 90% of the induced proteins (Muenzer et al., 1979), suggesting that an excess of basic PRPs is correlated with less granule condensation. Consistent with the proposed role of PRPs in the buffering of secretory granules, the internal pH of granules from isoproterenol-treated animals is higher than the pH of granules from untreated animals (Arvan and Castle, 1986).
(D) Sulfated Proteoglycans and Granule Storage
Sulfated proteoglycans are large polyanions that are stored in secretory granules of most cell types. The highly acidic proteoglycans have long been proposed to play a role in protein storage in exocrine secretory granules (Reggio and Dagorn, 1978). Sulfated proteoglycans are secreted from both parotid and submandibular glands (Iversen et al., 1987; Blair et al., 1991; Kamada et al., 1996). In the rat parotid gland, two sulfated proteoglycans have been characterized (Blair et al., 1991). The core proteins for these proteoglycans have been cloned (PRPG1, PRPG2). Both are acidic proline-rich proteins (aPRP) (Castle and Castle, 1993). The addition of acidic glycosaminoglycan chains to the acidic PRP core proteins may serve to balance the positive charges from basic PRPs in secretory granules (Blair et al., 1991). This may be of particular importance in tannin- or isoproterenol-treated animals, which exhibit high basic PRP levels.
The concentration of sulfur in secretory granules increases during their maturation (Izutsu et al., 1994), supporting the proposed function of proteoglycans in protein storage in secretory granules. Since proteoglycan synthesis in parotid acinar cells can be inhibited by xyloside analogs (Robinovitch et al., 1992), this approach has been used to test the role of sulfated proteoglycans in the storage of amylase and PSP in parotid secretory granules (Venkatesh and Gorr, 2002). Xyloside had little effect on the storage of proteins that were already stored in secretory granules, suggesting that the xyloside is not toxic to the parotid acinar cells. However, inhibition of proteoglycan synthesis reduced the storage of newly synthesized amylase and PSP by about 50%. This was reflected both in direct granule content and in stimulated secretion of these proteins (Venkatesh and Gorr, 2002). The effects of the proteoglycan synthesis inhibitors on protein storage are reversed when parotid tissue is treated with acetic acid (Venkatesh and Gorr, 2002). This suggests that acidification of secretory granules by a weak acid could substitute for the presence of sulfated proteoglycans.
(E) Acidic Proteins in Non-salivary Cell Types
The proposed role of the acidic sulfated proteoglycans acting in the sorting and storage of secretory proteins is not unique to the parotid gland. Thus, the acidic PRP is sorted more efficiently to endocrine secretory granules than is basic PRP (Castle and Castle, 1993). Sulfated glycoproteins may play a role in the storage of regulated secretory proteins in pancreatic zymogen granules (De Lisle, 2002), and heparin sulfate is necessary for the storage of cationic proteins in mast cell secretory granules (Humphries et al., 1999). In contrast, sulfated proteoglycans are not necessary for the sorting and storage of secretory proteins in endocrine cells (Burgess and Kelly, 1984; Gorr, 1996; A Castle and J Castle, 1998). Instead, most endocrine cells store significant amounts of chromogranin A in dense-core secretory vesicles. Chromogranin A is an acidic sulfated glycoprotein that has been proposed to play a role in the condensation of secretory granule contents (Gorr et al., 1987) and stabilization of granule pH (Fasciotto and Gorr, in preparation). The presence of chromogranin A in endocrine secretory granules may explain why sulfated proteoglycans are not necessary for the sorting of regulated secretory proteins in endocrine cells.
(F) Sulfated Glycoproteins in Other Cell Types
Sulfated proteoglycans and glycoproteins are not unique to the sorting of secretory proteins in parotid acinar cells. The major sulfated proteoglycans in mast cells are necessary for protein storage in secretory granules (Forsberg et al., 1999; Humphries et al., 1999). Similarly, a sulfated glycoprotein plays a role in protein storage in pancreatic exocrine cells (De Lisle, 2002). Indeed, the aggregation of regulated secretory proteins and glycosaminoglycans was first described for exocrine pancreatic secretory granules and was proposed to play a role in protein storage (Reggio and Dagorn, 1978). Unlike mast and exocrine cells, sulfated proteoglycans are not necessary for protein storage in secretory granules of pituitary AtT-20 cells (Burgess and Kelly, 1984) or pituitary GH4C 1 cells (Gorr, 1996).
