Abstract
Although slow and persistent viruses often escape host defenses infection may be prevented by live vaccines. To determine whether an attenuated “slow” strain of the Creutzfeldt–Jakob disease agent (SY) could block expression of a virulent “fast” strain (FU), outbred CD-1 mice were inoculated intracerebrally with low infectious doses of SY and challenged 80 days later with higher doses of FU. For comparison, the same SY and FU samples were inoculated in two parallel control groups. All 18 superinfected mice showed incubation times identical to those inoculated with only the SY strain, yielding clinical disease >110 days later than predicted for the FU strain. Neurological signs, such as scratching and an extended clinical phase, were also characteristic for SY but not FU infection. Moreover, the widespread cortical pathology of FU was not detectable in superinfected mice. Western blot analyses further showed no strain-specific differences in prion protein (PrP) band profiles for all experimental groups, although there was ∼10-fold more protease-resistant PrP (PrP-res) in FU brains during terminal disease. In contrast, infectivity assays revealed an ∼10,000-fold difference between SY and FU at terminal stages, indicating that PrP-res content does not correlate with infectivity. In summary, an attenuated strain of the Creutzfeldt–Jakob disease agent evokes substantial interference against a virulent agent. Because superinfected mice had little PrP-res just before the onset of clinical disease and retained abundant cellular PrP, cellular PrP was not the factor limiting FU replication. The mechanisms underlying SY interference are not understood but could be based on host recognition of foreign molecular features shared by this class of invasive agents involving antibody production, and possibly involve defective viral particles produced by attenuated variants.
Keywords: transmissible encephalopathies, prevention, agent strains, virus, prion
The introduction of live viruses to control infection was practiced by the Chinese for centuries and was brought from Constantinople by Lady Montague before Jenner’s work was published in 1798 (1). The vaccination strategy derived from the fundamental observation that less-fulminant infectious lesions contained attenuated agents able to prevent superinfection by more virulent ones. By the 1960s, attenuated mutant strains were selected by sequential passage in animals and cell cultures, leading to the live poliovirus vaccine (2). Today, genetic engineering provides the potential to design and manufacture more attenuated viral vaccines that lack pathogenicity. The effectiveness of many vaccines is based on their ability to provoke specific antiviral antibodies in the host. However, some slow and persistent viral infections such as HIV are not arrested by these host responses, and persistent and latent viruses exploit a variety of mechanisms to evade immune surveillance (3).
The persistent infectious agents causing the neurologically devastating and incurable Creutzfeldt–Jakob disease (CJD) of humans, as well as sheep scrapie and bovine encephalopathy (BSE), have an intracellular residence and escape typical immunological recognition. Hence, an invasive form of these agents may be required to achieve effective vaccination. Presumably mechanisms for strain interference, if observed, would depend on host recognition of the foreign agent or on the positioning of the attenuated agent on sites required for the life cycle of the virulent challenge agent. The latter concept was suggested in an original scrapie study 25 years ago (4). Since that time, interference effects have not been reported with any combination of strains other than scrapie 22A and 22C. The two other previous experiments were also complicated by host genetic factors because there was a reversal in the virulence of both agent strains in congenic VM mice as compared with other inbred mice. Moreover, incubation time was also the only data shown to support the inteference effect (5, 6), and host prion protein (PrP) was not analyzed. Host PrP has been postulated to convert into an infectious or “prion” form (7), although this idea remains controversial.
It was therefore important to find whether interference could be generalized to other distinct agent strains, whether it was independent of inbred mouse genetic characteristics, and whether the effect could be linked to specific changes in PrP. Recently, small differences in PrP gel band profiles have been used to classify different agent strains, such as those caused by cow-derived agents (8). However, these infectious agents may educe distinct pathological forms of PrP but be different from the protein. Notably, these infectious agents separate from PrP (9–11), and infectivity is associated with intact virus-like particles and molecules (12–14). Even prion proponents now acknowledge there is no detectable form of PrP that corresponds to infectivity (15). Moreover, all attempts to show that purified, recombinant, or transgenic PrP produce significant infectivity have failed (16–19).
