Abstract
Purpose
Optical coherence tomography (OCT) is a new technology that uses near-infrared light in an interferometer to produce approximately 10-μm resolution cross-sectional images of the tissue of interest. The authors performed repeated quantitative assessment of nerve fiber layer thickness in individuals with normal and glaucomatous eyes, and they evaluated the reproducibility of these measurements.
Methods
The authors studied 21 eyes of 21 subjects by OCT. Each subject underwent five repetitions of a series of scans on five separate occasions within a 1-month period. Each series consisted of three circular scans around the optic nerve head (diameters, 2.9, 3.4, and 4.5 mm). Each series was performed separately using internal (fixation with same eye being studied) and external (fixation with contralateral eye) fixation techniques. The eye studied and the sequence of testing were assigned randomly.
Results
Internal fixation (IF), in general, provides a slightly higher degree of reproducibility than external fixation (EF). Reproducibility was better in a given eye on a given visit than from visit to visit. Reproducibility as measured by intraclass correlation coefficients were as follows: circle diameter (CD), 2.9 mm, 0.51/0.57 (normal/glaucoma) (IF), 0.43/0.54 (EF); CD, 3.4 mm, 0.56/0.52 (IF), 0.43/0.61 (EF); CD, 4.5 mm, 0.53/0.43 (IF), 0.42/0.49 (EF).
Conclusions
Nerve fiber layer thickness can be reproducibly measured using OCT. Internal is superior to external fixation; each circle diameter tested provides adequate reproducibility.
Optical coherence tomography (OCT) is a new technology that allows cross-sectional imaging of the eye using light. This technology is noncontact and noninvasive and may have use in diagnosing and managing retinal disease and glaucoma. We previously reported on the quantification of nerve fiber layer (NFL) and retinal thickness using OCT; however, for this technology to provide meaningful data, measurements must be reproducible.
The goal of this study is to determine the reproducibility of measurements of NFL and retinal thickness made by OCT. We also sought to evaluate the optimal circle diameter for OCT NFL scans based on reproducibility of measurements and on disc size and to study fixation technique (internal or external) and its effect on the reproducibility of OCT measurements. In addition, we aimed to determine whether there was a difference in the variability of measurements in normal and glaucomatous eyes, how the NFL thickness changed with increasing distance from the optic nerve head (ONH), and whether there were regional differences in both NFL thickness and the reproducibility of measurements of that value.
Materials and Methods
Identification of Subjects
We examined one eye of each of 21 subjects (10 with glaucoma and 11 control subjects), following informed consent and using a protocol approved by the Human Investigation Review Committee of New England Medical Center. Each subject had a complete ophthalmologic examination, including medical and family history, visual acuity testing with refraction, intraocular pressure measurement, Humphrey 24-2 visual field, slit-lamp examination, dilated slit-lamp stereo biomicroscopy, indirect ophthalmoscopy, stereoscopic optic nerve head photography, and nerve fiber layer photography, in addition to OCT. Only those people with no history or evidence of intraocular surgery or laser, no history or evidence of retinal pathology or glaucoma, best-corrected visual acuity of 20/40 or better, normal Humphrey 24-2 visual field, intraocular pressure of 21 mmHg or lower, and normal-appearing optic nerve heads qualified as control subjects. Subjects with glaucoma had no history or evidence of intraocular surgery or laser; no history or evidence of retinal pathology; an abnormal Humphrey 24-2 visual field with a characteristic arcuate, Bjerrum, Seidel, and/or paracentral scotoma and/or nasal step on Humphrey 24-2 visual field; cupping of optic nerve heads corresponding to visual field loss; and/or a nerve fiber layer defect on stereo biomicroscopy.
Optical Coherence Tomography Technology
Optical coherence tomography was performed with a high-speed prototype system, which we have described previously.1–9 We used a fiber-optic delivery system to couple the OCT unit to a slit-lamp biomicroscope for in vivo tomography of the retina. Computer-controlled galvanometric scanners allowed manipulation of the beam directed into the eye. The beam passed through a 78-diopter Volk lens (Volk, Inc, Mentor, OH) for indirect imaging, and beam focus was coincident with the slit-lamp image plane, permitting visualization of the eye with a CCD camera while scanning. The tomogram was displayed in real-time on a computer monitor.
The OCT system used in this study acquired a typical 3-mm-deep × 100-pixel-wide image in under 2.5 seconds. The axial resolution of the OCT system was experimentally determined to be 14 μm in air by measuring the full width at half-maximum of the reflection obtained by imaging a mirror placed in the image plane of the slit-lamp biomicroscope. This measurement predicts a full width at half-maximum resolution of 10 μm in the retina due to the difference in refractive index between air and tissue. The actual ability of the instrument to distinguish between two closely spaced structures might be expected to be better than this figure when locating structures with high contrast (e.g., the vitreoretinal interface) or worse when identifying closely spaced structures with similar reflectivities (e.g., the retinal pigment epithelium and choriocapillaris). The lateral resolution was determined by the larger of the beam waist diameter in the retina, or the sampling distance between adjacent transverse pixels in the tomogram. The beam spot diameter in the retina used in this study was approximately 13 μm as computed from the Gullstrand-reduced schematic eye. For comparison, a typical 3.4-mm diameter circular tomogram contained 100 pixels spaced approximately 110 μm apart.
