Abstract
The virulence plasmid pJM1 enables the fish pathogen Vibrio anguillarum, a gram-negative polarly flagellated comma-shaped rod bacterium, to cause a highly fatal hemorrhagic septicemic disease in salmonids and other fishes, leading to epizootics throughout the world. The pJM1 plasmid 65,009-nucleotide sequence, with an overall G+C content of 42.6%, revealed genes and open reading frames (ORFs) encoding iron transporters, nonribosomal peptide enzymes, and other proteins essential for the biosynthesis of the siderophore anguibactin. Of the 59 ORFs, approximately 32% were related to iron metabolic functions. The plasmid pJM1 confers on V. anguillarum the ability to take up ferric iron as a complex with anguibactin from a medium in which iron is chelated by transferrin, ethylenediamine-di(o-hydroxyphenyl-acetic acid), or other iron-chelating compounds. The fatDCBA-angRT operon as well as other downstream biosynthetic genes is bracketed by the homologous ISV-A1 and ISV-A2 insertion sequences. Other clusters on the plasmid also show an insertion element-flanked organization, including ORFs homologous to genes involved in the biosynthesis of 2,3-dihydroxybenzoic acid. Homologues of replication and partition genes are also identified on pJM1 adjacent to this region. ORFs with no known function represent approximately 30% of the pJM1 sequence. The insertion sequence elements in the composite transposon-like structures, corroborated by the G+C content of the pJM1 sequence, suggest a modular composition of plasmid pJM1, biased towards acquisition of modules containing genes related to iron metabolic functions. We also show that there is considerable microheterogeneity in pJM1-like plasmids from virulent strains of V. anguillarum isolated from different geographical sources.
The fish pathogen Vibrio anguillarum strain 775 is the causative agent of vibriosis, a highly fatal hemorrhagic septicemic disease (3). This bacterium disseminates in the vertebrate host by using the otherwise unavailable iron bound by high-affinity iron binding proteins, such as transferrin and lactoferrin. Furthermore, V. anguillarum 775 has the ability to grow in vitro in media in which iron is chelated by transferrin, ethylenediamine-di(o-hydroxyphenyl-acetic acid), and other iron chelators (15, 40). The metabolic pathway supporting the ability of this bacterium to grow under iron-limiting conditions is linked to the presence in the bacterial cells of the virulence plasmid pJM1 (15).
Iron metabolic plasmids are rare; in addition to the pJM1-like plasmids only the pColV-K30 family of plasmids, identified in human clinical strains of Escherichia coli and other enteric bacteria, have been associated with iron metabolism. However, the pJM1 and pColV-K30 plasmid-mediated iron uptake systems are unrelated (17, 49). pJM1-like-plasmids, usually around 65 to 67 kbp, have been reported by us and others in different virulent V. anguillarum strains isolated from many epizootics throughout the world (30, 32, 46). In this family of plasmids the best characterized is the 65-kb pJM1 plasmid that has been isolated from the V. anguillarum strain 775 and is the one that we chose to sequence. The diverse biochemical features of the pJM1 gene products and the possibility of horizontal evolution provided by the existence of insertion elements surrounding some of these genes make this fascinating plasmid also of interest for the study of evolutionary ecology.
Here we report the nucleotide sequence and annotation of the entire pJM1 virulence plasmid from V. anguillarum strain 775.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
V. anguillarum strain 775 harboring the virulence plasmid pJM1 was grown in M9 minimal medium supplemented with mineral salts and Casamino Acids (Difco) as previously described (51). Plasmids carrying subclones from pJM1 were propagated in E. coli HB101 in Luria-Bertani medium in the presence of chloramphenicol (30 μg/ml). Plasmid DNA was extracted using the Qiagen Midi kit.
Determination of the pJM1 sequence.
Sequencing primers were designed using Oligo 6.8 primer analysis software and purchased from the Oregon Health and Science University-Molecular Microbiology and Immunology (OHSU-MMI) Research Core Facility (http://www.ohsu.edu/core) and Invitrogen. The DNA template used for sequencing reactions was either the complete pJM1 plasmid or fragments cloned using pBR325 as cloning vehicle. DNA sequencing reactions were carried out manually by the chain-termination method and by the OHSU-MMI Research Core Facility using a model 377 Applied Biosystems Inc. automated fluorescence sequencer. Base calling was performed on a Macintosh computer with the ABI base-calling program. Cycle sequencing was performed with AmpliTaq FS DNA polymerase with “Big-Dye” labeled terminators, both from Applied Biosystems Inc. The Sequencher software (Gene Code Corporation) was used for sequence assembly and editing. Codon usage tables were generated by the GCG Wisconsin Package. Coding regions were identified with BLAST (6). Coding sequences with associated ribosome binding sites were predicted with GENEMARK (9) and GLIMMER (http://www.tigr.org/software/glimmer/) and compared to the BLAST alignments to establish the coding sequence start. Regions lacking identified open reading frames (ORFs) and giving no BLAST results but revealing, on visual inspection, coding sequences with ribosomal binding sites were included in the annotation. G+C content analyses were performed with Oligo 6.8 primer analysis software.
Nucleotide sequences accession number.
The complete circular nucleotide sequence of pJM1 from V. anguillarum strain 775 has been deposited in the GenBank sequence library and assigned the accession number AY312585.
RESULTS
Nucleotide composition.
The circular plasmid is comprised of 65,009 bp, and the overall G+C content is 42.6%, which is slightly lower than the average G+C content of the two V. anguillarum 775 chromosomes (44%) (36). The G+C content is also reflected by the lack of restriction sites for AscI (GGCGCGCC), NotI (GCGGCCGC), and SrfI (GCCCGGGC) and the presence of only one site for FseI (GGCCGGCC). The bias towards A and T in the third codon position is also a consequence of this structural feature (data not shown).
