Abstract
The present study examined the effects of NIH 11082 ((−)-(1R,5R,9R)-5,9-dimethyl-2'-hydroxy-2-(6-hydroxyhexyl)-6,7-benzomorphan hydrochloride), a benzomorphan analogue, in the mouse tail suspension, an assay used to detect antidepressant agents. NIH 11082 significantly decreased immobility time during tail suspension, with a comparable magnitude as the tricyclic antidepressant desipramine. Importantly, NIH 11082 failed to elicit convulsions or other overt behavioral signs of toxicity. The delta-opioid receptor antagonist naltrindole (AD50=2.0 mg/kg), but not the nonselective mu-opioid receptor antagonist naltrexone or the kappa-opioid receptor antagonist nor-BNI, blocked the effects of NIH 11082 in the tail suspension test. These results reinforce the notion that delta opioid receptor agonists can produce significant effects in a behavioral model used to screen antidepressant drugs.
1. Introduction
Depression is a serious illness affecting millions of people, and though there are many pharmacological treatments available, there remains a need to develop therapeutic agents that effectively treat a large population of patients with fewer adverse side effects than currently available agents. Current antidepressant therapy has centered on the potentiation of serotonergic and noradrenergic signaling in the central nervous system. However, other classes of compounds have also been identified to have potential antidepressant activity as determined in animal models. In particular, studies have been performed using a variety of different opioid system-enhancing agents that suggest an antidepressant role for opioid receptor agonists (Fink et al., 1970; Kline et al., 1977; 1997).
Previous studies have shown that disregulation of the delta opioid receptor system may be related to depression or depressive symptoms and, therefore, may be a useful therapeutic target for treating depression. Interestingly, delta-opioid receptor deficient mice displayed altered emotional responses that are consistent with significant effects in the forced swim and conditioned suppression of motility paradigms (Filliol et al., 2000), which are sensitive to antidepressant drugs. Conversely, delta-opioid receptor agonists have been shown to possess significant effects in several animal models used to screen antidepressant agents. The selective delta-opioid receptor agonist Tyr-D-Ser-(O–C(CH3)3)-Gly-PheLeu-Thr-(O–C(CH3)3 (BUBU) produced significant effects in the learned helplessness model of depression (Tejedor-Real et al., 1998). Similarly, increasing levels of endogenous delta-opioid peptides with enkephalinase inhibitors such as RB101 revealed antidepressant-like effects in both mice and rats (Baamonde et al., 1992; Tejedor-Real et al., 1998). In addition, the nonpeptidic delta-opioid receptor agonists SNC80 ( (+)-4-(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1piperazinyl)-3-methoxybenzyl)N,N-diethylbenzamide) and (+)BW373U86 ((+)-4-[(alpha-R*)-alpha-[(2S*,5R*)-4-allyl-2,5-dimethyl-1-piperazinyl]-3-hydroxybenzyl]-N,N-diethylbenzamide) to rats elicited naltrindole-sensitive antidepressant-like properties, as assessed in the forced swim test (Broom et al., 2002a; Jutkiewicz et al., 2004; Jutkiewicz et al., 2005). Conversely, chronic administration of the potent, nonselective opioid receptor antagonist naltrexone to humans induced a self-reported mental depression in a placebo-controlled open study (Hollister et al., 1981), suggesting a general role for opioid systems in depression. An interpretation of these findings is that activation of delta-opioid receptors may have therapeutic potential to treat depression (Jutkiewicz, 2006).