Image and Data Processing
The images were corrected for artifacts due to involuntary subject motion during data acquisition using a standard image processing technique of cross-correlation scan registration.3 We developed an image processing computer program to quantitate total retinal thickness and retinal nerve fiber layer thickness for the cylindrical OCT sections obtained by scanning around the optic nerve head (because axial information was obtained with OCT, circular scans produced a cylinder in 3 dimensions). After subject motion in the longitudinal direction was corrected with a cross-correlation scan registration technique,3 a digital filter was applied to smooth the tomograms and reduce image speckle noise. Smoothing was accomplished by first applying a median filter to remove extreme values of reflectivity in the image. Two-dimensional linear convolution with a center-weighted kernel then was used to reduce remaining speckle variations. Retinal thickness was quantitated by computer for each scan in the image as the distance between the first reflection at the vitreoretinal interface and the anterior boundary of the red, reflective layer corresponding to the retinal pigment epithelium and choriocapillaris. Nerve fiber layer thickness was determined by computer; it was assumed to be correlated with the extent of the red, highly reflective layer at the vitreoretinal interface. Boundaries were located by searching for the first points on each scan where the reflectivities exceeded a certain threshold. For example, the inner limiting membrane was located by starting anteriorly and searching downward in the image. The posterior margin of the nerve fiber layer was located by starting within the photoreceptor layer and searching upward in the image. The retinal pigment epithelium was similarly located by starting within the photoreceptor layer and searching downward in the image. The location of the photoreceptor layer was assumed to lie at the position of minimum reflectivity within the neurosensory retina. Thresholds were separately determined by the computer for each scan in the image as two thirds of the maximum reflectivity in each smoothed A-scan evaluated on a logarithmic scale. Linear interpolation was performed to remove gaps in the boundaries resulting from shadowing due to blood vessels. The boundaries chosen by the computer were overlaid on a false-color display of the each image. The NFL and retinal pigment epithelial boundaries were highlighted with a blue line, and the superficial surface of the retina was denoted with a white line. The thicknesses were reported individually for each A-scan, as averages over each quadrant (superior, inferior, temporal, nasal), as averages for each clock hour, or as averages over the entire cylindrical section. We have shown good correlation, within 10 μm, between an NFL thickness measurement made in vivo and subsequent histopathologic measurement on the same tissue.9
Five × Five Study Protocol
After dilation with 1% tropicamide and 2.5% phenylephrine, each eye was scanned at three different circle diameters with two different fixation techniques five times, in random order, on a given occasion. In between the series of scans on a particular day, the subject sat back from the machine for at least 1 minute and then was repositioned. The three circle diameters investigated were 2.9, 3.4, and 4.5 mm (Figs 1 and 2). Scan diameters were calculated based on the Gullstrand schematic eye and were not corrected for variations in axial eye length between subjects. The five series of scans were repeated on five separate occasions during a 1-month period (Figs 3–7).
Figure 1.
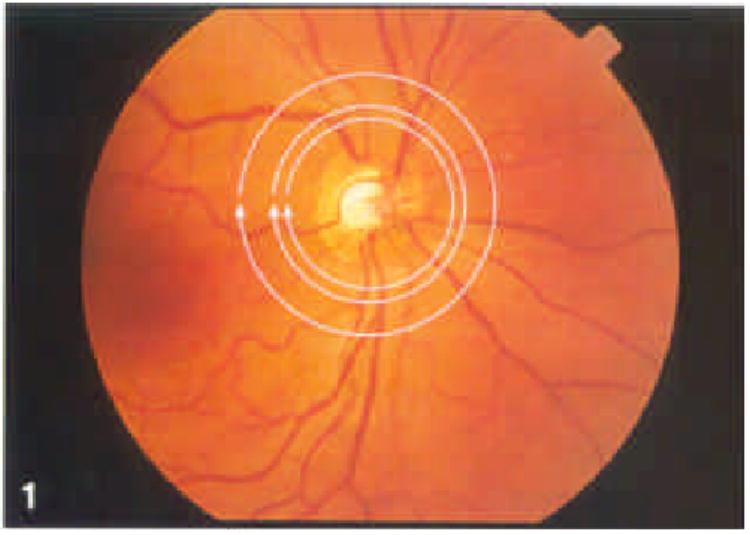
Optic nerve head photograph with three circles that depict regions scanned by optical coherence tomography. Innermost circle is 2.9 mm, center circle is 3.4 mm, and outermost circle is 4.5 mm.
Figure 2.