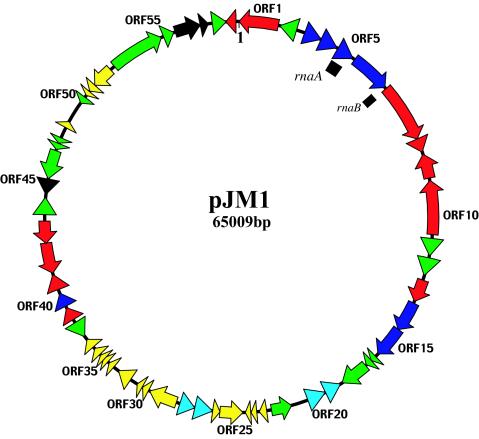
With no demonstrated origin of replication (see below), position 1 of the sequence was arbitrarily assigned to 290 nucleotides upstream of a BamHI site, which corresponds to the last base of the angM gene (ORF1). A total of 86.6% of the plasmid genome appears to have a coding function, and 41 of the 59 ORFs either correspond to biochemically characterized proteins or can be correlated with assigned or described functions by high degrees of similarity to sequences in the databases. Additionally, 18 ORFs predicted by GENEMARK, GLIMMER, or visual inspection but having no significant similarity to sequences in the databases may be functionally relevant (Table 1). Two additional genes, rnaA and rnaB, were found on the pJM1 plasmid and were shown to encode two antisense RNAs. The ORFs and the two antisense RNA genes are distributed between the strands in a ratio of 36 on one and 25 on the other strand.
TABLE 1.
Summary of ORFs identified by significant homology (BLAST search) or prediction or previously experimentally verified
| ORF | Length (amino acids) | CDS (start codon- stop codon)a | Gene or function of closest relative (source) | Data bank reference | Identityb (%) | e valuec |
|---|---|---|---|---|---|---|
| 1 | 706 | 1-2118c | NRPSd VibF (V. cholerae) | gb|AAG00566.1| | 209/535 (39) | e-103 |
| 2 | 302 | 2207-3115c | Transposase for ISV-A1 (V. anguillarum) | gb|AAA81774.1| | 301/302 (99) | e-173 |
| 3 | 314 | 3578-4522 | Iron transport protein FatD (V. anguillarun) | gb|AAA25641.1| | 314/314 (100) | e-141 |
| 4 | 317 | 4519-5472 | Iron transport protein FatC (V. anguillarum) | gb|AAA25642.1| | 317/317 (100) | e-139 |
| 5 | 322 | 5526-6494 | Iron transport protein FatB (V. anguillarum) | gb|AAA91580.1| | 322/322 (100) | e-178 |
| 6 | 726 | 6598-8778 | Iron transport protein FatA (V. anguillarum) | gb|AAA91581.1| | 726/726 (100) | 0.0 |
| 7 | 1,048 | 8863-12009 | NRPS AngR (V. anguillarum) | gb|AAA79860.1| | 1,048/1,048 (100) | 0.0 |
| 8 | 252 | 12006-12764 | NRPS AngT (V. anguillarum) | gb|AAA79861.1| | 252/252 (100) | e-150 |
| 9 | 442 | 12862-14190c | Rhizobactin biosynthesis protein RhbE (Sinorhizobium meliloti) | gb|AAK65920.1| | 237/435 (54) | e-143 |
| 10 | 956 | 14314-17184c | NRPS VibF (V. cholerae) | gb|AAG00566.1| | 332/931 (35) | e-157 |
| 11 | 306 | 17396-18316 | Transposase for ISV-A2 (V. anguillarum) | gb|AAA81776.1| | 305/306 (99) | 6-174 |
| 12 | 322 | 18437-19405 | Transposase (Vibrio vulnificus) | gb|AAO10886.1| | 301/322 (93) | e-152 |
| 13 | 386 | 19652-20854 | Histidine decarboxylase AngH (V. anguillarum) | emb|CAA83945.1| | 386/386 (100) | 0.0 |
| 14 | 536 | 20970-22580 | ABC transporter homologue (E. coli) | gb|AAN79727.1| | 137/548 (25) | 3e-27 |
| 15 | 560 | 22577-24259 | Putative ABC transporter (Streptomyces coelicolor) | emb|CAC17507.1| | 132/461 (28) | 2e-40 |
| 16 | 105 | 24444-24761 | Orf1 ISVme (V. metschnikovii) | gb|AAN33020.1| | 24/96 (25) | 1.4 |
| 17 | 117 | 24758-25111 | Orf2 ISVme (V. metschnikovii) | gb|AAN33021.1| | 41/103 (39) | e-15 |
| 18 | 513 | 25171-26712 | Orf3 ISVme (V. metschnikovii) | gb|AAN33022.1| | 217/526 (41) | 2e-99 |
| 19 | 304 | 26817-27731c | RepA (Aeromonas salmonicida) | gb|AAK97757.1| | 52/205 (25) | e-07 |
| 20 | 343 | 27728-28759c | Related to DNA mismatch repair protein (Streptococcus mutans) | gb|AAN59686.1| | 28/93 (30) | 0.67 |
| 21 | 362 | 29673-30761c | Transposase for IS801 (Pseudomonas syringae) | emb|CAA40540.1|| | 171/341 (50) | 8e-92 |
| 22 | 128 | 31031-31417 | Unknown (V. vulnificus) | gb|AAO07608.1| | 34/99 (34) | 0.014 |
| 23 | 99 | 31555-31854 | Conserved hypothetical protein (Pseudomonas putida) | emb|CAC86752.1| | 54/92 (58) | e-22 |
| 24 | 96 | 31878-32168 | Hypothetical protein (P. putida) | emb|CAC86751.1| | 48/83 (57) | 2e-21 |
| 25 | 409 | 32297-33526c | GLIMMER, GENEMARK prediction; no homology | |||
| 26 | 127 | 33513-33896c | GLIMMER prediction; no homology | |||
| 27 | 323 | 33902-34873c | ParB (Xylella fastidiosa) | gb|AAF85627.1| | 64/218 (29) | 5e-16 |
| 28 | 273 | 34870-35691c | ParA (Leptospira interrogans) | gb|AAN51545.1| | 74/247 (29) | 3e-22 |
| 29 | 514 | 35892-37436 | Putative bacteriophage protein (Salmonella enterica) | gb|AAO69541.1| | 101/307 (32) | 5e-32 |
| 30 | 115 | 37505-37852 | GLIMMER prediction; no homology | |||
| 31 | 103 | 37883-38194 | GLIMMER prediction; no homology | |||
| 32 | 264 | 38616-39410 | GLIMMER, GENEMARK prediction; no homology | |||
