Abstract
Synechococcus sp. strain RF-1 is a unicellular freshwater cyanobacterium that fixes N2 aerobically and exhibits a circadian rhythm for nitrogenase activity under a light-dark regimen. Synechococcus sp. strain RF-1 also utilizes nitrate, nitrite, or ammonium for growth. Under the diazotrophic growth, the nitrate uptake in Synechococcus sp. strain RF-1 was induced by nitrate or nitrite but repressed by ammonium. In contrast, a prominent nitrate reductase (NR) activity was detected in diazotrophically grown cells using the reduced methyl viologen assay. The NR activity was not inhibited by ammonium and only slightly enhanced by nitrate. The different expression patterns of nitrate uptake and NR in Synechococcus sp. strain RF-1 were reflected in general at the transcript level determined by reverse transcriptase PCR. Under both nitrate-induced and uninduced conditions, the in situ NR activity exhibited similar biphasic kinetics for nitrate. The recombinant NR encoded by the narB gene of Synechococcus sp. strain RF-1, expressed in E. coli, also showed the biphasic kinetics with similar pH and temperature profiles. By in-gel NR activity assay, the recombinant NarB was found to exist as a single form. Both the high- and low-affinity NR activities of the recombinant NarB showed the same thermostability. When modified at the N terminus by a polyhistidine tag, the recombinant NR activity was shifted from biphasic to hyperbolic kinetics and showed only a single Km for nitrate, indicating the functional importance of the NarB N-terminal structure in NR kinetics.
Cyanobacteria make up one of the most widespread groups of photoautotrophic bacteria, contributing significantly to the natural carbon and nitrogen cycle. They perform oxygenic photosynthesis to assimilate inorganic carbon and use primarily inorganic nitrogen sources (dinitrogen, nitrate, and ammonium) to fulfill their nitrogen requirement (6). Nitrate assimilation is one of the major processes of nitrogen acquisition in cyanobacteria (10). In the process, nitrate is transported into cells by an active transport system and reduced to ammonium by the sequential action of nitrate reductase (NR) and nitrite reductase (NiR). The resulting ammonium is fixed as the amide group of glutamine by the action of glutamine synthetase (GS). In many cyanobacteria, the nitrate assimilation-related genes encoding the nitrate transport system (nrtABCD or nrtP) and nitrate assimilation enzymes (narB and nirA) are usually cotranscribed as an operon (7, 23, 27). The nirA operon of cyanobacteria is subjected to an ammonium-promoted repression and to positive regulation by nitrate, nitrite (7, 37), or long-standing nitrogen starvation (23).
Assimilatory NR of cyanobacteria has been characterized as a single polypeptide with a molecular mass of 75 to 80 kDa that does not contain FAD or heme but contains an iron-sulfur cluster (17, 24). Recent evidence indicates that the NR of Synechococcus sp. strain PCC 7942 contains the bis-molybdopterin guanine dinucleotide (bis-MGD) cofactor and a [3Fe-4S] cluster (32, 33, 34). Ferredoxin is believed to be the physiological electron donor for the cyanobacterial NR (24, 31, 34). When dithionite-reduced methyl viologen (MV) or bromphenol blue is used as the electron donor, cyanobacterial NR exhibits a high pH optimum of 10.5 (11, 17, 22, 24, 31). A wide range of Km values (0.07 to 22 mM nitrate) has been observed for MV-NR in a variety of unicellular and heterocystous cyanobacteria (22). The NRs examined in a number of heterocystous, N2-fixing cyanobacteria showed biphasic kinetics for nitrate. In contrast, the NRs of studied unicellular cyanobacteria, except Synechocystis sp. strain PCC 6803, exhibited a hyperbolic kinetic behavior (22). Interestingly, Synechocystis sp. strain PCC 6803 contains only one copy of the NR gene, narB, in its genome (http://www.kazusa.or.jp/cyano/Synechocystis/index.html). In addition, the partially purified NR of ATCC 29413-P9 (a N2-fixing heterocystous cyanobacterium) appears as a monomeric enzyme exhibiting two independent kinetics (22). The results suggest that the biphasic kinetics in the cyanobacterial NRs is derived from a single narB gene. Although sequences of narB genes are available from some unicellular and heterocystous cyanobacteria (4, 26, 35, 39), little is known about the mechanism underlying the biphasic kinetics. In addition, the evolutionary relationship of NRs among heterocystous and unicellular cyanobacteria has not been determined.
Synechococcus sp. strain RF-1 is a unicellular freshwater cyanobacterium (13). It fixes N2 aerobically under continuous illumination at low light intensity (30 to 40 μmol of photosynthetically active radiation [PAR] m−2 s−1) and exhibits a circadian rhythm for nitrogenase activity under a light-dark regimen (15, 16). Synechococcus sp. strain RF-1 also utilizes nitrate, nitrite, or ammonium for growth. Little is known about nitrate assimilation in Synechococcus sp. strain RF-1.
In all cyanobacteria tested to date, the narB gene is located just downstream of nitrate transporter genes, and the encoded NR is subjected to repression by ammonium present in medium. In the present study, we show that the expression of NR is different from nitrate uptake at the activity and transcript levels in Synechococcus sp. strain RF-1. The narB gene of Synechococcus sp. strain RF-1 appears not to cluster with the nitrate transporter genes in an operon. Moreover, we provide evidence showing that NarB of Synechococcus sp. strain RF-1 exists as a single polypeptide and exhibits dual affinity for nitrate in in vitro or in situ MV-NR assay. The biphasic kinetics also associates with the recombinant NarB, but a shift toward a hyperbolic form of kinetics was observed in the modified recombinant NarB by adding a polyhistidine tag at the N terminus.
MATERIALS AND METHODS
Organisms and growth conditions.
Axenic culture of Synechococcus sp. strain RF-1 (PCC 8801) was maintained in BG-110 (nitrate free) medium under continuous light (30 to 35 μmol of PAR m−2 s−1) at 28°C as described previously (14). For batch cultures, cells were transferred and cultured in fresh BG-110 medium with aerating (70-ml airflow per min per 100 ml of culture) at 28°C under continuous illumination provided by cool-white fluorescent lamps (35 μmol of PAR m−2 s−1). Diazotrophically grown cells at the exponential growing phase (7 to 10 μg of chlorophyll a [Chl a]/ml) were used for experiments.
Nitrate uptake.