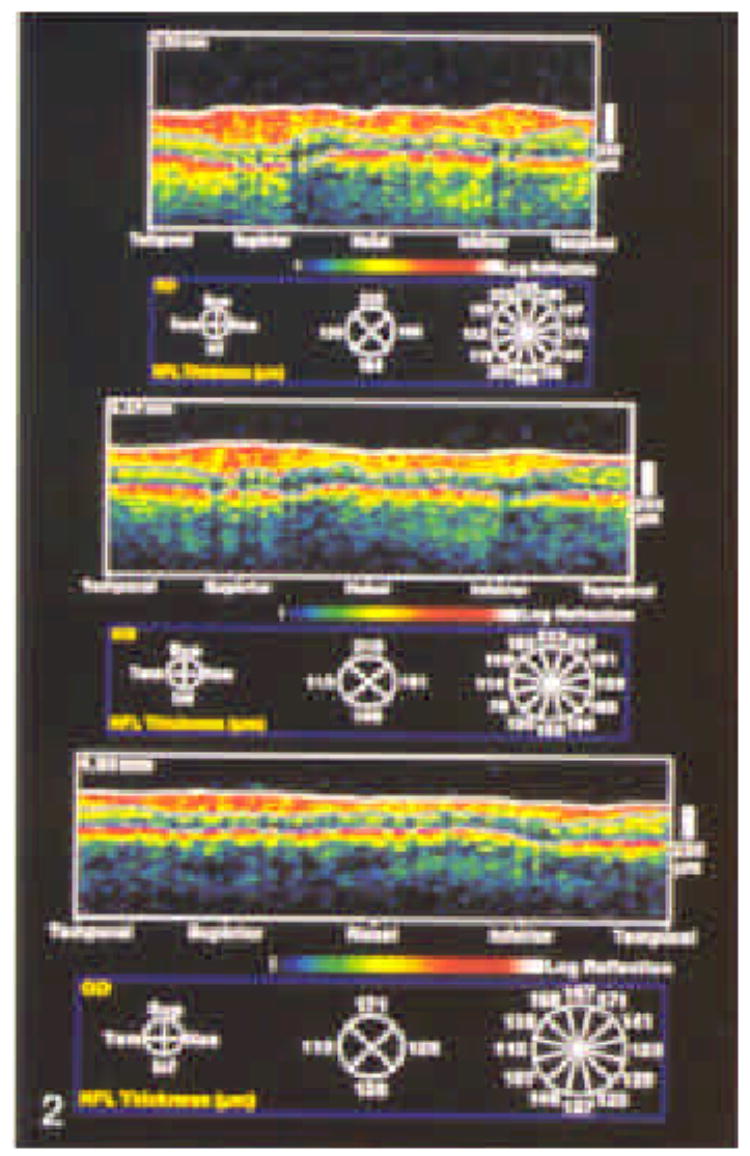
Optical coherence tomographies corresponding to circles are shown. Notice thinning of the nerve fiber layer with increasing distance from the optic nerve head. The computer algorithm used to quantitate nerve fiber layer thickness summarizes the data by quadrant and by clock hour.
Figure 3.
Stereoscopic optic nerve head photograph of a healthy individual.
Figure 7.
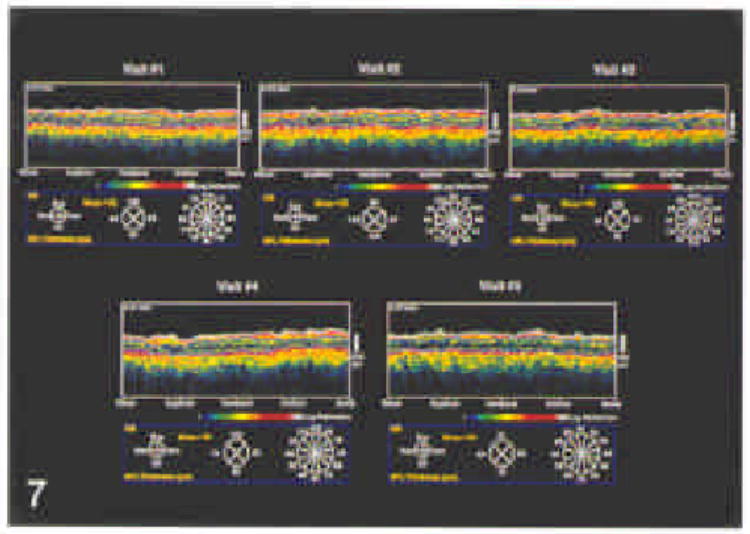
Series of five optical coherence tomographies taken using a circle diameter of 3.4 mm on five separate occasions within a 1-month period demonatrates thinning of the nerve fiber layer corresponding to visual field loss. Notice consistency of optical coherence tomography measurements on this series of scans.
Internal and external fixation techniques were used. With internal fixation, the examiner centers the circular scan on the optic nerve head while the subject fixates on a light shone in the eye being scanned that was offset from the scan. The subject attends to the aiming beam while the probe beam is centered around the optic nerve head. The offset information in the transverse direction between the fixation light and the scan pattern is recorded for automatic registration of subsequent scans. The second fixation technique, external fixation, involves the technician placing the scanning circle centered on the optic nerve head each time that the eye is studied. With this technique, the contralateral eye is fixated using a standard light which is mounted on the slit lamp. The scans were performed using near infrared illumination (840 nm) to minimize subject discomfort.
Nerve Fiber Layer and Retinal Quantification
An automated computer algorithm identified the anterior and posterior borders of the NFL and the posterior border of the retina and calculated NFL and total retinal thickness overall by quadrant and by clock hour.
Statistical Analysis
Both left and right eyes were analyzed; therefore, the data were adjusted such that clock hours could be appropriately assessed. Left eyes were treated as mirror images of right eyes. Therefore, in all tables of clock hours, 1:00 to 5:00 represents the nasal clock hours and 7:00 to 11:00 represents the temporal clock hours.
For each diagnosis group (glaucoma and control), diameter (2.9, 3.4, and 4.5 mm), fixation technique (internal and external), and region (superior, inferior, temporal, nasal, and overall mean), variance components and intraclass correlation coefficients (ICCs) were determined using repeated measures regression (proc mixed, SAS Institute, Inc, Cary, NC). In these models, NFL thickness, as measured by OCT, was considered to have three variance components, intersubject, intervisit (within-subject between-dates), and intravisit (within-subject within-date). The intraclass correlation coefficient is the ratio of the intersubject component of the variance to the total variance. It is commonly used as a measure of reliability. As a measure of the numeric variation of each type of measurement, we used the square root of the intrasubject variance (i.e., the sum of the intervisit and the intravisit variance components), which we will refer to as the intrasubject standard deviation.
To examine the effect of diagnosis, diameter, and fixation type on the reliability of the NFL thickness measurements, we used multiple regression, controlling for region. We examined multiple regressions using both ICC and logit(ICC) (i.e., ICC/[1−ICC]) as dependent variables. Because the results were essentially the same for both types of regressions, we present only the coefficients for ICC, which is more intuitive. In addition, we did similar analyses using the intrasubject standard deviation.