(VII) CONCLUDING REMARKS
It was recognized early that secretory proteins that are stored in secretory granules in one cell type can be stored in secretory granules in other cell types as well (Burgess et al., 1985). This finding has since been generalized by the expression of regulated secretory proteins in a variety of secretory cell types. Importantly, this general ability of regulated secretory proteins to be stored in different secretory granules does not correlate with the presence of universal sorting mechanisms or signals (Gorr et al., 2001). Calcium- and low-pH-induced aggregation has been recognized as a frequent property of regulated secretory proteins from multiple cell types. However, it is now clear that protein storage in parotid secretory granules involves a different mechanism (Venkatesh and Gorr, 2002; Venkatesh et al., 2004). Parotid secretory granules serve as a distribution center for regulated secretory proteins in the major and minor regulated secretory pathways and for constitutive-like secretory proteins (Fig.). A better understanding of the retention and release of secretory proteins from maturing granules is needed if we are to control the distribution of secretory proteins within these pathways. To achieve this, future work should focus on the roles of acidic and basic granule proteins and ion channels in regulating the internal ionic milieu and protein storage in secretory granules. Ionic control of protein storage could offer a suitable method for the regulation of protein release in engineered salivary acinar cells (Hoque et al., 2001).
Acknowledgments
Dr. C. Geetha is thanked for sharing unpublished data. Work in the authors’ laboratory was supported by PHS grant R01 DE 12205.
References
- Anderson R, Orci L. A view of acidic intracellular compartments. J Cell Biol. 1988;106:539–543. doi: 10.1083/jcb.106.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H+ pump activity. J Cell Biol. 1986;103:1257–1267. doi: 10.1083/jcb.103.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Chang A. Constitutive protein secretion from the exocrine pancreas of fetal rats. J Biol Chem. 1987;262:3886–3890. [PubMed] [Google Scholar]
- Arvan P, Rudnick G, Castle JD. Osmotic properties and internal pH of isolated rat parotid secretory granules. J Biol Chem. 1984;259:13567–13572. [PubMed] [Google Scholar]
- Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, et al. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. [PubMed] [Google Scholar]
- Baum BJ, Berkman ME, Marmary Y, Goldsmith CM, Baccaglini L, Wang S, et al. Polarized secretion of transgene products from salivary glands in vivo. Hum Gene Ther. 1999;10:2789–2797. doi: 10.1089/10430349950016528. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Voutetakis A, Wang J. Salivary glands: novel target sites for gene therapeutics. Trends Mol Med. 2004;10:585–590. doi: 10.1016/j.molmed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Beaudoin AR, Grondin G. Zymogen granules of the pancreas and the parotid gland and their role in cell secretion. Int Rev Cytol. 1992;132:177–222. doi: 10.1016/s0074-7696(08)62456-0. [DOI] [PubMed] [Google Scholar]
- Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem. 2004;279:20242–20249. doi: 10.1074/jbc.M310613200. [DOI] [PubMed] [Google Scholar]
- Blair EA, Castle AM, Castle JD. Proteoglycan sulfation and storage parallels storage of basic secretory proteins in exocrine cells. Am J Physiol. 1991;261:C897–C905. doi: 10.1152/ajpcell.1991.261.5.C897. [DOI] [PubMed] [Google Scholar]
- Blobel G. Protein targeting (Nobel lecture) Chem Bio Chem. 2000;1:86–102. doi: 10.1002/1439-7633(20000818)1:2<86::AID-CBIC86>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bloom GD, Carlson B, Danielsson A, Gustafsson H, Henriksson R. Quantitative structural analysis and the secretory behaviour of the rat parotid gland after long and short term isoprenaline treatment. Med Biol. 1979;57:224–233. [PubMed] [Google Scholar]