Batch-cultured Synechococcus sp. strain RF-1 cells were harvested by centrifugation and resuspended in fresh BG-110 medium at a cell concentration of 20 μg of Chl a per ml. The resuspended cells were incubated with aerating under continuous light (110 μmol of PAR m−2 s−1) at 30°C. Different nitrogen sources [4 mM NaNO3, 4 mM NaNO2, 2 mM (NH4)2SO4, or 1 mM NH4NO3] were supplemented at time zero. At various time intervals, aliquots of cell were withdrawn, centrifuged, washed with 10 mM tricine buffer (pH 8.0) containing 1 mM EDTA-Na2 and 100 μg of chloramphenicol (CA) per ml, and resuspended in fresh HEPES buffer (10 mM HEPES [pH 8.0], containing 1 mM NaHCO3, 0.3 mM MgSO4, 0.24 mM CaCl2). The resuspended cells were incubated under illumination (1,000 μmol of PAR m−2 s−1) at 30°C for 2 min to consume the residual internal nitrogen. For the maximum initial nitrate uptake assay, a saturating concentration of nitrate (250 μM) was added. Aliquots (0.5 ml) of cells were withdrawn at 5-min intervals and immediately passed through a syringe filter (0.45-μm pore size) by vacuum suction to stop the reaction. Nitrate depletion in the external medium was measured by the high-pressure liquid chromatography method as described previously (38). Uptake assay was carried out with two replicates, and each assay was performed for 15 min (three continuous 5-min intervals). The linear uptake phase was maintained for more than 30 min under the nitrate-saturated condition.
NR activity assay.
The NR activity was determined using dithionite-reduced MV as the electron donor as described previously (11, 22). The toluene-treated cells provided a measurement of in situ activity, and cell extracts were used for measurements of in vitro activity (22). The cell extract was obtained by disrupting the cells with a Mini-Beadbeater (5) in Tricine buffer (10 mM Tricine [pH 8.0], 2 mM MgSO4). After centrifugation, the supernatant was filtered through a 0.45-μm-pore-size membrane and diluted with the same tricine buffer to 1 mg of Chl a/ml. NR activity was assayed in triplicate at 30°C for 2 min in 1 ml of the reaction mixture containing 100 μmol of NaHCO3-Na2CO3 (pH 10.5), 40 μmol of KNO3, 6 μmol of methyl viologen, 15 μmol of Na2S2O4, and enzymes or cells. Aliquots of enzyme extract (1 to 1.5 μg of proteins) or toluene-treated cells (15 to 20 μg of Chl a) were preincubated in the reaction mixture without Na2S2O4 at 30°C for 5 min. The reaction was initiated by the addition of Na2S2O4 and stopped by vigorous agitation of the mixture. After the reaction was completed, cell debris was precipitated upon the addition of 0.2 ml of 0.5 M Zn(CH3COO)2, and the amount of nitrite produced was determined by using the diazo reaction (36).
Measurements of protein and chlorophyll.
Protein quantification was determined using Coomassie Plus reagent (Pierce, Rockford, Ill.) by photometric reading at 595 nm. Bovine serum albumin was used as the standard. Chl a contents were measured in ethanol extracts of toluene-treated cells or crude extracts as described previously (41).
Isolation of the narB gene.
Genomic DNA of Synechococcus sp. strain RF-1 was purified as previously described (5). The PCR methods, based on degenerate primers and chromosome walking (using the Genome Walker kit [Clontech, Palo Alto, Calif.]), were used to isolate the narB gene. The degenerate forward and reverse primers [5′-TGT CC(T/G) TA(T/C) TG(T/C) GG(T/A) GT(T/C/G) GG(T/C) TGT G-3′ and 5′-AC(A/T/G) GC(A/G) CA(A/G) GCT TTT A(A/G)T TC(T/C) GGT T-3′] were designed according to the conserved regions of narB genes from Synechococcus sp. strain PCC 7942 (26), Synechocystis sp. strain PCC 6803 (18), Anabaena sp. strain PCC 7120 (4), and Oscillatoria chalybea (39). The PCR was performed using a high-fidelity super-Taq polymerase (HT Biotechnology, Cambridge, United Kingdom) with an annealing temperature of 50°C. The amplified product, about 2 kb of partial narB gene, was cloned into pBluescript II KS+ (Stratagene). Two narB-specific primers (5′ TCC CAA CTG ACA CGA CGG AAA GAT TC-3′ and 5′ ATC GCG TCG AGG AGT AGC GCG GTT TC-3′), respectively, were then designed for amplification of 5′ and 3′ flanking sequences using the Genome Walker kit. A 3.2-kb DNA encompassing the entire narB ORF was thus assembled.
Isolation of NRT genes.
The nitrate transporter (NRT) gene cluster of Synechococcus sp. strain RF-1 was isolated using PCR approaches similar to that used for narB. The degenerate primers [5′-GGC AT(T/C) GGC T(A/T)(T/C) TTT AT(T/C) TGG GAT GCC TA-3′ and 5′-A(A/G)(A/G) GCA TC(T/C) A(A/G)(A/G/C) GC(A/G/C) CCA AA(G/C) GGT TCA TC-3′] were designed according to the conserved regions of nrtB identified in cyanobacteria (7, 18, 23, 26). Two clones were obtained, 26RFnrt and 30RFcmp, which have high peptide sequence similarity to the known gene clusters of nrtABCD and cmpABCD, respectively. The 26RFnrt is 2,064 bp in length and contains partial coding sequences for nrtB and nrtC; the 30RFcmp is 3,719 bp in length and contains the complete coding sequence for cmpB and partial coding sequences for cmpA and cmpC.
RT-PCR.
Culture of Synechococcus sp. strain RF-1 was transferred to fresh BG110 containing 4 mM of NaCl (nitrate free), NaNO3, or NH4Cl. The suspensions were maintained at 28°C for 4 h under continuous illumination (110 μmol of PAR m−2 s−1). Cells were harvested by centrifugation (10,000 × g for 5 min at 4°C) and washed once by vortexing with the buffer containing 10 mM of sodium acetate (pH 4.5) and EDTA-Na2. Total RNA was isolated from the washed cells (∼120 μg of Chl a), homogenized by a Mini-Beadbeater (5) using TRIzol reagent (Life Technologies, Invitrogen) and Absolutely RNA reverse transcriptase PCR (RT-PCR) Miniprep kit (Stratagene) according to the manufacturers' procedures. RNase-free DNase I was used to eliminate the genomic DNA.