Finally, we examined the relation between circle diameter and measured NFL, because, according to some hypotheses, the NFL thickness should be inverse to diameter. To do this, we multiplied each NFL thickness measurement by the diameter at which it was measured. If NFL thickness were inversely related to diameter, we would find that the product of NFL and diameter was constant. This hypothesis was examined using repeated measures regression with dummy variables for the diameters.
Results
Subject Basic Characteristics
We studied 21 eyes of 21 subjects. Eleven of the subjects were healthy and ten had glaucoma. For control subjects, the mean age was 28 ± 5 years, whereas for subjects with glaucoma the mean age was 66 ± 12 years. There were 14 men and 7 women; 7 of the men and 3 of the women had glaucoma. Five of the subjects were not white, and of these, two had glaucoma. Optical coherence tomography was well tolerated by all subjects at all visits.
Reproducibility of Measurements of Nerve Fiber Layer Thickness
Both NFL and retinal thickness, as measured by the OCT, varied by diagnosis, diameter, and fixation type (Table 1). The NFL thickness decreased with increasing circle diameter (Fig. 2). The total variance went up with circle diameter in the control subjects, but down with circle diameter in the subjects with glaucoma. The intersubject standard deviation was approximately constant among the control subjects but showed a tendency to drop with diameter among the subjects with glaucoma. Among the control subjects, the ICC was higher for internal fixation than for external fixation, although the opposite was true for the subjects with glaucoma. For both groups of subjects, 3.4 mm was the best diameter for each fixation type. There was a tendency for the ICC to be higher among subjects with glaucoma.
Table 1.
Mean Nerve Fiber Layer Thickness by Diagnosis, Diameter, and Fixation Type*
| Diagnosis | Diameter (mm) | Fixation Technique | Mean (μm) | Total Variance | Intersubject Standard Deviation (μm) | Intraclass Correlation Coefficient |
|---|---|---|---|---|---|---|
| Normal | 2.9 | E | 169 | 301 | 11 | 0.43 |
| I | 169 | 293 | 12 | 0.51 | ||
| 3.4 | E | 153 | 317 | 12 | 0.43 | |
| I | 153 | 319 | 13 | 0.56 | ||
| 4.5 | E | 124 | 363 | 12 | 0.42 | |
| I | 125 | 363 | 14 | 0.53 | ||
| Glaucoma | 2.9 | E | 120 | 1016 | 24 | 0.54 |
| I | 120 | 1103 | 25 | 0.57 | ||
| 3.4 | E | 111 | 831 | 23 | 0.61 | |
| I | 108 | 867 | 21 | 0.52 | ||
| 4.5 | E | 83 | 570 | 17 | 0.49 | |
| I | 82 | 666 | 17 | 0.43 |
E = external; I = internal.
Normal means, etc., are based on 258 to 276 measurements of 11 subjects. Glaucoma means, etc., are based on 228 to 300 measurements of 10 subjects.
Mean retinal thickness and its total variance also decreased with diameter, as did the intersubject standard deviation (Table 2). The ICC was higher far the subject with glaucoma than for the control subjects, and both were much higher than the ICC for the NFL thickness.
Table 2.
Mean Retinal Thickness by Diagnosis, Diameter, and Fixation Type*
| Diagnosis | Diameter (mm) | Fixation Technique | Mean (μm) | Total Variance | Intersubject Standard Deviation (μm) | Intraclass Correlation Coefficient |
|---|---|---|---|---|---|---|
| Normal | 2.9 | E | 303 | 624 | 22 | 0.80 |
| I | 301 | 617 | 23 | 0.88 | ||
| 3.4 | E | 286 | 547 | 22 | 0.84 | |
| I | 284 | 537 | 22 | 0.88 | ||
| 4.5 | E | 256 | 370 | 17 | 0.76 | |
| I | 254 | 346 | 17 | 0.86 | ||
| Glaucoma | 2.9 | E | 270 | 745 | 26 | 0.92 |
| I | 268 | 700 | 25 | 0.92 | ||
| 3.4 | E | 262 | 597 | 23 | 0.91 | |
| I | 260 | 551 | 22 | 0.91 | ||
| 4.5 | E | 240 | 407 | 18 | 0.77 | |
| I | 237 | 345 | 16 | 0.74 |
E = external; I = internal.
Normal means, etc., are based on 258 to 276 measurements of 11 subjects. Glaucoma means, etc., are based on 228 to 300 measurements of 10 subjects.
Multiple regression for ICC of NFL thickness, controlling far region, showed that ICC was higher for internal fixation (P = 0.02) and for subjects with glaucoma (P = 0.02) and lower for circle diameter 4.5 mm (P = 0.05) (Table 3).
Table 3.
Results of Regression Analysis of Intraclass Correlation Coefficients for Nerve Fiber Layer and Retinal Thickness as a Function of Diagnosis, Diameter, and Fixation Technique, Controlling for Region
| Measurement | Predictor | Coefficient | P |
|---|---|---|---|
| NFL thickness | Glaucoma* | 0.06 | 0.02 |
| Diameter, 2.9 mm† | 0.00 | 0.88 | |
| Diameter, 4.5 mm† | 0.06 | 0.05 | |
| Internal fixation‡ | 0.06 | 0.02 | |
| Retinal thickness | Glaucoma* | 0.00 | 0.90 |
| Diameter, 2.9 mm† | 0.00 | 0.97 | |
| Diameter, 4.5 mm† | 0.10 | 0.01 | |
| Internal fixation‡ | 0.05 | 0.13 |
NFL = nerve fiber layer.
Versus control subjects.
Versus 3.4 mm.