A promising opioid candidate that can elicit potential therapeutic effects and is apparently devoid of provoking seizures (Traynor et al, 2005) is NIH 11082 ((−)-(1R,5R,9R)-5,9-dimethyl-2'-hydroxy-2-(6-hydroxyhexyl)-6,7-benzomorphan hydrochloride). This compound produced antinociceptive activity in the paraphenylquinone mouse writhing assay (ED50 (95% CL) = 1.9 (0.7-5.3 mg/kg), but showed virtually no activity by itself in the tail-flick and hot-plate tests (Traynor et al., 2005; Aceto et al., in press). Naltrindole, a selective delta-opioid receptor antagonist, blocked the analgesic action of NIH 11082, while neither the kappa opioid selective antagonist nor-BNI nor the mu-opioid receptor antagonist beta-FNA reduced this effect (Aceto et al., in press). However, NIH 11082 has low binding affinity (Ki = 140 nM) and lacks potency and efficacy in the GTPγS assay for the delta-opioid receptor (Traynor et al., 2005). Thus, the effects of this drug are mediated through an allosteric site of action, the release of endogenous delta-opioid receptor ligands, or other indirect mechanisms of action. The goal of the present study was to investigate whether NIH 11082 would elicit antidepressant-like effects in the tail suspension assay. The tricyclic antidepressant agent desipramine, which blocks the uptake of the monoamines, was used as a positive control. Additionally, the opioid receptor antagonists naltrindole, nor-BNI, or naltrexone were given before NIH 11082 to ascertain the receptor mechanism of action.
2. Materials and methods
2.1. Subjects
Naïve male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME), served as subjects. All subjects weighed between 20 and 30 g, and were housed four or five animals per cage in a temperaturecontrolled (20-22°C) facility. Food and water were available ad libitum. A sample size of 8 mice per group was used in each experiment. This animal protocol was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
2.2 Drugs
Desipramine hydrochloride was obtained from RBI (Natick, MA) and (−)-(1R,5R,9R)-5,9-dimethyl-2'-hydroxy-2-(6-hydroxyhexyl)-6,7-benzomorphan hydrochloride (NIH 11082) was synthesized in our laboratory (Aceto et al., in press). Naltrindole hydrochloride, naltrexone hydrochloride, and norbinaltorphimine hydrochloride (nor-BNI) were obtained from Sigma (St. Louis, MO). All drugs were dissolved in saline and were administered through the i.p. route administration in a volume of 10 μl/g body weight 30 min before evaluation in the tail suspension test or for locomotor activity. The dose of desipramine used was selected from those reported in the literature, while the selection of doses of the opioid receptor antagonists were previously described from our laboratory in blocking the pharmacological effects of opioid receptor agonists (Lichtman et al., 1996).
2.3. The tail suspension test
The procedure followed in this study was previously described by Stéru et al. (Steru et al., 1987a; Steru et al., 1987b). An automated tail-suspension apparatus (Model TS100 Tail suspension, Hamilton Kinder, CA, USA) was used to measure the total sum of periods of immobility. Mice were suspended by the tail, using non-irritating adhesive scotch tape, to a hook connected to a strain gauge that transmitted the animal's movements to a central unit that calculated the total duration of immobility during a 6-min test. The following settings were used in all experiments: threshold 0.40 Newton, off delay 40 ms, full scale: 4.00 Newton. Four animals were tested simultaneously on separate units. The data were expressed as mean immobility time(s ± S.E.M.).
2.4.Assessment of Locomotor activity
Mice were given an i.p. injection of saline or NIH 11082 (16 or 32 mg/kg), and placed individually into clean 28 × 16 cm plastic cages inside of sound-attenuating chambers. The distance traveled and time spent mobile were recorded from 30-36 min (i.e., the same time block used in the tail suspension test) and analyzed by an Anymaze (Stoelting, Wood Dale, Illinois) video tracking system.
2.5. Data analysis
One-way analysis of variance (ANOVA) was used to analyze the data. Dunnett's test was used for post hoc analysis in the NIH 11082 dose-response experiment and the Scheffe test was used for post hoc analysis in the antagonism experiments. Differences were considered significant at the P < 0.05 level. The median antagonist dose (AD50) value with the 95% confidence interval of naltrindole was calculated using least-squares linear regression analysis.