| 33 | 104 | 39738-40052 | GLIMMER, GENEMARK prediction; no homology | |||
| 34 | 116 | 40152-40451 | GLIMMER prediction; no homology | |||
| 35 | 93 | 40396-40677 | GLIMMER prediction; no homology | |||
| 36 | 136 | 40727-41137 | GLIMMER prediction; no homology | |||
| 37 | 176 | 41246-41776 | GLIMMER prediction; no homology | |||
| 38 | 306 | 41945-42865c | Transposase for ISV-A2 (V. anguillarum) | gb|AAG33855.1| | 306/306 (100) | e-174 |
| 39 | 239 | 43053-43772 | NrgA (Photorhabdus luminescens) similar to EntD (E. coli) | gb|AAO17175.1| | 91/227 (40) | 3e-40 |
| 40 | 251 | 43873-44628 | Iron(III) ABC transporter (Vibrio parahaemolyticus) | dbj|BAC62003.1| | 183/250 (73) | e-101 |
| 41 | 287 | 44827-45690 | AngB/G (V. anguillarum) | gb|AAG33854.1| | 287/287 (100) | e-151 |
| 42 | 546 | 45741-47381c | DHBA-AMP ligase (V. cholerae) | gb|AAF93937.1| | 307/536 (57) | 0.0 |
| 43 | 393 | 47362-48543c | Isochorismate synthase (V. vulnificus) | gb|AAO07758.1| | 252/392 (64) | e-149 |
| 44 | 306 | 48880-49800 | Transposase for ISV-A2 (V. anguillarum) | gb|AAG33856.1| | 306/306 (100) | e-174 |
| 45 | 277 | 49943-50776c | DHAPe synthase (V. vulnificus) | gb|AAO07756.1| | 149/268 (55) | 2e-82 |
| 46 | 513 | 50796-52337c | Orf3 ISVme (V. metschnikovii) | gb|AAN33022.1| | 217/526 (41) | 2e-99 |
| 47 | 117 | 52397-52750c | Orf2 ISVme (V. metschnikovii) | gb|AAN33021.1| | 41/103 (39) | e-15 |
| 48 | 105 | 52747-53064c | Orf1 ISVme (V. metschnikovii) | gb|AAN33020.1| | 24/96 (25) | 1.4 |
| 49 | 115 | 53664-54011 | GLIMMER prediction; no homology | |||
| 50 | 153 | 55181-55561c | Putative transposase (Klebsiella pneumoniae) | emb|CAA09339.1| | 98/151 (64) | 4e-55 |
| 51 | 112 | 55679-55951c | Putative arylsulfatase regulatory protein (E. coli) | gb|AAN83151.1| | 24/97 (24) | 0.73 |
| 52 | 140 | 55971-56393c | GLIMMER prediction; no homology | |||
| 53 | 389 | 56383-57552c | Hypothetical protein (Pseudomonas putida) | gb|AAN662271.1| | 34/101 (33) | 0.002 |
| 54 | 980 | 57830-60772 | Transposase (Mesorhizobium loti) | dbj|BAB54451.1| | 431/965 (44) | 0.0 |
| 55 | 194 | 60891-61475 | Putative resolvase (Serratia marcescens) | gb|AAN52496.1| | 183/194 (94) | 8e-88 |
| 56 | 464 | 61553-62947 | SpnT (S. marcescens) | gb|AAN52497.1| | 430/464 (92) | 0.0 |
| 57 | 144 | 62944-63378 | RecX (E. coli) | dbj|BAA16560.1| | 54/146 (36) | e-18 |
| 58 | 299 | 63430-64329 | Transposase for ISV-A2 (V. anguillarum) (partial) | gb|AAG33855.1| | 293/293 (100) | e-166 |
| 59 | 175 | 64326-64853c | VibA (V. cholerae) (partial) | gb|AAF93939.1| | 91/159 (57) | 2e-42 |
CDS, coding sequence; c, complementary strand.
Shown as number of identical amino acids/total number of amino acids in the region of homology identified by BLAST.
An e value of >0.4 indicates no homology.
NRPS, nonribosomal peptide synthetase.
DHAP, 2-dehydro-3-deoxyphosphoheptonate aldolase.
ORF analysis.
A graphical representation of the 59 known or predicted ORFs, and the two antisense RNA genes, appears in Fig. 1. Their relationships to representative homologues in databases are detailed in Table 1. Two ORFs, identified by using GLIMMER software, are not included in Table 1 because they are encoded within ORF54 in the opposite strand, they show no homology with any known proteins or ORFs in GenBank, and no obvious Shine-Dalgarno sequence was identified upstream of the AUG of these ORFs.
FIG. 1.
Schematic representation of the ORFs on the two strands of pJM1 DNA. Red, ORFs related to biosynthesis of the siderophore; blue, ORFs related to transport of the siderophore; green, ORFs related to IS elements and composite transposon; cyan, ORFs related to replication and partitioning; yellow, conserved hypothetical ORFs and ORFs with no known functions; black, ORFs with functions that do not fall in any of the above categories. The black blocks represent genes encoding antisense RNAs: rnaA, antisense RNAα, and rnaB, antisense RNAβ.
Based on gene similarity search results, functions associated with ORFs may be classified into the following categories: utilization of iron, transposition, and replication-partition (Table 1).
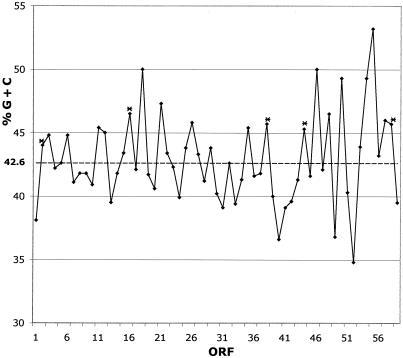
In Fig. 2 the G+C content of each ORF is shown, including the relative position of insertion sequence (IS) elements.
FIG. 2.
G+C contents of the ORFs of pJM1. Dashed line, mean G+C content of pJM1 DNA (42.6%). Asterisks indicate the highly related ISV-A1 and ISV-A2.
Utilization of iron.