Versus external fixation.
Similar analysis for ICC of retinal thickness measurements showed that ICC was lower for circle diameter 4.5 mm (P = 0.01) (Table 3). Internal fixation was suggestively associated with higher ICC (P = 0.13).
Analyses for the intrasubject standard deviation confirmed that internal fixation leads to lower intrasubject variation (Table 4). Glaucoma diagnosis and diameter 2.9 mm also resulted in higher intrasubject.
Table 4.
Results of Regression Analysis of Intrasubject Standard Deviation of Nerve Fiber Layer and Retinal Thickness as a Function of Diagnosis, Diameter, and Fixation Technique, Controlling for Region
| Measurement | Predictor | Coefficient | P |
|---|---|---|---|
| NFL thickness | Glaucoma* | 5.2 | 0.0001 |
| Diameter, 2.9 mm† | 1.9 | 0.008 | |
| Diameter, 4.5 mm† | −0.5 | 0.51 | |
| Internal fixation ‡ | −1.4 | 0.01 | |
| Retinal thickness | Glaucoma* | −1.2 | 0.18 |
| Diameter, 2.9 mm† | 1.5 | 0.16 | |
| Diameter, 4.5 mm † | 0.6 | 0.55 | |
| Internal fixation‡ | −2.2 | 0.02 |
NFL = nerve fiber layer.
Versus control subjects.
Versus 3.4 mm.
Versus external fixation.
Because internal fixation was more reliable (i.e., associated with higher ICC for NFL thickness), we present detailed results for NFL and retinal thickness using internal fixation only. Circle diameters other than 3.4 mm each proved less reliable than 3.4 mm on at least one analysis; therefore, we restricted ourselves to 3.4 mm.
For control subjects with circle diameters of 3.4 mm and with internal fixation, ICCs were near or above 0.5 for the mean and all quadrants except temporal (Table 5). For subjects with glaucoma, the temporal and nasal quadrants had low ICCs. Intraclass correlation coefficients for the individual clock hours were similar to those for the quadrants to which the clock hours belong.
Table 5.
Mean Total Variance, Percent of Variance in Each Variance Component, and Intersubject and Intrasubject Standard Deviations for Nerve Fiber Layer Thickness, by Quadrants and Clock Hours*
| Measurement | Mean (μm) | Total Variance | % Intersubject (ICC) | % Intervisit | % Intravisit | Intersubject Standard Deviation (μm) | Intrasubject Standard Deviation (μm) |
|---|---|---|---|---|---|---|---|
| Normal subjects | |||||||
| Mean NFL quadrants | 153 | 319 | 56 | 22 | 22 | 13 | 12 |
| Superior | 179 | 491 | 54 | 21 | 25 | 16 | 15 |
| Inferior | 175 | 435 | 47 | 21 | 32 | 14 | 15 |
| Temporal | 126 | 499 | 24 | 37 | 39 | 11 | 19 |
| Nasal | 131 | 1137 | 61 | 14 | 25 | 26 | 21 |
| Clock hours | |||||||
| 12 | 177 | 910 | 53 | 24 | 23 | 22 | 21 |
| 1 | 171 | 1184 | 68 | 10 | 21 | 28 | 19 |
| 2 | 147 | 1421 | 63 | 10 | 27 | 30 | 23 |
| 3 | 112 | 1172 | 47 | 17 | 35 | 24 | 25 |
| 4 | 136 | 1343 | 55 | 14 | 30 | 27 | 24 |
| 5 | 157 | 817 | 44 | 18 | 38 | 19 | 21 |
| 6 | 186 | 818 | 60 | 14 | 26 | 22 | 18 |
| 7 | 182 | 521 | 32 | 16 | 52 | 13 | 19 |
| 8 | 130 | 862 | 35 | 23 | 42 | 17 | 24 |
| 9 | 111 | 664 | 19 | 35 | 47 | 11 | 23 |
| 10 | 139 | 556 | 21 | 34 | 45 | 11 | 21 |
| 11 | 186 | 536 | 43 | 23 | 34 | 15 | 18 |
| Glaucoma subjects | |||||||
| Mean NFL quadrants | 108 | 867 | 52 | 30 | 18 | 21 | 20 |
| Superior | 128 | 1355 | 59 | 20 | 21 | 28 | 24 |
| Inferior | 131 | 1483 | 65 | 19 | 16 | 31 | 23 |
| Temporal | 86 | 897 | 17 | 48 | 34 | 12 | 27 |
| Nasal | 86 | 1124 | 31 | 32 | 37 | 19 | 28 |
| Clock hours | |||||||
| 12 | 128 | 1634 | 45 | 26 | 29 | 27 | 30 |
| 1 | 126 | 2712 | 65 | 17 | 19 | 42 | 31 |
| 2 | 102 | 1827 | 38 | 25 | 38 | 26 | 34 |
| 3 | 67 | 1175 | 21 | 27 | 53 | 16 | 31 |
| 4 | 88 | 1294 | 22 | 31 | 47 | 17 | 32 |
| 5 | 127 | 2071 | 44 | 35 | 21 | 30 | 34 |
| 6 | 140 | 2224 | 71 | 12 | 18 | 40 | 26 |
| 7 | 125 | 2176 | 60 | 19 | 21 | 36 | 30 |
| 8 | 79 | 871 | 5 | 31 | 64 | 7 | 29 |
| 9 | 74 | 952 | 2 | 47 | 51 | 5 | 31 |
| 10 | 103 | 1552 | 50 | 22 | 28 | 28 | 28 |
| 11 | 131 | 1710 | 55 | 14 | 31 | 31 | 28 |
NFL = nerve fiber layer.