- Brodsky FM, Chen C-Y, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Sorting and secretion of adrenocorticotropin in a pituitary tumor cell line after perturbation of the level of a secretory granule-specific proteoglycan. J Cell Biol. 1984;99:2223–2230. doi: 10.1083/jcb.99.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Craik CS, Kelly RB. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985;101:639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Castle JD. Novel secretory proline-rich proteoglycans from rat parotid. Cloning and characterization by expression in AtT-20 cells. J Biol Chem. 1993;268:20490–20496. [PubMed] [Google Scholar]
- Castle AM, Castle JD. Enhanced glycosylation and sulfation of secretory proteoglycans is coupled to the expression of a basic secretory protein. Mol Biol Cell. 1998;9:575–583. doi: 10.1091/mbc.9.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Stahl LE, Castle JD. A 13-amino acid n-terminal domain of a basic proline-rich protein is necessary for storage in secretory granules and facilitates exit from the endoplasmic reticulum. J Biol Chem. 1992;267:13093–13100. [PubMed] [Google Scholar]
- Castle AM, Huang AY, Castle JD. Passive sorting in maturing granules of AtT-20 cells: the entry and exit of salivary amylase and proline-rich protein. J Cell Biol. 1997;138:45–54. doi: 10.1083/jcb.138.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Huang AY, Castle JD. The minor regulated pathway, a rapid component of salivary secretion, may provide docking/fusion sites for granule exocytosis at the apical surface of acinar cells. J Cell Sci. 2002;115:2963–2973. [Google Scholar]
- Castle D, Castle A. Intracellular transport and secretion of salivary proteins. Crit Rev Oral Biol Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Castle JD. Protein secretion by rat parotid acinar cells: pathways and regulation. Ann NY Acad Sci. 1998;842:115–124. doi: 10.1111/j.1749-6632.1998.tb09639.x. [DOI] [PubMed] [Google Scholar]
- Castle JD, Castle AM. Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci. 1996;109:2591–2599. doi: 10.1242/jcs.109.10.2591. [DOI] [PubMed] [Google Scholar]
- Coore H, Hellman B, Pihl E, Taljedal I. Physicochemical characteristics of insulin secretion granules. Biochem J. 1969;111:107–113. doi: 10.1042/bj1110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley DJ, Moore YR, Darling DS, Joyce PB, Gorr SU. N- and C-terminal domains direct cell type-specific sorting of chromogranin A to secretory granules. J Biol Chem. 2000;275:7743–7748. doi: 10.1074/jbc.275.11.7743. [DOI] [PubMed] [Google Scholar]
- Cowley DJ, Chu M, Gorr S-U. Sorting of an exocrine secretory protein to the regulated secretory pathway in endocrine cells. Biochem Biophys Res Commun. 2002;299:98–101. doi: 10.1016/s0006-291x(02)02576-7. [DOI] [PubMed] [Google Scholar]
- Day R, Gorr S-U. Secretory granule biogenesis and chromogranin A: master gene, on/off switch or assembly factor? Trends Endocr Metabol. 2003;14:10–13. doi: 10.1016/s1043-2760(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Day R, Benjannet S, Matsuuchi L, Kelly RB, Marcinkiewicz M, Chretien M, et al. Maintained PC1 and PC2 expression in the AtT-20 variant cell line 6T3 lacking regulated secretion and POMC: restored POMC expression and regulated secretion after cAMP treatment. DNA Cell Biol. 1995;14:175–188. doi: 10.1089/dna.1995.14.175. [DOI] [PubMed] [Google Scholar]
- De Lisle RC. Role of sulfated O-linked glycoproteins in zymogen granule formation. J Cell Sci. 2002;115:2941–2952. [Google Scholar]
- Duden R. ER-to-Golgi transport: COP I and COP II function (review) Mol Membr Biol. 2003;20:197–207. doi: 10.1080/0968768031000122548. [DOI] [PubMed] [Google Scholar]
- Elsner M, Hashimoto H, Nilsson T. Cisternal maturation and vesicle transport: join the band wagon! Mol Membr Biol. 2003;20:221–229. doi: 10.1080/0968768031000114024. [DOI] [PubMed] [Google Scholar]