First-strand cDNA synthesis was performed using SuperScript II RNase H− reverse transcriptase (Life Technologies, Invitrogen) according to the manufacturer's procedures. Five micrograms of total RNA, 50 pmol of the gene-specific oligonucleotide, and 10 nmol of dNTP mix were used in 20 μl of total reaction volume. The gene-specific oligonucleotides for narB, 26nrtC, and 30cmpC are 5′-TCC TCA ATC TTG AAT CTA GCT GCA TCG-3′ (nucleotides 2544 to 2570), 5′-CGA TCG GCT AAT AGA AGG GCT TCA TC-3′ (nucleotides 1062 to 1088), and 5′-TCG GAT CAG ACA GTA ATA CGG CTT CAT C-3′ (nucleotides 2822 to 2849), respectively. Two microliters of first-strand cDNAs were then used for PCR using nested primer pairs and Taq polymerase in 50 μl of total reaction volume. PCR was performed with narB-specific nested primers 5′-ATG ACT AAC TCT GCC AAA ACT CTC-3′ (forward, nucleotides 622 to 645) and 5′-AGT CAT CGA GAC GAA TTT CGC AGT TAC-3′ (reverse, nucleotides 1457 to 1483), 26nrtC-specific nested primers 5′-GAT CGA GTA TTT TCT CTT CCC GAT GGT-3′ (forward, nucleotides 495 to 521) and 5′-ATC CCT CCA GAA AGT TCG GCG GGA TAC-3′ (reverse, nucleotides 866 to 892), and 30cmpC-specific nested primers 5′-AAG TCT TTC CCC TAA GTG ATG GCG GTG-3′ (forward, nucleotides 2259 to 2285) and 5′-ACG AAT AGC GAG GGC ACG GGC AAT AGC-3′ (reverse, nucleotides 2666 to 2792). The RT-PCR products derived from the narB, 26nrtC, and 30cmpC genes were measured after 25, 30, 35, and 40 cycles of PCR. Negative controls were performed using total RNA as the template without RT. The plasmid containing gene and diluted genomic DNA (0.1 μg) amplified by PCR only were carried out as the controls for expected sizes of RT-PCR products and amplification efficiencies. A 20-μl portion of each PCR product was detected on a 1.2% agarose gel.
Expression of recombinant NarB in E. coli.
The entire narB gene (length, 2,309 bp) was amplified from genomic DNA of Synechococcus sp. strain RF-1 by PCR using a mix of VentR (New England Biolabs, Beverly, Mass.) and super-Taq DNA polymerase at a ratio of 1/10 with an annealing temperature of 65°C. The amplified fragments were cloned through pBluescript II KS+ vector into the expression vector pET28a+ (Novagen, Madison, Wis.). The clones containing the narB gene were verified by sequencing. The PCR primers used include the forward primers of p1 (5′-CCA TGG CTA ACT CTG CCA AAA CTC TCT-3′) and p2 (5′-ATG ACA TAT GAC TAA CTC TGC CAA AAC-3′) and the reverse primers of p3 (5′-GAA TTC CAT CCA ATG GCA ATG GTA CTC CTA-3′) and p4 (5′-CTC GAG TTT ACG GGT CAA TGC TTG ATT TCT CTT-3′). The final narB gene constructs in pET vector were designated pRF-NarB1, pRF-NarB2, and pRF-NarB3, which were derived from PCR products using the p1-p3, p2-p3, and p1-p4 primer pairs, respectively. The NarB was expressed in Escherichia coli BL21 cells as wild-type N- and C-terminal His-tagged proteins from pRF-NarB1, pRF-NarB2, and pRF-NarB3, respectively. For expression of various recombinant NarB proteins, transformed BL21 cells were aerobically cultured in 2× YT medium (16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl per liter [pH 7.0] [35a]) containing Na2MoO4 (1 mM) and kanamycin (50 μg/ml) at 37°C and induced for 5 h by 50 μM of isopropyl-β-d-thiogalactopyranoside (IPTG) at the late-log growth phase. The pET28a+-transformed BL21 was used as the negative control.
Preparation of recombinant NarB.
Crude extracts of recombinant NarB protein were prepared from BL21 by simple vortexing and centrifugation. A pellet from a 1.8-ml culture was mixed with 0.1 g of glass beads (0.1 mm in diameter), 0.1 ml of toluene, and 0.2 ml of extraction buffer in a microtube (polypropylene, 2 ml) and was then subjected to vigorous vortexing at high speed for 5 min. The extraction buffer (pH 10.3) consisted of 5 mM CAPS (3-[cyclohexylamino]-1-propane sulfonic acid; J. T. Baker) and 10 μM Na2MoO4. By this method, the membrane fraction of the broken cells was dissolved in toluene, and soluble proteins were released into the extraction buffer. After centrifugation (14,000 × g for 5 min) at room temperature, the mixture was separated into three fractions. The middle fraction of soluble extract was transferred to a new microtube, and the upper fraction (gel-like toluene) and cell debris were discarded. The soluble fraction was subjected to an additional centrifugation after incubation on ice for 15 min. The supernatant was used directly for in-gel NR assay or diluted with extraction buffer at a protein concentration of 100 μg of protein per ml for in vitro NR assay.
In-gel NR assay.
About 30 to 60 μg of proteins per lane was used in nondenaturing gel electrophoresis. The stacking and separating polyacrylamide gels were 4 and 8%, respectively, and they were all buffered at pH 9.2 with 0.2 M Tris. The running buffer contained 0.6 g of Tris base and 2.88 g of glycine per liter (pH 8.8). After electrophoresis, the separating gel was washed, equilibrated with extraction buffer, and transferred to a transparent polyethylene bag containing the reaction mixture, thereby creating a limited oxygen condition for the detection of MV-dependent NR activity. The components of the reaction mixture were the same as those for the MV-NR assay, except that 1 mM or 20 mM NaNO3 was supplied. The NR activity was indicated as an achromatic zone in the blue gel. The processes of gel electrophoresis, equilibration, and enzyme detection were carried out at room temperature.
Thermostability of the recombinant narB.
For determining thermostability of the recombinant NarB, crude extract was prepared in CAPS buffer at a protein concentration of 50 μg/ml, incubated in a water bath at various temperatures (ranging from ∼30 to 80°C) for 30 min, and then returned to room temperature. After centrifugation for 10 min at 14,000 × g, residual protein content and NR activity were then measured to determine the NR specific activity.
Nucleotide sequence accession numbers.
Nucleotide sequences for narB, nrtBC, and cmpABC genes from Synechococcus sp. strain RF-1 are in the EMBL, GenBank, and DDBJ databases under accession numbers AY162268, AY283673, and AY283674, respectively.
RESULTS
Regulation of nitrate uptake.
Whereas most studies of Synechococcus sp. strain RF-1 have focused on physiological regulation of the nitrogenase and the strategy to protect it from oxygen inactivation (16, 29), little is known about nitrate assimilation of this unicellular freshwater cyanobacterium. We therefore examined the regulation of its nitrate assimilation at steps of both nitrate uptake and nitrate reduction.