The predicted metabolic abilities linked to pJM1 center on iron uptake and utilization (Fig. 1). We have demonstrated that the genes fatDCBA (ORF3 to ORF6) confer on pJM1-carrying bacteria the ability to utilize ferric anguibactin and so are part of the pathway of iron utilization (2, 4, 5, 26). We also demonstrated that the genes angM (ORF1), angR (ORF7), angT (ORF8), angN (ORF10), and angBG (ORF41) encode nonribosomal peptide synthetases while angU (ORF9) and angH (ORF13) encode tailoring enzymes for the biosynthesis of anguibactin, since transposon insertions in each of these genes resulted in no siderophore production (17, 40-42). Other ORFs encoded on pJM1 (ORF39 to ORF43 and ORF59) show high similarity to siderophore biosynthetic genes found in other bacteria, and some of them could intervene in anguibactin biosynthesis. The homologies in Table 1 suggest that the predicted polypeptides of these ORFs could play a role in the early stages of anguibactin biosynthesis: ORF39 (AngD) is a phosphopantetheinyl transferase; ORF41 (AngBG) possesses the isochorismate lyase and aryl carrier protein (ArCP) domains; ORF42 (AngE) is the 2,3-dihydroxybenzoic acid (DHBA) AMP ligase; and ORF43 (AngC) is an isochorismate synthetase. ORF40 may have a function in the transport of ferric anguibactin into the cell cytosol. Upstream of angM (ORF1) there is another ORF (ORF59) with similarity to a stretch of 159 amino acids within the amino acid sequence of the 2,3-dihydro-DHBA dehydrogenase of Vibrio cholerae, the last enzyme in the DHBA biosynthetic pathway (52). Further upstream of ORF59 is a small (23-amino-acid) ORF that corresponds to the amino-terminal end of the 2,3-dihydro-DHBA dehydrogenase. It is thus possible that ORF59 could have been generated by a frameshift of the original full-length gene, resulting in a shorter ORF truncated at the amino-terminal end. The small ORF upstream of ORF59 has not been included in Table 1.
Retrobiosynthesis of anguibactin indicates that it is composed of one molecule of DHBA, one of cysteine, and one of N-hydroxyhistamine (1, 22). Cysteine is converted to a thiazoline ring, through cyclization, in the process of synthesis, and N-hydroxyhistamine is obtained from the modification of histidine.
In V. anguillarum strain 775, the anguibactin precursor DHBA is synthesized by chromosome-encoded proteins, as shown by the ability of the plasmidless strain to produce DHBA (11), although some virulent strains of V. anguillarum, such as 531A, rely on the pJHC1 (a pJM1-like plasmid)-encoded AngBG protein for the synthesis of this precursor (50). The amino terminus of the pJHC1-encoded AngBG possesses the isochorismate lyase activity, thereby explaining the need for this protein for the synthesis of DHBA in this strain (50). Analysis of mutations in the angB ORF of pJHC1 provided evidence that, in addition to angB, an overlapping gene, angG, exists at this locus and that it encodes polypeptides which are in frame to the carboxy-terminal end of the isochorismate lyase. The carboxy terminus of AngBG encodes an ArCP domain that is also present in the internal AngG polypeptides and is where phosphopantetheinylation occurs at a conserved serine residue, the phosphopantetheinylate moiety acting as an acceptor of an activated aryl or amino acid group (50). In strain 775 the angBG gene is also found on the pJM1 plasmid (ORF41); however, we do not know at present if the product of this gene is essential for anguibactin biosynthesis, as is the case for strain 531A.
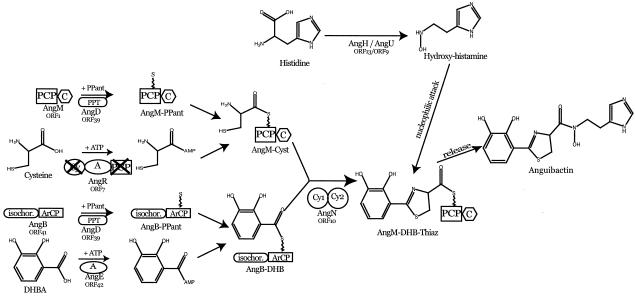
The enzymology of anguibactin biosynthesis is still under investigation; however, predictions can be made based on our knowledge of the structure of this siderophore, genetic evidence, and functions of potential biosynthetic proteins inferred by homology studies (17, 39). The results of this combined genetic and in silico analysis are shown in the model in Fig. 3. In the biosynthesis of other phenolic siderophores, activation of DHBA, before loading on the specific ArCP domain, occurs by the action of a 2,3-dihydroxybenzoate-AMP ligase, VibE in vibriobactin biosynthesis in V. cholerae, and EntE in enterobactin biosynthesis in E. coli. ORF42 in pJM1 shows homology with VibE and EntE adenylation domains (Table 1), suggesting that it could act as the 2,3-dihydroxybenzoate-AMP ligase in anguibactin biosynthesis. The activated DHBA is then ligated to the phosphopantetheinyl moiety in AngBG. Phosphopantetheinylation of this protein might have occurred by the action of the pJM1-encoded AngD (ORF39), showing homology to phosphepantotheinyl transferases. The pJM1 plasmid-encoded proteins AngR (ORF7), AngM (ORF1), and AngN (ORF10) must play a role in subsequent biosynthetic steps, although AngR, in addition to its biosynthetic function, is also essential for regulation of iron transport gene expression (13, 16-18, 34, 39, 42, 51). Cysteine, one of the anguibactin precursors, is likely activated by the A domain of AngR. Activated cysteine is then loaded onto the peptidyl carrier protein (PCP) domain of AngM. Although the AngR amino acid sequence also contains a PCP domain, this may not be functional because an essential serine is replaced by alanine in this domain. The condensation (C) domain of AngM then catalyzes the formation of a peptide bond between DHBA and cysteine, resulting in the DHBA-cysteine dipeptide bound to the PCP domain of AngM. Another plasmid-encoded protein, AngN, contains two tandem cyclization (Cy) domains that are involved in the cyclization of the cysteine moiety to form the thiazoline ring. Another Cy domain is found in the AngR protein; however, the essential first aspartic acid is replaced by asparagine in the highly conserved Cy motif, suggesting that also this domain of AngR is not functional. Anguibactin is released from the PCP domain of AngM by nucleophilic attack of N-hydroxyhistamine to the phosphopantetheinyl arm of the PCP domain of AngM. N-Hydroxyhistamine is produced by modification of histidine catalyzed by the histidine decarboxylase AngH (ORF13) (41) and a monooxygenase homologue, AngU (ORF9). AngT (ORF8), the thioesterase identified in this system, is not included in this model because it does not appear to be strictly necessary for anguibactin production, since an angT mutant results in only a 17-fold-lower yield of anguibactin (17, 51).
FIG. 3.
Model of anguibactin biosynthesis. The predicted ORF numbers from Table 1 are given under the protein designations. Abbreviations: DHB, DHBA and radicals thereof such as a diphenolic ring; Cyst, cysteine; Thiaz, thiazoline ring; PPant, phosphopantetheinyl moiety; isochor., isochorismate.