Based on 276 measurements from 11 control subjects and 300 measurements from 10 glaucoma subjects; circle diameter 3.4 mm, internal fixation.
The pattern for the intrasubject standard deviation was similar, except that the smaller areas had larger variances (i.e., the quadrants had larger variances than the overall mean, and the hours had larger variances than the quadrants).
The intrasubject standard deviation among control subjects was similar to the intersubject standard deviation. It was considerably lower than the difference between control subjects and those with glaucoma. The clinical implications of these findings are (1) do at least three measurements at a session to cut down intrasubject variation; (2) do not leap to conclusions based on small changes.
Nerve Fiber Layer Thickness and Circle Diameter. Nerve fiber layer thickness did not vary exactly inversely with diameter, although this is approximately true (Table 6). For example, the product of mean NFL thickness and circle diameter among control subjects was 489 for diameter 2.9 mm, 516 for diameter 3.4 mm, and 558 to 563 (external–internal fixation) for diameter 4.5 mm. Among control subjects, NFL, both mean and for quadrants, was always significantly lower at a diameter of 2.9 mm than predicted by this hypothesis (compared with diameter 3.4 mm), whereas it was higher at 4.5 mm (also significant, except for the nasal quadrant). Among subjects with glaucoma, the measurements at diameter 2.9 mm also were lower than expected, but the results at 4.5 mm were mixed.
Table 6.
Effects of Circle Diameter on Nerve Fiber Layer Thickness
| Diameter 2.9mm
|
Diameter 4.5 mm
|
||||
|---|---|---|---|---|---|
| Diagnosis | NFL Variable | Coefficient* | P | Coefficient* | P |
| Normal | Mean | −21 | 0.0000 | 43 | 0.0000 |
| Superior | −31 | 0.0000 | 47 | 0.0000 | |
| Inferior | −20 | 0.0000 | 45 | 0.0000 | |
| Temporal | −21 | 0.0000 | 71 | 0.0000 | |
| Nasal | −13 | 0.01 | 8 | 0.10 | |
| Glaucoma | Mean | −17 | 0.0000 | 3 | 0.37 |
| Superior | −25 | 0.0000 | 5 | 0.23 | |
| Inferior | −16 | 0.0001 | 8 | 0.04 | |
| Temporal | −19 | 0.0000 | 18 | 0.0001 | |
| Nasal | −7 | 0.15 | −20 | 0.0001 | |
NFL = nerve fiber layer.
The coefficient gives the amount by which the product of NFL thickness and circle diameter differs from the value for diameter 3.4 mm, in repeated measures regression.
Discussion
A critical test for any technology is whether its measurements can be made reproducibly. The field of ophthalmic instrumentation, especially with regard to optic nerve head analysis, is replete with devices once introduced, only later to be withdrawn due to marginally reproducible results. Optical coherence tomography offers a device with a standard deviation of measurements of 10 to 20 μm for mean overall NFL thickness measurements and 15 to 30 μm for measurements of each clock hour.
Comparison with Existing Technologies
The reproducibility of OCT measurements compares well with other currently available ophthalmic instrumentation designed for optic nerve and NFL analysis. For the Glaucoma-Scope (Ophthalmic Imaging Systems, Sacramento, CA), the major source of between-visit variability is patient re-alignment and instrument adjustment, whereas the primary cause of image-to-image variation rests in the instrument and computer analysis.10 Of course, the Glaucoma-Scope cannot measure NFL thickness and can only provide information about the optic nerve head itself.
Confocal scanning laser ophthalmoscopes provide axial resolution superior to the Glaucoma-Scope, but are also limited to coronal sections of the optic nerve head and retina. Cross-sectional imaging of the fundus with confocal laser-scanning ophthalmoscopes and tomographs is limited by ocular aberrations and numeric aperture available through the pupil to 300-μm axial resolution. In a model eye in vitro, the standard deviation of measurements made with the Heidelberg retinal tomograph (Heidelberg Engineering GmbH, Heidelberg, Germany) is 31 to 56 μm11; the in vivo variability of the Heidelberg retinal tomograph on a single scan is 25 to 40 μm in vivo.12,13 The topographic scanning system (Laser Diagnostic Technologies, San Diego, CA) could be expected to perform in a similar fashion, being derived from a similar parent device. Variability may be improved through repetitive imaging.13 These devices, which were developed to quantitate cupping, can measure rim area and provide indices of structure, but have not been shown to differentiate between normal and glaucomatous nerve heads, or to detect changes over time.
The nerve fiber analyzer (NFA, Laser Diagnostic Technologies, San Diego, CA) measures the change in polarization of light reflected from the eye. A recent study suggests that the NFA can differentiate between normal and glaucomatous eyes, with a reproducibility of approximately 5% in normal eyes.14 Reproducibility has not been reported in glaucomatous eyes using this device. The NFA uses the principle of NFL birefringence to rotate polarized light, and from this rotation derives NFL thickness. Future studies are needed to show the effects of changes in the cornea and lens, also birefringent, on NFA measurements.
Optical coherence tomography differs from each of the above technologies in producing cross-sectional images of the retina, from which direct measurements of NFL thickness are made. An automated computer algorithm quantitates NFL and total retinal thickness, summarized by quadrant, clock hour, and overall. Because OCT is based on near-infrared interferometry, it is not affected by refractive status or axial length of the eye, nor is it affected by changes in nuclear sclerotic cataract density or similar media opacity; however, posterior subcapsular and cortical cataracts do impair the ability to perform OCT.