As shown in Fig. 1A, the cells of Synechococcus sp. strain RF-1 exhibited a low rate of nitrate uptake in nitrate-free medium (BG-110). Under continuous light (110 μmol of PAR m−2 s−1), the nitrogen fixation of cells was inhibited (16). Nitrate uptake in Synechococcus sp. strain RF-1 does not appear to be induced by long-standing (22 h) nitrogen starvation. However, when 4 mM nitrate was supplemented, the rate of net nitrate uptake was readily increased and peaked at 3 to 6 h. The decrease in nitrate uptake after 3 to 6 h of nitrate induction might be due to metabolic feedback inhibition since the substrate was not exhausted (nitrate concentration of more than 2 mM was still detected). Nitrite at a concentration of 4 mM is also capable of inducing nitrate uptake in Synechococcus sp. strain RF-1, although at a more modest level than that caused by nitrate induction. A feedback inhibition was observed for the nitrite induction at an earlier time point than was recorded for nitrate induction (Fig. 1A). Similar results have been demonstrated previously in cyanobacteria (7, 19). The induction of nitrate uptake by nitrate was eliminated by CA, a typical protein synthesis inhibitor found in prokaryotes. Only a constitutively low nitrate uptake rate was observed in CA-treated cells throughout a 22-h period (Fig. 1A), suggesting that newly synthesized protein(s) is involved in the nitrate-induced nitrate uptake. The constant low level of nitrate uptake activity in the presence of CA indicates a low turnover rate for the nitrate transporter activity in Synechococcus sp. strain RF-1. As in many other cyanobacteria, nitrate uptake of the Synechococcus sp. strain RF-1 was subjected to repression by ammonium, even when nitrate was present (Fig. 1A). In our experimental condition, 1 mM ammonium was usually exhausted within 22 h (data not shown); the slight increase of nitrate uptake at 22 h of ammonium repression was likely due to the consumption of ammonium.
FIG. 1.
Effects of nitrogen sources on nitrate uptake (A) and NR activity (B) in Synechococcus sp. strain RF-1 cells. As indicated for each nitrogen treatment, 4 mM NaNO3 or NaNO2, 2 mM (NH4)2SO4, and 1 mM NH4NO3 was added at time zero. NaCl at 4 mM was added in nitrate-free treatment (BG-110). The concentration of CA added was 100 μg/ml. Rates of nitrate uptake and in situ NR activity of cells were estimated at each time point of nitrogen treatments.
Regulation of NR activity.
In contrast to the regulation of nitrate uptake in Synechococcus sp. strain RF-1, a prominent, constitutive in situ NR activity was detected when cells were incubated in nitrate-free (BG-110) or ammonium-containing media (Fig. 1B). The in situ NR activity was increased about onefold after adding nitrate, and the induction was not repressed in the presence of ammonium (Fig. 1B). The modest induction of NR activity was not observed in the presence of CA, and de novo protein synthesis also appears to be required. Only about half of the initial NR activity remained at 22 h after adding CA, which indicated a slow turnover rate for the NR proteins (Fig. 1B).
Biphasic enzyme kinetics of NR in Synechococcus sp. strain RF-1.
In the nitrate-induced and uninduced cells of Synechococcus sp. strain RF-1, the in situ NR activity showed biphasic kinetics for nitrate (Fig. 2). The apparent kinetic components calculated from the Hofstee plots exhibited two independent kinetic systems. Under an uninduced condition (BG-110), Km (I) and Km (II) were calculated to be 0.12 and 4.17 mM for nitrate, respectively. The ratio of Vmax (I)/Vmax (t) was 0.269, where Vmax (t) = Vmax (I) + Vmax (II). Very similar kinetic components were detected when the in situ NR activity was measured under a nitrate-induced condition. The Km (I) and Km (II) were 0.13 and 4.26 mM for nitrate, respectively, and the ratio of Vmax (I)/Vmax (t) was 0.268. It appears that under both conditions the high-affinity component had lower catalytic activity than the low-affinity component. The similarity of kinetic components suggests that the same enzyme, and not the induction of an additional enzyme, is responsible for the detected NR activities under induced and uninduced conditions.
FIG. 2.
Hofstee plots of the effect of nitrate concentration on in situ NR activity of Synechococcus sp. strain RF-1. The NR assays were conducted in cells induced by 4 mM NaNO3 for 5 h (▪) and in cells without induction (□). The concentrations of NaNO3 used in NR assays are in the range of 50 μM to 32 mM.
Synechococcus sp. strain RF-1 contains a single nitrate reductase gene, narB.
To examine whether the dual-affinity kinetics of NR in Synechococcus sp. strain RF-1 is derived from a single gene, the NR gene narB was isolated, and the encoded protein was then expressed and characterized in E. coli. A 3,175-bp genomic fragment containing the narB gene was isolated from Synechococcus sp. strain RF-1 by using PCR approaches. Sequence analyses revealed a 2,241-bp open reading frame encoding the putative NarB with 746 amino acids and a calculated molecular mass of 83.4 kDa and pI of 7.3. Unlike known nrtABCD-narB gene clusters, where the narB is immediately clustered downstream of nrtABCD, the 5′ and 3′ flanking sequences of narB from Synechococcus sp. strain RF-1 are 621 and 313 bp in length, respectively, and they contain no sequences that are homologous to genes encoding nitrate transporters or assimilation enzymes from cyanobacteria. The deduced NarB protein showed high sequence identities (3) compared to NarB from various cyanobacteria, ranging from 57 to 77% (data not shown). It has higher sequence identities and similarities to the NarB from nitrogen-fixing cyanobacteria Oscillatoria chalybea (76%) and Anabaena sp. strain PCC 7120 (74%) than to the NarB from non-nitrogen-fixing cyanobacteria Synechocystis sp. strain PCC 6803 (66%) and Synechococcus sp. strain PCC 7942 (65%). Similar to known NarB sequences, conserved iron-sulfur (2 to 68 amino acids [aa]) and molybdopterin guanine dinucleotide binding domains (604 to 715 aa) were found at the N and C terminus, respectively, of Synechococcus sp. strain RF-1 NarB. DNA blot analyses indicated that Synechococcus sp. strain RF-1 genome contains a single copy of the narB gene (data not shown).
Isolation of partial nrtABCD and cmpABCD gene clusters from Synechococcus sp. strain RF-1.
To examine RNA expression as a parallel study to nitrate uptake and NR activities, we attempted to isolate the nrtABCD gene cluster from Synechococcus sp. strain RF-1. Two genomic fragments, 26RFnrt and 30RFcmp, were obtained by using PCR approaches. These fragments have high similarities of deduced peptide sequences compared to the known gene clusters of cyanobacterial nrtABCD and cmpABCD (which encode the bicarbonate transporter; for a review, see reference 28), respectively. The 26RFnrt is 2,064 bp in length and contains partial coding sequences for NrtB (108 aa of the C-terminal portion) and NrtC (533 aa of the N-terminal portion); the 30RFcmp is 3,719 bp in length and contains the complete coding sequence for CmpB (with a deduced peptide sequence of 280 aa in length) and partial coding sequences for CmpA (387 aa of the C-terminal portion) and CmpC (498 aa of the N-terminal portion). Synechococcus sp. strain RF-1 NrtB and NrtC have higher sequence identities to corresponding NrtB and -C (54 to 77%) from various cyanobacteria compared to CmpB and C (58 to 64%). Similarly, Synechococcus sp. strain RF-1 CmpA, -B, and -C have higher sequence identities and similarities to corresponding CmpA, -B, and -C (62 to 83%) from various cyanobacteria compared to NrtA, -B, and -C (50 to 64%). In agreement with these findings, as examined by RT-PCR, Synechococcus sp. strain RF-1 cmpC transcripts were expressed similarly to the cmpABCD in Synechococcus sp. strain PCC 7942 (see below) (28).