3. Results
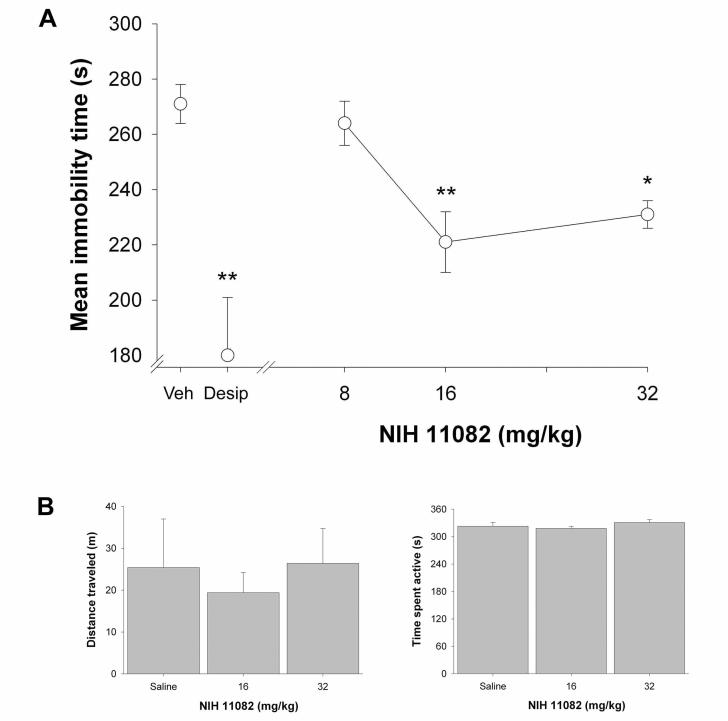
As shown in Fig. 1a, acute administration of a wide dose range of NIH 11082 (8-32 mg/kg) significantly decreased the immobility time in the tail suspension test, F(4, 31) = 6.8, P < 0.001. Significant effects occurred at the 16 (P < 0.01) and 32 (P< 0.05) mg/kg NIH 11082 as compared to the vehicle-treated group. Consistent with previous reports (Shearman et al., 2003), the positive control desipramine (16 mg/kg) significantly decreased the immobility time compared to vehicle treatment (P < 0.01; Fig. 1a). However, NIH 11082 (16 or 32 mg/kg) treatment failed to elicit any significant effects on general locomotor activity with respect to distance traveled (P = 0.83) or time spent mobile (P = 0.97) during the 6 min evaluation period (Fig. 1b). Additionally, there was no apparent convulsive behavior (e.g., head nodding, isolated twitches, tail extension, forelimb clonus, loss of posture, etc.) or other behavioral signs of toxicity (e.g., diarrhea or writhing) in any mice treated with NIH 11082.
Fig. 1.
Panel A. NIH 11082 (8, 16, and 32 mg/kg) or desipramine (Desip; 16 mg/kg) produces antidepressant-like effects in mice, as reflected by significant decreases of the immobility time in the tail suspension test. Panel B. NIH 11082 did not produce significant locomotor activity effects on either the total distanced traveled (left) or the amount of time spent mobile (right) during a six min test. Values are expressed as mean ± S.E.M. immobility time. * P < 0.05 and ** P < 0.01 as compared to vehicle; N = 8 mice per group.
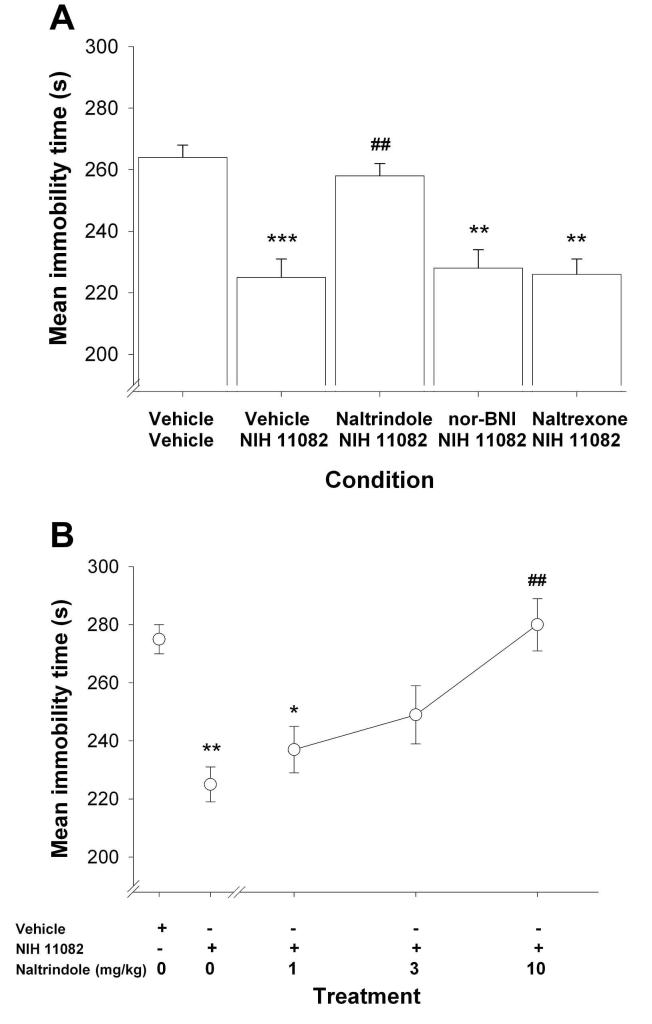
In the next set of experiments, we assessed whether mu, delta, or kappa opioid receptor antagonists would block the effects of NIH 11082 in the tail suspension test. For simplicity, the data from the respective vehicle-vehicle groups were pooled, as were the data from the vehicle-NIH 11082 groups, because the values for each of these respective conditions did not significantly differ across experiments. ANOVA revealed a significant main effect of drug treatment, F(4,59) = 13.1, P < 0.001. As shown in Fig. 2a, naltrindole (10 