Future Directions
Based on our results, a circle diameter of 3.4 mm was selected as our standard for future studies, because it was large enough to avoid overlap with the optic nerve head in nearly all eyes and allowed measurement of NFL in a thicker area than the 4.5-mm diameter circle. This potentially permitted a higher sensitivity to subtle NFL defects with a 3.4-mm circle diameter as compared with 4.5 mm. Additionally, reproducibility was significantly better at this circle diameter than at 2.9 mm.
Internal fixation was significantly less variable than the external fixation technique in this study. The high reproducibility of measurements made with external fixation spoke to the experience of the OCT operator. Internal fixation required operator placement of the OCT probe beam only on the first scan; all subsequent scans used the same placement, stored in the computer. This study validated the internal fixation technique, which will be used in future work.
Reproducibility results for OCT measurement of NFL thickness were encouraging. The standard deviation of measurements made with OCT in this study was approximately 10 to 20 μm for overall NFL thickness, 5 to 9 μm for retinal thickness. Based on the findings that reproducible measurements of NFL thickness can be made with OCT, the stage is set for larger scale cross-sectional and longitudinal studies in normal and glaucomatous eyes to develop a normative database of NFL thickness, stratified by age, to characterize NFL defects in glaucoma and to correlate these defects with conventional findings of automated perimetry, optic nerve head, and NFL appearance.
Figure 4.
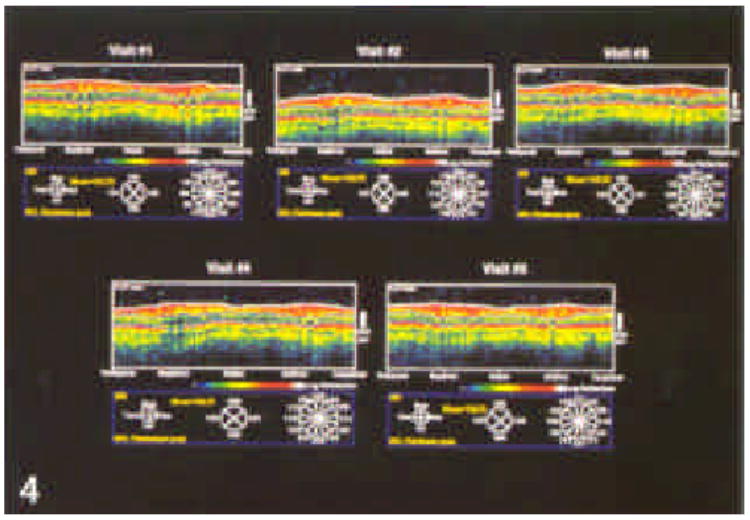
Series of five optical coherence tomographies taken using a circle diameter of 3.4 mm on five separate occasions from the individual whose optic nerve head is shown in Figure 3.
Figure 5.
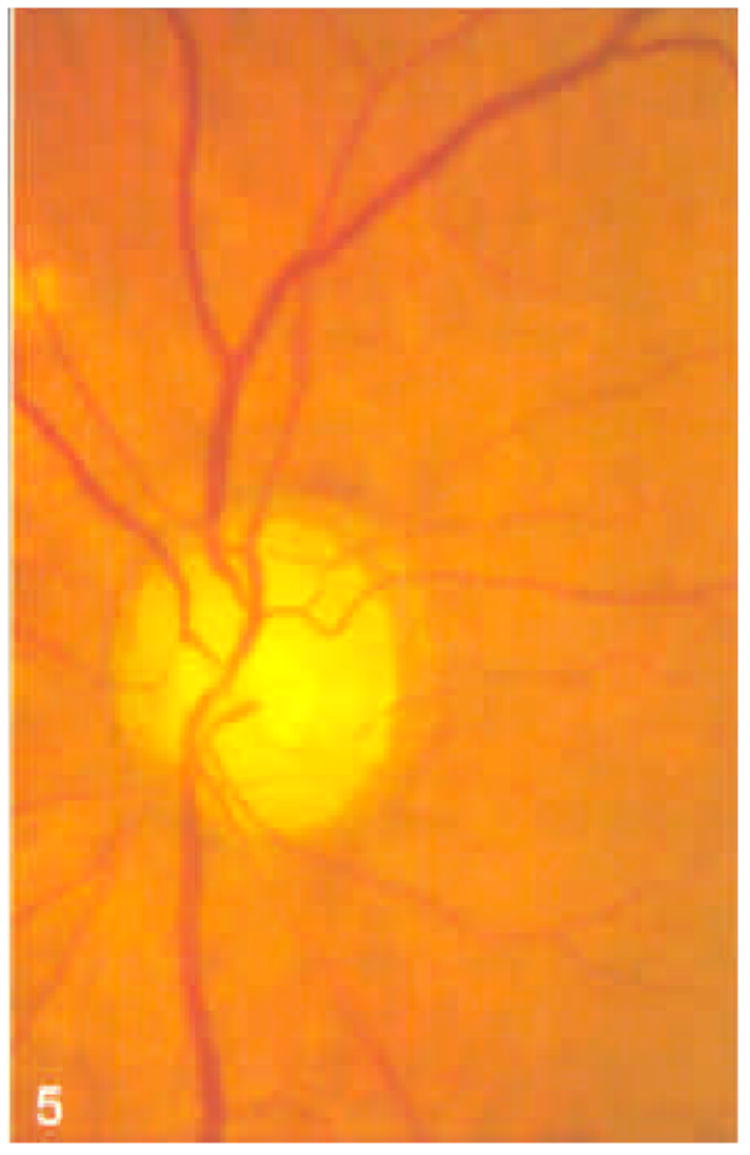
Stereoscopic optic nerve head photograph of a glaucomatous eye.
Figure 6.