Expression of narB and nrtC transcripts.
Whether regulation of nitrate uptake and NR activities was reflected at the steady-state transcript level in response to availability of nitrogen source was examined by RT-PCR. As shown in Fig. 3, similar to NR activity examined, narB transcripts were detected in all treatments of nitrogen sources, and an increment of narB transcripts was observed in the nitrate-treated cells. Whereas nrtC transcripts were not detected in NH4+-treated cells, the transcripts were detected with cells of nitrate-free and nitrate treatments. In contrast, cmpC transcripts were detected at similar and constantly high levels in cells incubated in nitrate-free (BG-110 and NaCl) medium or in NaNO3- or NH4Cl-containing medium, which is very similar to the expression of cmpABCD in Synechococcus sp. strain PCC 7942 (28).
FIG. 3.
Expressions of Synechococcus sp. strain RF-1 narB, nrtC, and cmpC transcripts. The steady-state level of transcripts of narB, nrtC, and cmpC were examined by RT-PCR. After 40 cycles of PCR amplification, 20 μl of the reaction mixture was resolved on 1.2% agarose gel containing ethidium bromide. The treatments of Synechococcus sp. strain RF-1 cells by NaNO3 (NO3−), NH4Cl (NH4+), or NaCl (−N) were described in Materials and Methods. No products were detected in the negative controls (without RT).
Characterization of recombinant NarB of Synechococcus sp. strain RF-1.
That the narB gene encodes a genuine nitrate reductase was verified by determination of NR activity with E. coli expressing NarB (Fig. 4). Only at pH 7 was a very low endogenous NR activity detected in nontransformed E. coli (data not shown), which did not interfere the measurement of heterologous NR activity assayed at pH 10.5. The NarB of Synechococcus sp. strain RF-1 was successfully expressed in E. coli (Fig. 4A). Compared to the nontransformed, the E. coli containing the wild-type NarB construct (pRF-NarB1) expressed an extra protein with a molecular mass of 82.5 kDa. The recombinant NarB was mostly in insoluble forms and partly in soluble forms (Fig. 4A). High NR activity was only associated with the recombinant and not with empty vector-transformed E. coli (Fig. 4B). The recombinant NarB has enzymatic properties very similar to the native NR in Synechococcus sp. strain RF-1 (Fig. 5). The in situ NR and the in vitro recombinant NR in E. coli gave very similar reaction profiles for different pH values (Fig. 5A) and temperatures (Fig. 5B). The optimum pH and temperature were 10 and 30°C, respectively.
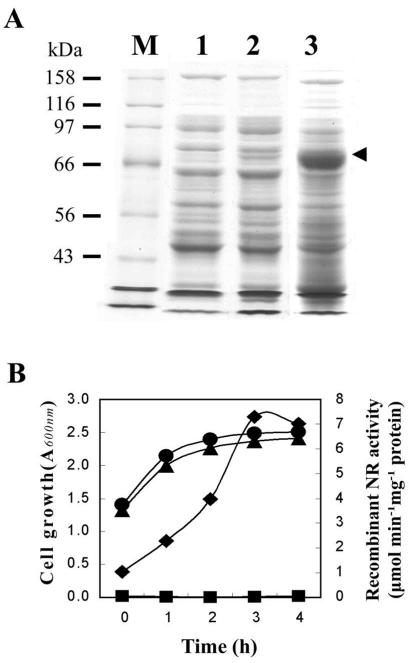
FIG. 4.
Expression of the cyanobacterial NarB in E. coli BL21 cells. The expression of NarB was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (A) and in vitro NR assay (B). Four hours IPTG-induced extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (10% polyacrylamide running gel). Lane M, protein size markers; lane 1, soluble extract of BL21 carrying pET28a+; lanes 2 and 3, soluble and insoluble fractions, respectively, for IPTG-induced BL21 extracts carrying the pRF-narB1. The arrowhead indicates the overexpressed recombinant NarB proteins (molecular mass, 83 kDa). IPTG at 50 μM was added at the late-log growth phase and designated as time zero. Growth curves of BL21 carrying pET28a+ (•) and pRFnarB1 (▴) were determined after IPTG induction. NR activity was determined at indicated time intervals after IPTG induction for BL21 extracts carrying pET28a+ (▪) and pRFnarB1 (⧫).
FIG. 5.
Effects of pH (A) and temperature (B) on the NR activities of Synechococcus sp. strain RF-1 cells (○) and recombinant NarB expressed in E. coli (•). NR activity of Synechococcus sp. strain RF-1 was determined with in vitro and in situ assay in panels A and B, respectively. NR activity of recombinant NarB was determined with in vitro assay.
The monomeric recombinant NarB exhibited dual affinity for nitrate.
The cyanobacterial NR was purified and characterized as a monomer with a molecular mass of 75 to 85 kDa in Synechococcus sp. strain PCC 7942 and Plectonema boryanum (17, 24, 31). In agreement with this finding, the recombinant NarB of Synechococcus sp. strain RF-1, preincubated with 0, 1, or 20 mM nitrate, mobilized as a single form on native gel electrophoresis as examined by in-gel NR activity assay (Fig. 6). The possible involvement of multimer formation in the biphasic kinetics of the NR in Synechococcus sp. strain RF-1 thus seems remote.
FIG. 6.

In-gel NR activity of recombinant NarB. Crude extracts of recombinant NarB were incubated with the extracting buffer containing 0, 1, and 20 mM nitrate at room temperature for 15 min before electrophoresis. Gel staining was performed as described in Materials and Methods. Albumin of bovine serum dimer (132 kDa), monomer (66 kDa), and chicken egg (45 kDa) were used as the protein markers (A 8654 and A 8529; Sigma).