- Flashner Y, Schramm M. Retention of amylase in the secretory granules of parotid gland after extensive release of Ca[++] by ionophore A-23187. J Cell Biol. 1977;74:789–793. doi: 10.1083/jcb.74.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- Freedman SD, Scheele GA. Regulated secretory proteins in the exocrine pancreas aggregate under conditions that mimic the trans-Golgi network. Biochem Biophys Res Commun. 1993;197:992–999. doi: 10.1006/bbrc.1993.2577. [DOI] [PubMed] [Google Scholar]
- Giannattasio G, Zanini A, Meldolesi J. Molecular organization of rat prolactin granules. I. In vitro stability of intact and “membraneless” granules. J Cell Biol. 1975;64:246–251. doi: 10.1083/jcb.64.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine ID, German MS, Tseng HC, Wang J, Bolaffi JL, Chen JW, et al. The endocrine secretion of human insulin and growth hormone by exocrine glands of the gastrointestinal tract. Nat Biotechnol. 1997;15:1378–1382. doi: 10.1038/nbt1297-1378. [DOI] [PubMed] [Google Scholar]
- Golovan SP, Hayes MA, Phillips JP, Forsberg CW. Transgenic mice expressing bacterial phytase as a model for phosphorus pollution control. Nat Biotechnol. 2001;19:429–433. doi: 10.1038/88091. [DOI] [PubMed] [Google Scholar]
- Gorr S-U. Differential storage of prolactin, granins (chromogranin B and secretogranin II), and constitutive secretory markers in rat pituitary GH4C1 cells. J Biol Chem. 1996;271:3575–3580. doi: 10.1074/jbc.271.7.3575. [DOI] [PubMed] [Google Scholar]
- Gorr S-U, Darling DS. An N-terminal hydrophobic peak is the sorting signal of regulated secretory proteins. FEBS Lett. 1995;361:8–12. doi: 10.1016/0014-5793(95)00142-v. [DOI] [PubMed] [Google Scholar]
- Gorr S-U, Moore YR. Sorting of a constitutive secretory protein to the regulated secretory pathway of exocrine cells. Biochem Biophys Res Commun. 1999;257:545–548. doi: 10.1006/bbrc.1999.0504. [DOI] [PubMed] [Google Scholar]
- Gorr S-U, Tseng SY. Aggregation and concentration-dependent sorting of exocrine regulated secretory proteins. Biochem Biophys Res Commun. 1995;215:82–88. doi: 10.1006/bbrc.1995.2436. [DOI] [PubMed] [Google Scholar]
- Gorr SU, Kumarasamy R, Dean WL, Cohn DV. New suggestions for the physiological role of secretory protein-I. Bone Miner. 1987;2:251–255. [PubMed] [Google Scholar]
- Gorr SU, Dean WL, Radley TL, Cohn DV. Calcium-binding and aggregation properties of parathyroid secretory protein-I (chromogranin A) Bone Miner. 1988;4:17–25. [PubMed] [Google Scholar]
- Gorr SU, Shioi J, Cohn DV. Interaction of calcium with porcine adrenal chromogranin A (secretory protein-I) and chromogranin B (secretogranin I) Am J Physiol. 1989;257:E247–E254. doi: 10.1152/ajpendo.1989.257.2.E247. [DOI] [PubMed] [Google Scholar]
- Gorr SU, Hamilton JW, Cohn DV. Regulated, but not constitutive, secretory proteins bind porcine chymotrypsinogen. J Biol Chem. 1992;267:21595–21600. [PubMed] [Google Scholar]
- Gorr SU, Huang XF, Cowley DJ, Kuliawat R, Arvan P. Disruption of disulfide bonds exhibits differential effects on trafficking of regulated secretory proteins. Am J Physiol. 1999;277:C121–C131. doi: 10.1152/ajpcell.1999.277.1.C121. [DOI] [PubMed] [Google Scholar]
- Gorr S-U, Jain RK, Kuehn U, Joyce PBM, Cowley DJ. Comparative sorting of neuroendocrine secretory proteins: a search for common ground in a mosaic of sorting models and mechanisms. Mol Cell Endocrinol. 2001;172:1–6. doi: 10.1016/s0303-7207(00)00342-7. [DOI] [PubMed] [Google Scholar]
- Grabe M, Oster G. Regulation of organelle acidity. J Gen Physiol. 2001;117:329–344. doi: 10.1085/jgp.117.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresz V, Kwon TH, Gong H, Agre P, Steward MC, King LS, et al. Immunolocalization of AQP5 in rat parotid and submandibular salivary glands after stimulation or inhibition of secretion in vivo. Am J Physiol Gastrointest Liver Physiol. 2004;287:G151–G161. doi: 10.1152/ajpgi.00480.2003. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Nomizu M, Roque E, Lee S, Jung DW, Yamada Y, et al. Laminin-1 and Laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J Biol Chem. 1998;273:28633–28641. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- Hoque AT, Baccaglini L, Baum BJ. Hydroxychloroquine enhances the endocrine secretion of adenovirus-directed growth hormone from rat submandibular glands in vivo. Hum Gene Ther. 2001;12:1333–1341. doi: 10.1089/104303401750270986. [DOI] [PubMed] [Google Scholar]