mg/kg), a selective delta-opioid receptor antagonist, blocked the decreased immobility time in mice treated with NIH 11082 (P < 0.01). In contrast, neither the nonselective muopioid receptor antagonist naltrexone (1 mg/kg) nor the kappa-opioid receptor antagonist nor-BNI (20 mg/kg) blocked the antidepressant-like activity of NIH 11082 (P = 1.00 for both; Fig. 2a). The mean immobility times ± S.E.M. for naltrindole, naltrexone, and nor-BNI administered alone (n = 6 mice/group) were 270 ± 13, 281 ± 14, and 272 ± 9 s, respectively. These immobility times of the opioid antagonists administered alone did not significant differ (P = 0.82) from the 282 ± 8 mean immobility time ± S.E.M of the vehicle control group. As shown in Fig. 2b, naltrindole (1, 3 or 10 mg/kg) dose-dependently antagonized the effects of NIH 11082 in the tail suspension test, F(4, 35) = 8.6, P < 0.001. The AD50(95% CL) of naltrindole in blocking the effects of NIH 11082 (16 mg/kg) was 2.0(1.1-3.8 mg/kg).
Fig. 2.
Panel A. Effects of delta-opioid (10 mg/kg naltrindole), kappa-opioid (20 mg/kg nor-BNI), and the nonselective mu-opioid (1 mg/kg naltrexone) receptor antagonists administered before NIH 11082 (16 mg/kg) in the tail suspension test. Values are expressed as mean ± S.E.M. immobility time (s). ** P < 0.01 and *** P < 0.001, as compared to vehicle-vehicle; ## P < 0.05, as compared to the vehicle-NIH 11082 group. N = 18 for the Vehicle + Vehicle group, 18 for the Vehicle + NIH 11082 group, 10 for the Naltrindole + NIH 11082 group, 10 for the Naltrindole + NIH 11082 group, 8 for the NIH + nor-BNI group, and 10 for the Naltrexone + NIH 11082 group. Panel B. The antidepressant-like effects of NIH 11082 (16 mg/kg) are mediated through a delta-opioid mechanism(s) of action. The delta opioid receptor antagonist, naltrindole (1, 3, or 10 mg/kg) significantly blocked the decreased immobility time produced by NIH 11082 in dose-related fashion. Values are expressed as mean ± S.E.M. immobility time (s). *p < 0.05, as compared to vehicle; ** P < 0.01, as compared to the NIH 11082-treated group; ##p <0.0001, as compared to the NIH 11082-treated group, N = 8 mice per group.