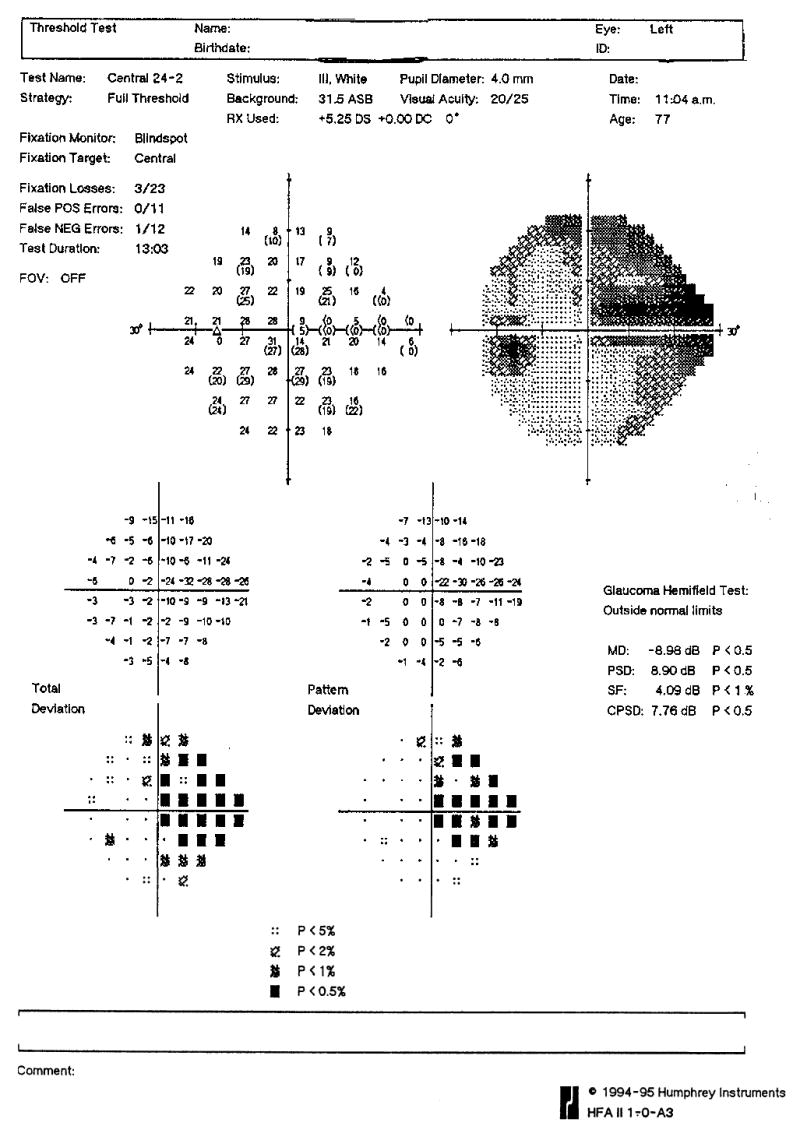
Humphrey 24-2 visual field from the eye in Figure 5. Notice the visual field defect corresponding to the area of cupping.
Footnotes
Supported by NIH 1-R29-EY11006-01 and NIH 9-RO1-EY11289, Bethesda, Maryland, MFEL N00014-94-1-0717, Arlington, Virginia, and Research to Prevent Blindness, Inc, New York, NY.
Presented in part at the American Academy of Ophthalmology Annual Meeting, Atlanta, Oct/Nov 1995, and at the ARVO Annual Meeting, Ft. Lauderdale, May 1995.
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izatt JA, Hee MR, Huang D, et al. Ophthalmic diagnostics using optical coherence tomography. In: Ren Q, Pavel JM, editors. Proceedings of Ophthalmic Technologies III; January 1992; Los Angeles, CA. Bellingham, WA: SPIE; 1993. pp. 136–44. (Progress in biomedical optics; Proceedings of SPIE; vol. 1877.) [Google Scholar]
- 3.Swanson EA, Izatt JA, Hee MR, et al. In vivo retinal imaging using optical coherence tomography. Opt Lett. 1993;18:1864–6. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- 4.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 5.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112:1584–9. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 6.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 7.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography: a pilot study. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 8.Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–56. doi: 10.1016/s0161-6420(95)30959-1. [DOI] [PubMed] [Google Scholar]
- 9.Schuman JS. Imaging optic nerve and nerve fiber layer: optical coherence tomography. In: Bucci MG, editor. Glaucoma: Decision Making in Therapy. Springer-Verlag; Milan: (in press) [Google Scholar]
- 10.Hoskins HD, Hetherington J, Glenday M, et al. Repeatability of the glaucoma-scope measurements of optic nerve head topography. J Glaucoma. 1994;3:17–27. [PubMed] [Google Scholar]
- 11.Dreher AW, Tso PC, Weinreb RN. Reproducibility of topographic measurements of the normal and glaucomatous optic nerve head with the laser tomographic scanner. Am J Ophthalmol. 1991;111:221–9. doi: 10.1016/s0002-9394(14)72263-9. [DOI] [PubMed] [Google Scholar]
- 12.Kruse FE, Burk RO, Volcker HE, et al. Reproducibility of topographic measurements of the optic nerve head with laser tomographic scanning. Ophthalmology. 1989;96:1320–4. doi: 10.1016/s0161-6420(89)32719-9. [DOI] [PubMed] [Google Scholar]
- 13.Weinreb RN, Lusky M, Bartsch DU, et al. Effect of repetitive imaging on topographic measurements of the optic nerve head. Arch Ophthalmol. 1993;111:636–8. doi: 10.1001/archopht.1993.01090050070031. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119:627–36. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]