After anguibactin is synthesized, it is secreted to the extracellular space. Two ORFs (ORF14 and ORF15), downstream of angH, related to the ABC-type transporter, are perhaps involved in secretion as part of the complex that exports anguibactin to the extracellular space. After the siderophore is secreted and bound to iron, the ferric siderophore complex is transported to the cytosol via a highly specific transport system (2, 4, 5, 26, 40, 44, 45). In V. anguillarum 775 this system includes the outer membrane receptor FatA (ORF6), which binds ferric anguibactin and shuttles it to the periplasm (2, 5). The energy necessary for this transport is mediated by a chromosomally encoded TonB-ExbB-ExbD complex, which interacts with the FatA protein (M. Stork, M. L. Lemos, and J. H. Crosa, unpublished data).
The next step in internalizing the ferric siderophore complex involves the periplasmic binding protein FatB (ORF5). FatB is a lipoprotein (4) that is anchored to the inner membrane, unlike the E. coli homologues FhuD and FepB, which are free in the periplasm (25, 37).
We believe that the last step in internalization of ferric anguibactin involves the inner membrane proteins FatD (ORF3) and FatC (ORF4) and that these catalyze the transport of ferric anguibactin from the periplasm to the cytosol (5, 26). In other systems, such as the ferrichrome and enterobactin systems, the energy for this transport through the inner membrane is provided by an ATP-binding protein (7, 24, 31). ORF40 shows homology with many of these iron(III) ATP-binding proteins and could provide the missing ATP-binding domain of the permease complex, although the activity of chromosomally encoded ATP-binding proteins cannot be ruled out.
The genes fatABCD together with the genes angR and angT are located in the iron transport-biosynthesis (ITB) operon (Fig. 1) (51). Regulation of this operon is carried out at the transcriptional level by the positive regulator AngR and the chromosomally encoded negative regulator Fur and at the posttranscriptional level by two antisense RNAs, RNAα and RNAβ, encoded within this operon by the rnaA and rnaB genes, respectively (Fig. 1) (12, 35, 47, 48). These two genes have been shown to encode RNAs that are not translated. Another positive regulator of the ITB operon, the trans-acting factor (TAF), is encoded in a region noncontiguous to this operon encompassing ORF29 to ORF53 (40, 45, 50). The TAF determinants have not been identified as yet, and no ORF included in the TAF region shows homology with regulatory proteins, with the exception of ORF51, which shows very low homology (24% identity) to an E. coli arylsulfatase regulatory protein.
Insertion elements and transposons.
The ITB operon and the biosynthetic genes angU (ORF9) and angN (ORF10) are located within a structure that resembles a transposon, flanked by the almost identical ISV-A1 and ISV-A2 elements (44). There is a second putative composite transposon on pJM1 that contains homologues of genes (ORF39 to ORF43) involved in the synthesis of DHBA, one of the anguibactin precursors (Table 1). These genes are also organized as a cluster that is flanked by identical ISV-A2 sequences. 2 shows very clearly that the ISV-A2-flanked DNA has an average G+C content of 39.3%, significantly lower than the average G+C content of the pJM1 plasmid, suggesting a horizontal acquisition of this composite gene region. A third set of ORFs, ORF1 (angM) and ORF59, is flanked by ISV-A1 and ISV-A2 sequences, although the ISV-A2 element is interrupted at the 5′ end of the putative transposase gene by an insertion of a sequence containing four ORFs (ORF54 to ORF57). It is of interest that one of these ORFs (ORF54) shows homology to a transposase gene, while another, ORF55, shows high similarity to a resolvase. ORF56 shows similarity to spnT, a Serratia marcescens gene encoding a protein with no homologues, although when overexpressed it affected both sliding motility and prodigiosin production in this bacterium (21). The other ORF in this cluster, ORF57, encodes a predicted protein with homology to RecX, a recA regulator (38). Furthermore this cluster is flanked by 5-bp direct repeats, suggesting that the interruption of the ISV-A2 transposase gene (ORF58) could have resulted from insertion of a transposon containing these four ORFs.
Besides ISV-A1 and ISV-A2, pJM1 carries several hypothetical IS elements (Table 1). Two of them show homology to a Vibrio metschnikovii ISVme insertion sequence, and they both contain all three ORFs found in this IS element. These two identical ISVme-like sequences flank a region of 24,085 bp containing 27 ORFs (from ORF19 to ORF45), almost half of the coding capacity of pJM1. Another IS element, RS1 carrying ORF21, which shows homology to a transposase, is found as a single copy in pJM1. Two other ORFs, ORF12 and ORF50, show similarity to transposases; however, no other components of ISs were identified.
Replication and partition.
The pJM1 plasmid replicates at a copy number of 1 to 2 in V. anguillarum cells (10). ORF19 to ORF28, encoding hypothetical proteins that could be involved in replication and partition, are clustered adjacent to the TAF region. ORF19 shows homology with the RepA protein of Aeromonas salmonicida plasmid pRAS3 (28). The presence of four 21-bp direct repeats in the proximity of ORF19, spanning bp 29013 to 29136, and the relatively low G+C content of 33.7% are consistent with the existence of a possible oriV in this region. The predicted amino acid sequences of ORF27 and ORF28 show similarity to those of ParB and ParA, respectively. In other systems, the plasmid-encoded ParA and ParB proteins form one operon autoregulated by the Par proteins. The ParA protein is an ATPase that assembles with ParB subunits and forms a nucleoprotein complex that binds to a cis-acting centromere-like site, parS (27). As predicted for ParA proteins, ORF28 exhibits at its N terminus an ATP-GTP-binding consensus sequence (19). In other bacteria, homologues of parA and parB map adjacent to the chromosomal and plasmid origin regions of replication (29, 33), and this is the case for pJM1. Experiments are being carried out to characterize the functions of the pJM1 replication region.
The microheterogeneity of pJM1-like plasmids in strains of V. anguillarum.
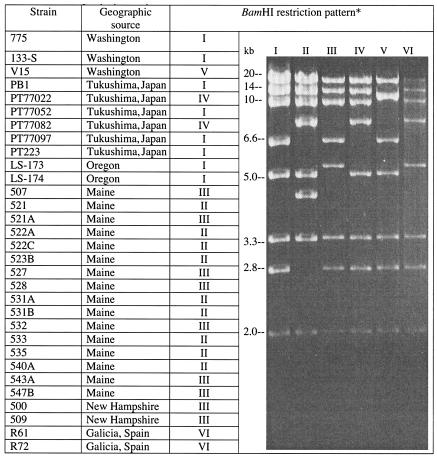
Different virulent V. anguillarum strains harboring the pJM1-like-plasmids showed microheterogeneity in their restriction endonuclease patterns. A listing of the BamHI restriction fragment length polymorphisms as well as the BamHI profiles for several naturally occurring pJM1-like virulence plasmids that we have examined is given in Fig. 4.