- Huang AY, Castle AM, Hinton BT, Castle JD. Resting (basal) secretion of proteins is provided by the minor regulated and constitutive-like pathways and not granule exocytosis in parotid acinar cells. J Biol Chem. 2001;276:22296–22306. doi: 10.1074/jbc.M100211200. [DOI] [PubMed] [Google Scholar]
- Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- Iversen JM, Keller PJ, Kauffman DL, Robinovitch MR. The presence of chondroitin sulfate in parotid secretory granules and saliva of the rat. Cell Tissue Res. 1987;250:221–226. doi: 10.1007/BF00214675. [DOI] [PubMed] [Google Scholar]
- Izutsu KT, Cantino ME, Johnson DE. A review of electron probe x-ray microanalysis studies of salivary gland cells. Microsc Res Techn. 1994;27:71–79. doi: 10.1002/jemt.1070270106. [DOI] [PubMed] [Google Scholar]
- Jain RK, Joyce PBM, Gorr S-U. Aggregation chaperones enhance aggregation and storage of secretory proteins in endocrine cells. J Biol Chem. 2000;275:27032–27036. doi: 10.1074/jbc.M000095200. [DOI] [PubMed] [Google Scholar]
- Jain RK, Chang WT, Geetha C, Joyce PB, Gorr SU. In vitro aggregation of the regulated secretory protein chromogranin A. Biochem J. 2002;13:605–610. doi: 10.1042/BJ20021195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Alvares OF, Etzel KR, Kalu DN. Regulation of salivary proteins. J Dent Res. 1987;66:576–582. doi: 10.1177/00220345870660023201. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Haga HJ, Gordon TP. Current concepts on diagnosis, autoantibodies and therapy in Sjögren’s syndrome. Scand J Rheumatol. 2000;29:341–348. doi: 10.1080/030097400447525. [DOI] [PubMed] [Google Scholar]
- Jung D, Hecht D, Ho S, O’Connell B, Kleinman H, Hoffman M. PKC and ERK1/2 regulate amylase promoter activity during differentiation of a salivary gland cell line. J Cell Physiol. 2000;185:215–225. doi: 10.1002/1097-4652(200011)185:2<215::AID-JCP6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Kagami H, O’Connell BC, Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther. 1996;7:2177–2184. doi: 10.1089/hum.1996.7.17-2177. [DOI] [PubMed] [Google Scholar]
- Kamada A, Tamura I, Okazaki J, Matsukawa F, Sakaki T. Characteristics and localization of rat submandibular gland proteoglycans. Arch Oral Biol. 1996;41:951–958. doi: 10.1016/s0003-9969(96)00031-3. [DOI] [PubMed] [Google Scholar]
- Keeler C, Hodsdon ME, Dannies PS. Is there structural specificity in the reversible protein aggregates that are stored in secretory granules? J Mol Neurosci. 2004;22:43–50. doi: 10.1385/JMN:22:1-2:43. [DOI] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng J, Eiden LE, Loh YP. Chromogranin A, an “on/off’ switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn U, Cohn D, Gorr S. Polarized secretion of the regulated secretory protein chromogranin A. Biochem Biophys Res Commun. 2000;270:631–636. doi: 10.1006/bbrc.2000.2469. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel D, Grondin G, Paquette J. In vitro stability of pancreatic zymogen granules: roles of pH and calcium. Biol Cell. 1988;63:343–353. [PubMed] [Google Scholar]
- Leblond FA, Viau G, Laine J, LeBel D. Reconstitution in vitro of the pH-dependent aggregation of pancreatic zymogens en route to the secretory granule: implications of GP-2. Biochem J. 1993;291:289–296. doi: 10.1042/bj2910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem IH, Olmos RA, Balm AJ, Keus RB, van Tinteren H, Takes RP, et al. Evidence for early and persistent impairment of salivary gland excretion after irradiation of head and neck tumours. Eur J Nucl Med. 1996;23:1485–1490. doi: 10.1007/BF01254473. [DOI] [PubMed] [Google Scholar]