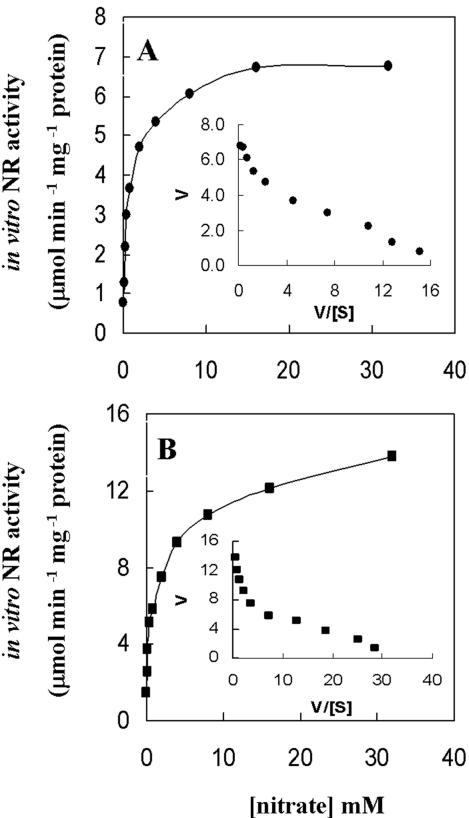
The enzyme kinetics of the wild-type recombinant NarB was determined using the same MV-assay as that used for measuring in situ NR activity in Synechococcus sp. strain RF-1. The recombinant NarB also exhibited two very similar kinetic components compared to the in situ NR activity (Fig. 2 and data not shown). Apparent Km (I) and Km (II) values of recombinant NarB for nitrate were 0.11 and 4.52 mM, respectively, and the ratio of Vmax (I)/Vmax (t) was 0.248.
The two kinetic components of the wild-type recombinant NarB exhibited similar thermostability.
The possibility of differentiation of the thermostability for the two kinetic components of the recombinant NarB was examined. When measured with low and high substrate concentrations, the thermostabilities of the two kinetic components were found to be very similar (Table 1). Both high- and low-affinity NR exhibited similar thermostabilities at temperatures below 70°C, where greater than 90% of NR specific activities were retained. Only 3 to 4% specific activity was retained for both high- and low-affinity of NR after treatment at 80°C. Whereas a sharp drop in NR activity above 30°C was found (Fig. 5B), the retention of high NR specific activity at temperature treatments below 70°C indicates that the denatured NR can be readily renatured when the optimum temperature is restored.
TABLE 1.
Recovery of NR activity of the heat-treated recombinant NarB
| Temp (°C) | NR sp acta
|
|||
|---|---|---|---|---|
| High-affinity NR
|
Low-affinity NR
|
|||
| μmol min−1 mg−1 of proteinb | % | μmol min−1 mg−1 of proteinb | % | |
| 30 | 0.64 ± 0.01 | 100 | 2.16 ± 0.08 | 100 |
| 40 | 0.57 ± 0.01 | 88 | 1.99 ± 0.02 | 92 |
| 50 | 0.56 ± 0.03 | 88 | 2.15 ± 0.05 | 99 |
| 60 | 0.58 ± 0.03 | 90 | 2.03 ± 0.09 | 94 |
| 70 | 0.59 ± 0.04 | 92 | 1.93 ± 0.05 | 89 |
| 80 | 0.02 ± 0.01 | 4 | 0.06 ± 0.003 | 3 |
Extracts of recombinant NarB were incubated in a water bath at specified temperatures for 30 min and then returned to room temperature. Activities of in vitro MV-NR were then measured at pH 10.25, 30°C. Substrate concentrations were 0.2 and 20 mM nitrate for the high- and low-affinity NR, respectively.
Values are means ± standard deviations of three replicates of preparations.
The biphasic kinetics of wild-type recombinant NarB was altered when polyhistidine was fused to the terminus of enzyme.
The NarB of Synechococcus sp. strain RF-1 was also expressed in E. coli as N- and C-terminal polyhistidine-tagged versions, designated RF-NarB2 and RF-NarB3, respectively. The polyhistidine-tagged proteins were also tested for MV-NR activity, and kinetics was tested for nitrate. The kinetics of NarB was altered when the polyhistidine was fused to either the N- or C-terminal end of NarB. The N-terminal-tagged NarB (RF-NarB2) gave a hyperbolic kinetics with a single apparent Km of 0.38 mM for nitrate (Fig. 7A). In contrast, the C-terminal-tagged NarB (RF-NarB3) still exhibited biphasic kinetics but with an altered apparent Km for high- and low-affinity components (Fig. 7B): 0.20 and 1.78 mM, respectively. Considering the near-end positions of NarB for iron-sulfur cluster and bis-MGD binding domain (30, 34), the alteration of enzymatic kinetics is likely due to structural interference posed by the polyhistidine.
FIG. 7.
Effect of nitrate concentration on the NR activity of His-tagged NarB variants. NR activity was determined in the extracts of recombinant NarB variants either His-tagged at the N terminus (A) or C terminus (B). The Eadie-Hofstee plots are shown as insets.
DISCUSSION
The related nitrate assimilatory genes, for nitrate reduction and nitrate uptake, in cyanobacteria are in general clustered as a nirA operon (nirA-nrtABCD-narB), which is transcriptionally activated by withdrawing the combined nitrogen source and by inhibiting ammonium assimilation. In addition, expression of the nitrate assimilatory operon is repressed by the presence of ammonium (4, 7, 12, 27). In general, nitrate or nitrite is not required for transcriptional activation. Interestingly, induction of nitrate uptake activity in Synechococcus sp. strain RF-1 strictly requires nitrate or nitrite, which in turn could be repressed by ammonium (Fig. 1A). The regulation of nitrate uptake in Synechococcus sp. strain RF-1 appears to be different from that of the Synechococcus sp. strain PCC 7942, Anabaena sp. strain PCC 7120, and Phormidium laminosum strains (4, 7, 23, 27). Regulation of Synechococcus sp. strain RF-1 nitrate uptake, in general, is reflected on expression of the nrtC transcripts. First, the nrtC transcripts were not detected in the presence of ammonium as examined by the steady-state level of nrtC transcripts using RT-PCR (Fig. 3). In addition, the expression of nrtC in nitrate treatment is higher than in nitrate-free treatment. However, the relatively more modest level of nitrate induction observed with nrtC transcripts compared to the high level of nitrate-induced nitrate uptake suggests a role of posttranscriptional regulation. In contrast, the in situ NR activity of Synechococcus sp. strain RF-1 was only modestly induced by nitrate and was not repressed by ammonium (Fig. 1B). Similarly, the narB transcripts were detected in all the nitrogen treatments, including the ammonium treatment. The nitrate-enhanced expression of narB transcripts was also observed. From the activity assay, it appears that nitrate uptake is more tightly regulated by the available nitrogen sources than is NR. This indicates that the nitrate assimilation is primarily regulated at the step of nitrate uptake in Synechococcus sp. strain RF-1.