FIG. 4.
Microheterogeneity of pJM1-like plasmids from V. anguillarum strains. *, “kb” indicates sizes in kilobase pairs of each of the pJM1 BamHI restriction endonuclease fragments.
DISCUSSION
The presence of genes of a complex biosynthetic pathway for a siderophore and the carriage of the iron transport operon together with its role in virulence of V. anguillarum 775 are unique features of plasmid pJM1 (15, 16, 17). The 65,009-nucleotide sequence, with an overall G+C content of 42.6%, revealed 59 genes and ORFs encoding functions associated with utilization of iron, transposition, and partition. Approximately a third of these ORFs and genes are related to iron metabolic functions, including ferric anguibactin transport proteins, nonribosomal peptide enzymes, and other proteins essential for the biosynthesis of the siderophore anguibactin. The majority of the ORFs encoding iron metabolic functions were experimentally demonstrated to be involved in anguibactin-mediated iron metabolism (ORF1 to ORF13). Other ORFs with homology to proteins involved in the biosynthesis and activation of the precursor of anguibactin DHBA, such as ORF39 to ORF43, were also identified. Our recent work has demonstrated that one of these, ORF42, named angE, encodes a 2,3-dihydroxybenzoate-AMP ligase that could act in activating DHBA. In a previous publication we reported that the angE homologue in plasmid pJHC1, a pJM1-like plasmid found in V. anguillarum strain 531A, was not essential for anguibactin production (50). A deletion that eliminated a region including the angB and angE genes was complemented by just the angB gene, demonstrating the existence of a chromosomal homologue of the angE gene in this strain (50). We have recently identified in the 775 strain chromosome homologues of angE and other genes involved in the production, activation, and incorporation of DHBA in the anguibactin biosynthetic pathway; therefore, the pJM1 plasmid-carried angE gene in the 775 strain might not be essential for anguibactin biosynthesis (unpublished data). It is of interest that the amino acid sequence of this chromosomally encoded AngE is identical to that reported previously (reference 20 and our unpublished results). It is also of interest that ORF39 to ORF43 are organized as a cluster that is flanked by ISV-A2 sequences and that the DNA in this cluster shows an average G+C content of 39.3%, significantly lower than the average G+C content of the pJM1 plasmid. It is tempting to speculate that this ISV-A2-flanked structure is a transposon that has been horizontally acquired from other bacteria. Furthermore, the existence of chromosome homologues for some of these ORFs suggests the attractive possibility that there could also have been an integration event in one of the two V. anguillarum chromosomes. Curiously, the ITB operon, fatDCBA-angRT (ORF3 to ORF8, respectively), and other anguibactin biosynthetic genes, angU (ORF9) and angN (ORF10), located downstream of this operon, are also bracketed by the highly related ISV-A1 and ISV-A2 ISs. Nevertheless, the fact that ORFs and genes on pJM1 are flanked by IS elements does not necessarily imply that the composite structures are transposons, since the relative orientation of the flanking IS elements is not always as found with the IS elements flanking known composite transposons such as Tn5, Tn7, and Tn10 (8, 14, 23). The positions of IS elements forming a composite transposon-like structure, corroborated by the G+C content of the pJM1 sequence, suggest a modular composition of the virulence plasmid pJM1 of V. anguillarum strain 775 biased towards acquisition of transposon modules containing genes related to iron metabolic functions.
An important final step is the secretion of the siderophore to the extracellular milieu, a still-unsolved question for siderophore-mediated iron transport systems. In this vein, although not yet proven, ORF14 and ORF15 could function in the export of anguibactin, since these ORFs show homology to ABC transporters involved in efflux. Elucidation of their function, if any, will have to await current genetic analysis.
Regions that have features consistent with replication and partitioning functions have been identified adjacent to the TAF region. It is possible that the product of ORF19 is a replication protein that interacts with the 21-bp repeats located in proximity. Whether these sequences are truly involved in these functions await further analysis, which is currently being carried out.
We also report in this work considerable microheterogeneity in pJM1 plasmids isolated from many parts of the world, even from both the east and west coasts of the United States. Previous work has also shown microheterogeneity in many other examples of pJM1-like plasmids (30, 32, 42, 43). For instance the pJM1-like plasmid pJHC1 from strain 531A shows two major changes, two extra insertions of the RS1 sequence and also an angR gene that has a single nucleotide change that results in an amino acid substitution in the AngR protein (H267N). This change was associated with an increased anguibactin production not only in the 531A strain but also in other strains in which the AngR protein shows this H-to-N alteration (42). In other cases the existing microheterogeneity has only been characterized by restriction endonuclease analysis without further genetic analysis (30, 32, 43).
Recently a partial sequence of a pJM1-like plasmid (pEIB1) was submitted to GenBank (accession number AY255699). This plasmid is another example of the microheterogeneity of the pJM1-like plasmids: pEIB1 differs from pJM1, in the sequence of at least two anguibactin biosynthesis genes. The angR sequence has the H267N substitution found in several pJM1-like plasmids that leads to a higher siderophore production phenotype (42), while the angN sequence shows an internal deletion compared with the angN gene present in pJM1. The deletion in this pJM1-like plasmid resulted in a frameshift splitting the angN gene (ORF10 in pJM1) into two ORFs, truncating the first Cy domain of AngN while leaving the second Cy domain intact. It would be of interest to know whether the strain harboring the pJM1-like plasmid with this angN deletion is still able to produce anguibactin. Since there is no information on the origin of the strain, we cannot identify whether this plasmid is one of the already described pJM1-like plasmids. Furthermore, this partial sequence submission erroneously assigns homology of the ORF JM15, which is 100% homologous to our ORF56, to the TcbA insecticidal toxin from Photorhabdus luminescens (GenBank accession number AAC38627). By our analysis ORF56, and thus JM15, shows only low homology (20% identity) to an 110-amino-acid stretch of the 2,504-amino-acid-long TcbA protein. Conversely, we found a strikingly high homology (92% identity [Table 1]) of the predicted 464-amino-acid product of ORF56, and thus JM15, to the 464-amino-acid-long SpnT (GenBank accession number AAN52497), an S. marcescens protein possibly involved in both sliding motility and prodigiosin production in this bacterium (21).