- Mastrangeli A, O’Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol. 1994;266:G1146–G1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- Muenzer J, Bilstein C, Gleason M, Carlson DM. Purification of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979;254:5623–5628. [PubMed] [Google Scholar]
- Natori S, King A, Hellwig A, Weiss U, Iguchi H, Tsuchiya B, et al. Chromogranin B (secretogranin I), a neuroendocrine-regulated secretory protein, is sorted to exocrine secretory granules in transgenic mice. EMBO J. 1998;17:3277–3289. doi: 10.1093/emboj/17.12.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg SG, Izutsu KT, Truelove EL. Human parotid saliva protein composition: dependence on physiological factors. Am J Physiol. 1982;242:G231–G236. doi: 10.1152/ajpgi.1982.242.3.G231. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Anderson RGW. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature. 1987;326:77–79. doi: 10.1038/326077a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;489:347–357. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Patton L, Wellner R. Established salivary cell lines. In: Dobrosielski-Vergona K, editor. Biology of the salivary glands. Boca Raton: CRC Press; 1993. pp. 319–341. [Google Scholar]
- Pelham HR. The retention signal for soluble proteins of the endoplasmic reticulum. TIBS. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Phillips C, Tandler B, Nagato T. Evolutionary divergence of salivary gland acinar cells: a format for understanding molecular evolution. In: Dobrosielski-Vergona K, editor. Biology of the salivary glands. Boca Raton: CRC Press; 1993. pp. 39–80. [Google Scholar]
- Prasad KN, Edwards-Prasad J, Carvalho E, La Rosa FG, Balbinder E, Meyers A, et al. Establishment of primary cultures of rat and human parotid epithelial cells for transfection experiments. In Vitro Cell Dev Biol. 1992;28A:493–499. doi: 10.1007/BF02634132. [DOI] [PubMed] [Google Scholar]
- Quissell DO. Stimulus-exocytosis coupling mechanism in salivary gland cells. In: Dobrosielski-Vergona K, editor. Biology of the salivary glands. Boca Raton: CRC Press, Inc; 1993. pp. 181–200. [Google Scholar]
- Quissell DO, Barzen KA, Redman RS, Camden JM, Turner JT. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev Biol Anim. 1998;34:58–67. doi: 10.1007/s11626-998-0054-5. [DOI] [PubMed] [Google Scholar]
- Reggio H, Dagorn C. Ionic interactions between bovine chymotrypsinogen A and chondroitin sulfate ABC. J Cell Biol. 1978;78:951–957. doi: 10.1083/jcb.78.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinovitch MR, Keller PJ, Johnson DA, Iversen JM, Kauffman DL. Changes in rat parotid salivary proteins induced by chronic isoproterenol administration. J Dent Res. 1977;56:290–303. doi: 10.1177/00220345770560031501. [DOI] [PubMed] [Google Scholar]
- Robinovitch MR, Iversen JM, Izutsu KT. Inhibition of synthesis of rat parotid secretory proteoglycan in a gland slice system. Arch Oral Biol. 1992;37:209–214. doi: 10.1016/0003-9969(92)90090-u. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Royce LS, Kibbey MC, Mertz P, Kleinman HK, Baum BJ. Human neoplastic submandibular intercalated duct cells express an acinar phenotype when cultured on a basement membrane matrix. Differentiation. 1993;52:247–255. doi: 10.1111/j.1432-0436.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Samuelson LC. Transgenic approaches to salivary gland research. Annu Rev Physiol. 1996;58:209–229. doi: 10.1146/annurev.ph.58.030196.001233. [DOI] [PubMed] [Google Scholar]
- Sato F, Kanno T, Nagasawa S, Yanaihara N, Ishida N, Hasegawa T, et al. Immunohistochemical localization of chromogranin A in the acinar cells of equine salivary glands contrasts with rodent glands. Cells Tissues Organs. 2002;172:29–36. doi: 10.1159/000064389. [DOI] [PubMed] [Google Scholar]