4. Discussion
In the present study, acute treatment of NIH 11082 significantly decreased the immobility time in the tail suspension test, a widely employed animal mode for assessing antidepressant drugs. The magnitude of this effect was comparable to that of desipramine. Naltrindole, a selective delta-opioid receptor antagonist, but not naltrexone or nor-BNI, significantly and dose-dependently blocked the effects of NIH 11082 on immobility time, indicating a delta-opioid receptor mechanism of action. Importantly, we report here that naltrindole possessed a similar potency in blocking the antidepressant-like effects of NIH 11082 (AD50 (95% C.I.) = as 2.0(1.1-3.8) mg/kg) as found in blocking the analgesic effects in the PBQ writhing test (AD50(95% C.I.) = 0.75 (0.26 − 2.20) mg/kg; Aceto et al., in press). While NIH 11082 did produce anti-hyperalgesic effects in the rat Freund's adjuvant arthritic model, it was ineffective in the tail-flick and hot plate tests, which are nociceptive assays that are sensitive to muopioid receptor antagonists (Traynor et al., 2005; Aceto et al., in press).
Several studies have identified a possible role of delta-opioid receptor agonists in treating depression. Early clinical experiments demonstrated that exogenously administered opioid peptides had antidepressant activity in human patients (Broom et al., 2002a; Kline et al., 1977). Also, enkephalinase inhibitors, which prevent the degradation of endogenous enkephalins, produced antidepressant-like effects mediated through the delta-opioid receptor in animal models of depression. More recently, the selective non-peptidic delta-opioid receptor agonists SNC80 and (+) BW373U86 were found to have antidepressant-like activity in the forced swim assay in rats (Broom et al., 2002a; Jutkiewicz et al., 2005).
In addition to antidepressant-like effects, delta-opioid receptor agonists have been demonstrated to elicit convulsions in mice, rats, and monkeys (Broom et al., 2002a; Comer et al., 1993; Dykstra et al., 1993; Hong et al., 1998; Jutkiewicz et al., 2005; Negus et al., 1994; Pakarinen et al., 1995). It has been suggested that these drugs may produce antidepressant-like effects and convulsions through a similar mechanism (Comer et al., 1993). However, Broom et al. (2002b) demonstrated a dissociation between antidepressant-like and convulsive events. Specifically, they found that pretreatment with the benzodiazepine midazolam to block convulsions did not alter the antidepressant-like properties of the delta opioid receptor agonist (+)BW373U86. In another study, Jutkiewicz et al. (2005) reported that different mechanisms of action generate the non-peptidic delta-opioid receptor agonist-induced convulsions and antidepressant-like effects, as these behaviors were differentially influenced by whole brain drug concentration, drug administration rate, and tolerance development. Specifically, slow infusion rates of delta-opioid receptor agonists nearly eliminated convulsions without altering the antidepressant-like properties of these compounds. These findings taken together highlight the independent natures of the convulsive and antidepressant-like effects and demonstrate that these two behavioral outcomes can be separated (Jutkiewicz et al., 2005). Moreover, these results suggest that it may be feasible to minimize undesirable convulsive effects, while retaining the antidepressant-like properties of delta opioid receptor agonists. While the anticonvulsant effects of SNC80 have not been reported in the mouse tail suspension test, this drug elicited significant effects in the rat forced swim test at 3.2 and 10 mg/kg (Jutkiewicz et al., 2005). Unlike direct acting delta-opioid receptor agonists described above, NIH 11082, which binds poorly to delta opioid receptors, produced significant effects in the tail suspension test through a naltrindole sensitive mechanism of action and failed to elicit either convulsions or other overt signs of behavioral toxicity (Traynor et al., 2005). This pattern of findings supports the hypothesis of Broom and colleagues (2002b) that delta opioid receptor agonist antidepressant-like effects do not require a convulsive event, though it should be noted that it is unknown whether NIH 11082 would lower the threshold of chemically or electrically provoked seizures. In conclusion, the results of the present study suggest that NIH 11082 may be a better therapeutic agent than direct-acting delta opioid receptor agonists for the treatment of depression.