Our complete sequence analysis and annotation of pJM1 present for the first time the entire sequence of an iron metabolic regulon that is in its majority encoded on a plasmid and that is an essential factor of virulence in V. anguillarum infections of fish.
Acknowledgments
This project was supported by National Public Health Service awards from the National Institutes of Health, AI19018-19 and GM60400-01 to J.H.C.
We thank Thomas Keller and the excellent technical support provided by the OHSU-MMI Research Core Facility.
REFERENCES
- 1.Actis, L. A., W. Fish, J. H. Crosa, K. Kellerman, S. R. Ellenberger, F. M. Hauser, and J. Sanders-Loehr. 1986. Characterization of anguibactin, a novel siderophore from Vibrio anguillarum 775(pJM1). J. Bacteriol. 167:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Actis, L. A., S. A. Potter, and J. H. Crosa. 1985. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J. Bacteriol. 161:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Actis, L. A., M. E. Tolmasky, and J. H. Crosa. 1999. Vibriosis, p. 523-557. In P. Woo and D. Bruno (ed.), Fish diseases and disorders. Viral, bacterial, and fungal infections, vol. 3. CAB International Publishing, Wallingford, United Kingdom. [Google Scholar]
- 4.Actis, L. A., M. E. Tolmasky, L. M. Crosa, and J. H. Crosa. 1995. Characterization and regulation of the expression of FatB, an iron transport protein encoded by the pJM1 virulence plasmid. Mol. Microbiol. 17:197-204. [DOI] [PubMed] [Google Scholar]
- 5.Actis, L. A., M. E. Tolmasky, D. H. Farrell, and J. H. Crosa. 1988. Genetic and molecular characterization of essential components of the Vibrio anguillarum plasmid-mediated iron-transport system. J. Biol. Chem. 263:2853-2860. [PubMed] [Google Scholar]
- 6.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 7.Becker, K., W. Koster, and V. Braun. 1990. Iron(III) hydroxamate transport of Escherichia coli K12: single amino acid replacements at potential ATP-binding sites inactivate the FhuC protein. Mol. Gen. Genet. 223:159-162. [DOI] [PubMed] [Google Scholar]
- 8.Berg, D. E. 1989. Transposon Tn5, p. 185-210. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 9.Borodovsky, M., and J. McIninch. 1993. GeneMark: parallel gene recognition for both DNA strands. Comput. Chem. 17:123-133. [Google Scholar]
- 10.Chen, Q. 1995. Ph.D. thesis. Oregon Health and Science University, Portland.
- 11.Chen, Q., L. A. Actis, M. E. Tolmasky, and J. H. Crosa. 1994. Chromosome-mediated 2,3-dihydroxybenzoic acid is a precursor in the biosynthesis of the plasmid-mediated siderophore anguibactin in Vibrio anguillarum. J. Bacteriol. 176:4226-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Q., and J. H. Crosa. 1996. Antisense RNA, fur, iron, and the regulation of iron transport genes in Vibrio anguillarum. J. Biol. Chem. 271:18885-18891. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Q., A. M. Wertheimer, M. E. Tolmasky, and J. H. Crosa. 1996. The AngR protein and the siderophore anguibactin positively regulate the expression of iron-transport genes in Vibrio anguillarum. Mol. Microbiol. 22:127-134. [DOI] [PubMed] [Google Scholar]
- 14.Craig, N. L. 1989. Transposon Tn7, p. 211-225. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 15.Crosa, J. H. 1980. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature 284:566-568. [DOI] [PubMed] [Google Scholar]
- 16.Crosa, J. H. 1997. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev. 61:319-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosa, J. H., and C. T. Walsh. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 66:223-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell, D. H., P. Mikesell, L. A. Actis, and J. H. Crosa. 1990. A regulatory gene, angR, of the iron uptake system of Vibrio anguillarum: similarity with phage P22 cro and regulation by iron. Gene 86:45-51. [DOI] [PubMed] [Google Scholar]
- 19.Gerdes, K., J. Moller-Jensen, and R. Bugge Jensen. 2000. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol. 37:455-466. [DOI] [PubMed] [Google Scholar]
- 20.Holmstrom, K., and L. Gram. 2003. Elucidation of the Vibrio anguillarum genetic response to the potential fish probiont Pseudomonas fluorescens AH2, using RNA-arbitrarily primed PCR. J. Bacteriol. 185:831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horng, Y. T., S. C. Deng, M. Daykin, P. C. Soo, J. R. Wei, K. T. Luh, S. W. Ho, S. Swift, H. C. Lai, and P. Williams. 2002. The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol. 45:1655-1671. [DOI] [PubMed] [Google Scholar]
- 22.Jalal, M., D. Hossain, D. van der Helm, J. Sanders-Loehr, L. A. Actis, and J. H. Crosa. 1989. Structure of anguibactin, a unique plasmid-related bacterial siderophore from the fish pathogen Vibrio anguillarum. J. Am. Chem. Soc. 111:292-296. [Google Scholar]
- 23.Kleckner, N. 1989. Transposon Tn10, p. 227-268. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 24.Koster, W. 1991. Iron(III) hydroxamate transport across the cytoplasmic membrane of Escherichia coli. Biol. Met. 4:23-32. [DOI] [PubMed] [Google Scholar]
- 25.Koster, W., and V. Braun. 1990. Iron (III) hydroxamate transport into Escherichia coli. Substrate binding to the periplasmic FhuD protein. J. Biol. Chem. 265:21407-21410. [PubMed] [Google Scholar]
- 26.Koster, W. L., L. A. Actis, L. S. Waldbeser, M. E. Tolmasky, and J. H. Crosa. 1991. Molecular characterization of the iron transport system mediated by the pJM1 plasmid in Vibrio anguillarum 775. J. Biol. Chem. 266:23829-23833. [PubMed] [Google Scholar]
- 27.Kwong, S. M., C. C. Yeo, and C. L. Poh. 2001. Molecular analysis of the pRA2 partitioning region: ParB autoregulates parAB transcription and forms a nucleoprotein complex with the plasmid partition site, parS. Mol. Microbiol. 40:621-633. [DOI] [PubMed] [Google Scholar]