- Sharp R, Richards E. Analysis of the carbon-13 and proton NMR spectra of bovine chromaffin granules. Biochim Biophys Acta. 1977a;497:14–28. doi: 10.1016/0304-4165(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Sharp R, Richards E. Molecular mobilities of soluble components in the aqueous phase of chromaffin granules. Biochim Biophys Acta. 1977b;497:260–271. doi: 10.1016/0304-4165(77)90159-3. [DOI] [PubMed] [Google Scholar]
- Shennan KIJ. Intracellular targeting of secretory proteins in neuroendocrine cells. Biochem Soc Trans. 1996;24:535–539. doi: 10.1042/bst0240535. [DOI] [PubMed] [Google Scholar]
- Stahl L, Wright R, Castle J, Castle A. The unique proline-rich domain of parotid prolinerich proteins functions in secretory sorting. J Cell Sci. 1996;109:1637–1645. doi: 10.1242/jcs.109.6.1637. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Evidence for a divalent cation dependent catecholamine storage complex in chromaffin granules. Biochem Biophys Res Commun. 1983;116:663–668. doi: 10.1016/0006-291x(83)90576-4. [DOI] [PubMed] [Google Scholar]
- Tu ZJ, Lazowski KW, Ehlenfeldt RG, Wu G, Lin HH, Kousvelari E, et al. Isoproterenol/tannin-dependent R15 expression in transgenic mice is mediated by an upstream parotid control region. Gene Exp. 1993;3:289–305. [PMC free article] [PubMed] [Google Scholar]
- Venkatesh SG, Gorr SU. A sulfated proteoglycan is necessary for storage of exocrine secretory proteins in the rat parotid gland. Am J Physiol Cell Physiol. 2002;283:C438–C445. doi: 10.1152/ajpcell.00552.2001. [DOI] [PubMed] [Google Scholar]
- Venkatesh SG, Cowley DJ, Gorr S-U. Differential aggregation properties of secretory proteins that are stored in exocrine secretory granules of the pancreas and parotid glands. Am J Physiol Cell Physiol. 2004;286:C365–C371. doi: 10.1152/ajpcell.00338.2003. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Castle JD. Protein sorting among two distinct export pathways occurs from the content of maturing exocrine storage granules. J Cell Biol. 1987;105:2675–2684. doi: 10.1083/jcb.105.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Castle AM, Castle JD. Ammonium chloride alters secretory protein sorting within the maturing exocrine storage compartment. J Biol Chem. 1989;264:6566–6571. [PubMed] [Google Scholar]
- Wallach D, Tessler R, Schramm M. The proteins of the content of the secretory granules of the rat parotid gland. Biochim Biophys Acta. 1975;382:552–564. doi: 10.1016/0005-2736(75)90222-9. [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Riviere MA, Rustvold DL, Lauten JD, Madden TE, David LL. Two-dimensional liquid chromatography study of the human whole saliva proteome. J Proteome Res. 2004;3:1017–1023. doi: 10.1021/pr049911o. [DOI] [PubMed] [Google Scholar]
- Yaku Y. Ultrastructural studies on the development of human fetal salivary glands. Arch Histol Jpn. 1983;46:677–690. doi: 10.1679/aohc.46.677. [DOI] [PubMed] [Google Scholar]
- Yao Y, Berg EA, Costello CE, Troxler RF, Oppenheim FG. Identification of protein components in human acquired enamel pellicle and whole saliva using novel proteomics approaches. J Biol Chem. 2003;278:5300–5308. doi: 10.1074/jbc.M206333200. [DOI] [PubMed] [Google Scholar]
- Yeh C, Mertz PM, Oliver C, Baum BJ, Kousvelari EE. Cellular characteristics of long-term cultured rat parotid acinar cells. In Vitro Cell Dev Biol. 1991;27A:707–712. doi: 10.1007/BF02633215. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Albanesi JP. Ca2+-induced conformational change and aggregation of chromogranin A. J Biol Chem. 1990;265:14414–14421. [PubMed] [Google Scholar]
- Zanini A, Giannattasio G, Nussdorfer G, Margolis R, Margolis R, Meldolesi J. Molecular organization of prolactin granules. II. Characterization of glycosaminoglycans and glycoproteins of the bovine prolactin matrix. J Cell Biol. 1980;86:260–272. doi: 10.1083/jcb.86.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Hoffman M, McMillan T, Kleinman H, O’Connell B. Growth factor regulation of the amylase promoter in a differentiating salivary acinar cell line. J Cell Physiol. 1998;177:628–635. doi: 10.1002/(SICI)1097-4652(199812)177:4<628::AID-JCP13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]