Acknowledgements
This work was supported by the National Institute on Drug Abuse DA017259, DA015683, DA1-7725 and DA3-8823
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto MD, May EL, Harris LS, Bowman ERC. Pharmacological studies with a nonpeptidic, delta-opiate (−)-(1R,5R,9R)-5,9-Dimethyl-2′-hydroxy-2-(6-hydroxyhexyl)-6,7-benzomorphan hydrochloride (NIH 11082) Eur. J. Pharmacol. doi: 10.1016/j.ejphar.2007.03.008. C.D., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baamonde A, Dauge V, Ruiz-Gayo M, Fulga I, Turcaud S, Fournie-Zalushi M-C, Roques B. Antidepressanttype effects of endogenous enkephalins protected by systemic RB101 are mediated by opioid and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Broom D, Jutkiewicz E, Folk J, Traynor J, Rice K, Woods J. Convulsant activity of a nonpeptidic delta-opioid receptor agonist is not required for antidepressant-like effects. Psychopharmacology (Berl) 2002b;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom D, Jutkiewicz E, Rice K, Traynor J, Woods J. Behavioral effects of delta-opioid receptor agonists: potential antidepressants? Jpn J Pharmacol. 2002a;90:1–6. doi: 10.1254/jjp.90.1. [DOI] [PubMed] [Google Scholar]
- Comer S, Hoenicke E, Sable A, McNutt R, Chang K, DeCosta B, Mosberg H, Woods J. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–895. [PubMed] [Google Scholar]
- Dykstra L, Schoenbaum G, Yarbrough J, McNutt R, Chang K-J. A novel ä-opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther. 1993;267:875–882. [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes H, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer B. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fink M, Simeon J, Itil T, Freedman A. Clinical antidepressant activity of cyclazocine - a narcotic antagonist. Clin Pharmac Ther. 1970;11:41–48. doi: 10.1002/cpt197011141. [DOI] [PubMed] [Google Scholar]
- Hollister L, Johnson K, Boukhabza D, Gillespie H. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- Hong E, Rice K, Calderon S, Woods J, Traynor J. Convulsive behavior of nonpeptide ä-opioid ligands: comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Jutkiewicz E, Eller E, Folk J, Rice K, Traynor J, Woods J. Delta -opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz E, Rice K, Traynor J, Woods J. Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacology (Berl) 2005;182:588–596. doi: 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant -like effects of delta-opioid receptor agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kline N, Li C, Lehmann H, Lajtha A, Laski E, Cooper T. Beta-Endorphin-induced changes in schizophrenic and depressed patients. Arch Gen Psychiatry. 1977;34:1111–1113. doi: 10.1001/archpsyc.1977.01770210125012. [DOI] [PubMed] [Google Scholar]
- Kline N, Li C, Lehmann H, Lajtha A, Laski E, Cooper T. Beta-Endorphin induced changes in schizophrenic and depressed patients. Arch Gen Psychiat. 1997;34:1111–1113. doi: 10.1001/archpsyc.1977.01770210125012. [DOI] [PubMed] [Google Scholar]
- Negus S, Butelman E, Chang K-J, DeCosta B, Winger G, Woods J. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Pakarinen E, Woods J, Moerschbaecher J. Repeated acquisition of behavioral chains in squirrel monkeys: comparisons of a mu, kappa and delta opioid agonist. J Pharmacol Exp Ther. 1995;272:552–559. [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Mico J, Lenegre A, Steru M, Simon P, Porsolt R. The automated Tail Suspension Test: a computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1987a;11:659–671. doi: 10.1016/0278-5846(87)90002-9. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Mico JA, Lenegre A, Steru M, Simon P, Porsolt RD. The automated Tail Suspension Test: a computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1987b;11:659–671. doi: 10.1016/0278-5846(87)90002-9. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Mico J, Smadja C, Maldonado R, Roques B, Gilbert-Rahola J. Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol. 1998;354:1–7. doi: 10.1016/s0014-2999(98)00423-3. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Fantegrossi W, Woods JH. Evaluation of Compounds for Opioid Activity. In: Dewey WL, editor. Problems of Drug Dependence, 2004: Proceedings of the 66th Annual Scientific Meeting; The Committee on Problems of Drug Dependence, Inc., NIDA Research Monograph; Rockville, MD: U.S. Department of Health and Human Services; 2005. pp. 131–159. [Google Scholar]