- 28.L'Abee-Lund, T. M., and H. Sorum. 2002. A global non-conjugative Tet C plasmid, pRAS3, from Aeromonas salmonicida. Plasmid 47:172-181. [DOI] [PubMed] [Google Scholar]
- 29.Lin, D. C., and A. D. Grossman. 1998. Identification and characterization of a bacterial chromosome partitioning site. Cell 92:675-685. [DOI] [PubMed] [Google Scholar]
- 30.Olsen, J. E., and J. L. Larsen. 1990. Restriction fragment length polymorphism of the Vibrio anguillarum serovar O1 virulence plasmid. Appl. Environ. Microbiol. 56:3130-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozenberger, B. A., M. S. Nahlik, and M. A. McIntosh. 1987. Genetic organization of multiple fep genes encoding ferric enterobactin transport functions in Escherichia coli. J. Bacteriol. 169:3638-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen, K., T. Tiainen, and J. L. Larsen. 1996. Plasmid profiles, restriction fragment length polymorphisms and O-serotypes among Vibrio anguillarum isolates. Epidemiol. Infect. 117:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picardeau, M., J. R. Lobry, and B. J. Hinnebusch. 2000. Analyzing DNA strand compositional asymmetry to identify candidate replication origins of Borrelia burgdorferi linear and circular plasmids. Genome Res. 10:1594-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salinas, P. C., and J. H. Crosa. 1995. Regulation of angR, a gene with regulatory and biosynthetic functions in the pJM1 plasmid-mediated iron uptake system of Vibrio anguillarum. Gene 160:17-23. [DOI] [PubMed] [Google Scholar]
- 35.Salinas, P. C., L. S. Waldbeser, and J. H. Crosa. 1993. Regulation of the expression of bacterial iron transport genes: possible role of an antisense RNA as a repressor. Gene 123:33-38. [DOI] [PubMed] [Google Scholar]
- 36.Schiewe, M. H., J. H. Crosa, and E. J. Ordal. 1977. Deoxyribonucleic acid relationships among marine vibrios pathogenic to fish. Can. J. Microbiol. 23:954-958. [DOI] [PubMed] [Google Scholar]
- 37.Stephens, D. L., M. D. Choe, and C. F. Earhart. 1995. Escherichia coli periplasmic protein FepB binds ferrienterobactin. Microbiology 141:1647-1654. [DOI] [PubMed] [Google Scholar]
- 38.Stohl, E. A., J. P. Brockman, K. L. Burkle, K. Morimatsu, S. C. Kowalczykowski, and H. S. Seifert. 2003. Escherichia coli RecX inhibits RecA recombinase and coprotease activities in vitro and in vivo. J. Biol. Chem. 278:2278-2285. [DOI] [PubMed] [Google Scholar]
- 39.Stork, M., M. Di Lorenzo, T. J. Welch, L. M. Crosa, and J. H. Crosa. 2002. Plasmid-mediated iron uptake and virulence in Vibrio anguillarum. Plasmid 48:222-228. [DOI] [PubMed] [Google Scholar]
- 40.Tolmasky, M. E., L. A. Actis, and J. H. Crosa. 1988. Genetic analysis of the iron uptake region of the Vibrio anguillarum plasmid pJM1: molecular cloning of genetic determinants encoding a novel trans activator of siderophore biosynthesis. J. Bacteriol. 170:1913-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolmasky, M. E., L. A. Actis, and J. H. Crosa. 1995. A histidine decarboxylase gene encoded by the Vibrio anguillarum plasmid pJM1 is essential for virulence: histamine is a precursor in the biosynthesis of anguibactin. Mol. Microbiol. 15:87-95. [DOI] [PubMed] [Google Scholar]
- 42.Tolmasky, M. E., L. A. Actis, and J. H. Crosa. 1993. A single amino acid change in AngR, a protein encoded by pJM1-like virulence plasmids, results in hyperproduction of anguibactin. Infect. Immun. 61:3228-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolmasky, M. E., L. A. Actis, A. E. Toranzo, J. L. Barja, and J. H. Crosa. 1985. Plasmids mediating iron uptake in Vibrio anguillarum strains isolated from turbot in Spain. J. Gen. Microbiol. 131:1989-1997. [DOI] [PubMed] [Google Scholar]
- 44.Tolmasky, M. E., and J. H. Crosa. 1995. Iron transport genes of the pJM1-mediated iron uptake system of Vibrio anguillarum are included in a transposonlike structure. Plasmid 33:180-190. [DOI] [PubMed] [Google Scholar]
- 45.Tolmasky, M. E., and J. H. Crosa. 1984. Molecular cloning and expression of genetic determinants for the iron uptake system mediated by the Vibrio anguillarum plasmid pJM1. J. Bacteriol. 160:860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolmasky, M. E., P. C. Salinas, L. A. Actis, and J. H. Crosa. 1988. Increased production of the siderophore anguibactin mediated by pJM1-like plasmids in Vibrio anguillarum. Infect. Immun. 56:1608-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldbeser, L. S., Q. Chen, and J. H. Crosa. 1995. Antisense RNA regulation of the fatB iron transport protein gene in Vibrio anguillarum. Mol. Microbiol. 17:747-756. [DOI] [PubMed] [Google Scholar]
- 48.Waldbeser, L. S., M. E. Tolmasky, L. A. Actis, and J. H. Crosa. 1993. Mechanisms for negative regulation by iron of the fatA outer membrane protein gene expression in Vibrio anguillarum 775. J. Biol. Chem. 268:10433-10439. [PubMed] [Google Scholar]
- 49.Waters, V. L., and J. H. Crosa. 1991. Colicin V virulence plasmids. Microbiol. Rev. 55:437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welch, T. J., S. Chai, and J. H. Crosa. 2000. The overlapping angB and angG genes are encoded within the trans-acting factor region of the virulence plasmid in Vibrio anguillarum: essential role in siderophore biosynthesis. J. Bacteriol. 182:6762-6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wertheimer, A. M., W. Verweij, Q. Chen, L. M. Crosa, M. Nagasawa, M. E. Tolmasky, L. A. Actis, and J. H. Crosa. 1999. Characterization of the angR gene of Vibrio anguillarum: essential role in virulence. Infect. Immun. 67:6496-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyckoff, E. E., J. A. Stoebner, K. E. Reed, and S. M. Payne. 1997. Cloning of a Vibrio cholerae vibriobactin gene cluster: identification of genes required for early steps in siderophore biosynthesis. J. Bacteriol. 179:7055-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]