The different regulation associated with nitrate uptake and nitrate reduction in Synechococcus sp. strain RF-1 can partly be explained by the dissociation of narB from nrtABCD gene cluster in the genome. For all cyanobacteria examined, nirA and narB are generally located upstream and downstream of the genes encoding nitrate transporter (nrtABCD or nrtP), respectively, within the nirA operon (8, 19, 24, 31, 33, 36, 40). Synechocystis sp. strain PCC 6803 is an exception, the nirA being found ∼400 kb apart from the nrtABCD-narB operon (18). No sequences homologous to any of nitrate transporter genes were found in the examined flanking sequences of narB in Synechococcus sp. strain RF-1. In addition, attempts to amplify the sequence between narB and nrtABCD in two possible orientations (nrtABCD-narB or narB-nrtABCD) by PCR also failed.
Expression of the ammonium-repressible genes commonly requires the NtcA protein, a Crp-type transcriptional regulator, which is a critical component of a global nitrogen control in cyanobacteria (see reference 12 for a review). The strict requirement of nitrate or nitrite for induction of nitrate uptake in Synechococcus sp. strain RF-1 (Fig. 1A) indicates a possible difference in NtcA-mediated gene regulation. In Synechococcus sp. strain PCC 7942, nitrate and nitrite are also not essential for the expression of nirA-operon. However, NtcB (LysR family transcription factors), in cooperation with NtcA, is involved in nitrite-promoted regulation (1, 19, 21). In contrast, in Anabaena sp. strain PCC 7120 and Synechocystis sp. strain PCC 6803, NtcB acts as a nitrite-independent enhancer and is required for high-level expression of the nitrate assimilatory genes (2, 8). These indicate strain-specific diversities in the NtcB-mediated regulation in cyanobacteria. Whether NtcB is involved in the nitrite-induced nitrate uptake of Synechococcus sp. strain RF-1 is not known. However, the efficiency of the nitrite-induced nitrate uptake of Synechococcus sp. strain RF-1 was lower than that of nitrate (Fig. 1A). This suggests that nitrate per se or nitrate reduction has additional positive effects on the promotion of nitrate uptake in Synechococcus sp. strain RF-1.
Several lines of evidence indicate that the dual-affinity kinetics for nitrate is intrinsic to the NR of Synechococcus sp. strain RF-1. First, cyanobacterial NarB protein has been characterized as a single polypeptide (17, 24, 31) which contains a single molybdenum per enzyme (24, 34) and one bis-MGD binding domain, as predicted previously (30). One conserved bis-MGD binding domain at the C-terminal end of NarB protein in Synechococcus sp. strain RF-1 is also found by protein sequence analysis, indicating the presence of a single catalytic site in monomeric NarB. Second, in situ NR activity assays under either nitrate-induced or uninduced conditions both gave very similar biphasic kinetics for nitrate. The Km values for both high (0.13 mM) and low affinity (4.2 mM) and the ratio of Vmax (I)/Vmax (t) (0.27) are quite similar. Third, the wild-type recombinant NarB has enzymatic properties that are very similar to the native NarB as assessed by optimum pH and temperature profiles, which also produced biphasic kinetics with similar Km values for high (0.11 mM) and low affinity (4.52 mM) and a similar ratio of Vmax (I)/Vmax (t) (0.25) compared to the in situ enzymatic activity (Fig. 2). Fourth, the two kinetic components of NarB were found to have very similar thermostability in the MV-NR assay (Table 1), and monomeric NarB was found by in-gel NR assay (Fig. 6). Fifth, even if the enzyme was not pretreated with nitrate before native electrophoresis, monomeric-type NarB was still detected by in-gel NR assay by using 1 or 20 mM nitrate as substrate (data not shown). These indicate that the monomeric enzyme catalyzed nitrate reduction both at low and high concentrations of substrate. Sixth, the biphasic kinetics of the recombinant NarB was drastically altered by structural modification by terminal fusion of polyhistidine. Finally, Synechococcus sp. strain RF-1 likely contains only a single copy of the narB gene in the genome.
The molecular mechanism for the biphasic kinetics of NarB is presently unknown. From the above-mentioned evidence, it does not appear to be due to the presence of two different enzymes as described previously (22). It is likely not caused by multimer formation either, since only a single form of recombinant NarB was detected under low and high nitrate concentrations by native electrophoresis and in-gel NR assay. We propose that a novel regulatory nitrate-binding site may exist (distinct from the catalytic site), which is responsible for the change of NR affinity to nitrate due to a slight alternation of NR conformation. Alternatively, the dual affinity of NarB can be explained by multimodal kinetics described previously (25).
As reported by Martin-Nieto et al. (22), the nitrate reductases of examined multicellular nitrogen-fixing cyanobacteria exhibit the biphasic kinetics. In contrast, except for Synechocytis sp. strain PCC 6803, unicellular, non-nitrogen-fixing cyanobacteria exhibit single-component hyperbolic kinetics, such as the NarB of Synechococcus sp. strain PCC 7942 (23, 30). Only 65% identity of NarB protein sequences is found between Synechococcus sp. strain PCC 7942 and Synechococcus sp. strain RF-1. What differentiates the kinetic behavior for evolutionarily related NarB-type NR from various cyanobacteria is presently unknown. It appears that biphasic kinetics is not unique to nitrate reductase in cyanobacteria. Arabidopsis nitrate transporter CHL1 and K+ transporter AtKUP1 has been found to exhibit biphasic kinetics as well (9, 20).
Acknowledgments
We are grateful to Tan-Chi Huang for supplying Synechococcus sp. strain RF-1 (PCC 8801) and Rong-Fong Lin and Hsien-Jung Chen for technical assistance.
This work was supported by research grants from the National Science Council and Academia Sinica, Taipei, Taiwan to Y.J.S. and a graduate fellowship to T.H.W. from the National Science Council (1995 to 2002).
REFERENCES
- 1.Aichi, M., and T. Omata. 1997. Involvement of NtcB, a LysR family transcription factor, in nitrite activation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 179:4671-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aichi, M., N. Takatani, and T. Omata. 2001. Role of NtcB in activation of nitrate assimilation genes in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 183:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search program. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, Y., and C. P. Wolk. 1997. Nitrogen deprivation of Anabaena sp. strain PCC 7120 elicits rapid activation of a gene cluster that is essential for uptake and utilization of nitrate. J. Bacteriol. 179:258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, H. M., C. Y. Chien, and T. C. Huang. 1996. Regulation and molecular structure of a circadian oscillating protein located in the cell membrane of the prokaryote Synechococcus RF-1. Planta 199:520-527. [DOI] [PubMed] [Google Scholar]
- 6.Flores, E., and A. Herrero. 1994. Assimilatory nitrogen metabolism and regulation, p. 487-517. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 7.Frías, J. E., E. Flores, and A. Herrero. 1997. Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 179:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frías, J. E., E. Flores, and A. Herrero. 2000. Activation of the Anabaena nir operon promoter requires both NtcA (CAP family) and NtcB (LysR family) transcription factors. Mol. Microbiol. 38:613-625. [DOI] [PubMed] [Google Scholar]
- 9.Fu, H. H., and S. Luan. 1998. AtKUP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell 10:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero, M. G., J. M. Vega, and M. Losada. 1981. The assimilatory nitrate-reducing system and its regulation. Annu. Rev. Plant Physiol. 32:169-204. [Google Scholar]
- 11.Herrero, A., E. Flores, and M. G. Guerrero. 1981. Regulation of the nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119, and Nostoc sp. strain 6719. J. Bacteriol. 145:175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrero, A., A. M. Muro-Paster, and E. Flores. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, T. C., and T. J. Chow. 1986. New type of N2-fixing unicellular cyanobacterium (blue-green alga). FEMS Microbiol. Lett. 36:109-110. [Google Scholar]
- 14.Huang, T. C., and T. J. Chow. 1988. Comparative studies from rice field. J. Gen. Microbiol. 20:23-26. [Google Scholar]
- 15.Huang, T. C., J. Tu, T. J. Chow, and T. H. Chen. 1990. Circadian rhythm of the prokaryote Synechococcus sp. RF-1. Plant Physiol. 92:531-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, T. C., and N. Grobbelaar. 1995. The circadian clock in the prokaryote Synechococcus sp. RF-1. Microbiology 141:535-540. [Google Scholar]
- 17.Ida, S., and B. Mikami. 1983. Purification and characterization of assimilatory nitrate reductase from the cyanobacterium Plectonema boryanum. Plant Cell Physiol. 24:649-658. [Google Scholar]
- 18.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi, H., M. Aichi, I. Suzuki, and T. Omata. 1996. Positive regulation by nitrite of the nitrate assimilation operon in the cyanobacteria Synechococcus sp. strain PCC 7942 and Plectonema boryanum. J. Bacteriol. 178:5822-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, K. H., C. Y. Huang, and Y. F. Tsay. 1999. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, S., Y. Kawaguchi, T. Ohe, and T. Omata. 1998. cis-acting sequences required for NtcB-dependent, nitrite-responsive positive regulation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 180:4080-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martín-Nieto, J., E. Flores, and A. Herrero. 1992. Biphasic kinetic behavior of nitrate reductase from heterocystous, nitrogen-fixing cyanobacteria. Plant Physiol. 100:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merchán, F., K. L. Kindle, M. J. Llama, J. L. Serra, and E. Fernández. 1995. Cloning and sequencing of the nitrate transport system from the thermophilic filamentous cyanobacterium Phormidium laminosum: comparative analysis with the homologous system from Synechococcus sp. PCC 7942. Plant Mol. Biol. 28:759-766. [DOI] [PubMed] [Google Scholar]
- 24.Mikami, B., and S. Ida. 1984. Purification and properties of ferredoxin-nitrate reductase from the cyanobacterium Plectonema boryanum. Biochim. Biophys. Acta. 791:294-304. [Google Scholar]
- 25.Nissen, P., and J. Martín-Nieto. 1998. “Multimodal” kinetics: cyanobacterial nitrate reductase and other enzyme, transport and binding systems. Physiol. Plant. 104:503-511. [Google Scholar]
- 26.Omata, T., X. Andriesse, and A. Hirano. 1993. Identification and characterization of a gene cluster involved in nitrate transport in the cyanobacterium Synechococcus sp. PCC 7942. Mol. Gen. Genet. 236:193-202. [DOI] [PubMed] [Google Scholar]
- 27.Omata, T. 1995. Structure, function and regulation of the nitrate transport system of the cyanobacterium Synechococcus sp. PCC 7942. Plant Cell Physiol. 36:207-213. [DOI] [PubMed] [Google Scholar]
- 28.Omata, T., G. D. Price, M. R. Badger, M. Okamura, S. Gohta, and T. Ogawa. 1999. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc. Natl. Acad. Sci. USA 96:13571-13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy, K. J., B. Haskell, D. M. Sherman, and L. A. Sherman. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 175:1284-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson, D. J., B. C. Berks, D. A. Russell, S. Spiro, and C. J. Taylor. 2001. Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell. Mol. Life Sci. 58:165-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubio, L. M., A. Herrero, and E. Flores. 1996. A cyanobacteria narB gene encodes a ferredoxin-dependent nitrate reductase. Plant Mol. Biol. 30:845-850. [DOI] [PubMed] [Google Scholar]
- 32.Rubio, L. M., E. Flores, and A. Herrero. 1998. The narA locus of Synechococcus sp. strain PCC 7942 consists of a cluster of molybdopterin biosynthesis genes. J. Bacteriol. 180:1200-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubio, L. M., E. Flores, and A. Herrero. 1999. Molybdopterin quanine dinucleotide cofactor in Synechococcus sp. nitrate reductase: identification of mobA and isolation of a putative moeB gene. FEBS Lett. 462:358-362. [DOI] [PubMed] [Google Scholar]
- 34.Rubio, L. M., E. Flores, and A. Herrero. 2002. Purification, cofactor analysis, and site-directed mutagenesis of Synechococcus ferredoxin-nitrate reductase. Photosynth. Res. 72:13-26. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto, T., K. Inoue-Sakamoto, and D. A. Bryant. 1999. A novel nitrate/nitrite permease in the marine cyanobacterium Synechococcus sp. strain PCC 7002. J. Bacteriol. 181:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Scholl, R. L., J. E. Harper, and R. H. Hageman. 1974. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 53:825-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki, I., T. Sugiyama, and T. Omata. 1993. Primary structure and transcriptional regulation of the gene for nitrite reductase from the cyanobacterium Synechococcus PCC 7942. Plant Cell Physiol. 34:1311-1320. [Google Scholar]
- 38.Thayer, J. R., and R. C. Huffaker. 1980. Determination of nitrate and nitrite by high-pressure liquid chromatography: comparison with other methods for nitrate determination. Anal. Biochem. 102:110-119. [DOI] [PubMed] [Google Scholar]
- 39.Unthan, M., W. Klipp, and G. H. Schmid. 1996. Nucleotide sequence of the narB gene encoding assimilatory nitrate reductase from the cyanobacterium Oscillatoria chalybea. Biochim. Biophys. Acta 1305:19-24. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Q., H. Li, and A. Post. 2000. Nitrate assimilation genes of the marine diazotrophic, filamentous cyanobacterium Trichodesmium sp. strain WH9601.J. Bacteriol. 182:1764-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wintermans, L. F. G. M., and A. De Mots. 1965. Spectrophotometric characteristics of chlorophyll and their pheophytins in ethanol. Biochim. Biophys. Acta 109:448-453. [DOI] [PubMed] [Google Scholar]