Although PrP is an important pathological marker, we also considered this protein might be instead a necessary host “receptor” or intracellular docking site for these agents (20, 21), a concept consistent with the resistance of PrP knockout mice to infection (22, 23). Many other viruses cannot invade or multiply without a particular host protein (21). On this fundamental level, it was necessary to determine whether limiting amounts of PrP might be responsible for preventing a challenge infection. We were also particularly concerned with the growing epidemic of BSE and the possibility that a new virulent agent strain could infect humans (24). Indeed, the newly evolved BSE agent has infected a variety of species and has been linked most recently to an atypical form of CJD in younger people (25). Useful strategies to subvert these infections are needed. To explore the potential of preventive vaccines in these infections a classic viral interference strategy was used to show that an attenuated strain of CJD can prevent superinfection by a more virulent CJD agent passaged in the same species. Moreover, the “vaccination” CJD agent derived from a sporadic human case (26) whereas the challenge agent derived from a patient with a Leu-102 substitution in host PrP and a plaque-rich phenotype (27). This mutation is not present in the CD-1 mice used herein, yet both agent strains were clearly discriminated because only the challenge agent evoked histological deposits of PrP. Finally, direct brain inoculation was chosen as the most rigorous test of interference. The normal brain is immunologically isolated and is also the place where these agents are most concentrated and destructive.
RESULTS AND DISCUSSION
Because these infectious agents are still molecular enigmas, strains were selected by biological methods, i.e., passage in animals. Notably, all abnormal PrP conformations that can be assayed, including more protease-resistant PrP (PrP-res) and β-pleated amyloid forms, fail to predict or correlate with infectious titers (9, 15, 28–30). In this context two agents with the same PrP-res band profiles on gels but with large differences in virulence, as judged by incubation time and titer, were selected. Along with incubation time and titer differences these two strains could be clearly discriminated by their neurological effects. The slow and relatively attenuated strain (designated SY) that elicited unique clinical symptoms and sparse vacuoles in the cerebral cortex in both outbred and inbred mice was used for vaccination. In contrast, the challenge virulent strain (designated FU) killed mice after a much shorter incubation time (∼130 days for FU versus ∼370 days for SY) and additionally provoked fulminant cortical vacuolization as depicted (14). These differences in incubation provided ample time to establish the slow SY infection while still being able to distinguish disease caused by superinfection with FU. Moreover, SY and FU strains had been isolated from humans living in geographically separated regions of the United States and Asia, respectively, and quasispecies or revertants that might obscure the discrimination of strains was thereby minimized. In contrast, the 22A and 22C scrapie strains used previously were isolated from a single sheep, possibly minimizing strain differences and interference effects. For stabilization of agent properties, the SY strain (from “sporadic” CJD) was first isolated in guinea pigs and then passaged in CD-1 (Swiss) mice for four passages (26). The “familial” Gerstmann–Straussler–Scheinker disease isolate FU (for the Fukuoka-1 strain) was first isolated in rats and then subsequently continuously passaged in mice (27). It was passaged once again in CD-1 mice before doing tests of interference. At the same time, mice were inoculated with normal brain (1%). These mice survived for up to 865 days and none showed CJD pathology.
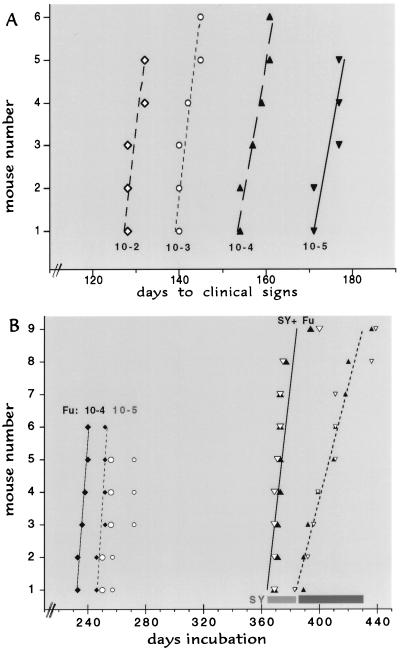
Control inoculations with each strain were first evaluated to determine infectious titers and incubation times. To ensure accurate comparisons, a single-aliquot vial of SY and FU 10% brain homogenates (10−1)was used for all titer, challenge, and analytic experiments herein described. After rapid thawing from −80°C, a sample from the SY and FU vials were each serially diluted and inoculated intracerebrally as described (26), starting with the maximal inoculum well tolerated by mice (30 μl diluted 10−2). With the maximal dose, SY gave an incubation time to clinical disease of >360 days (range 367–400 days). Moreover, SY-infected terminally ill mice often show stereotypic compulsive scratching without parasitic or other infections as described (26). Again, seven of 10 SY-infected mice showed the same repetitive behavior that led to destruction the skin on the back of the neck. SY mice remained alive with variable terminal signs for ∼35 days after clinical signs started. In contrast, no FU-infected animals exhibited scratching at any dilution (n = 77) but, instead, showed startle myoclonus with an invariably rapid progression within 12.5 days (±1.5 days, mean ± SEM) to a moribund state characterized by lack of feeding and grooming and profound inactivity. Incubation times for every inoculated mouse at the most pertinent serial dilutions of FU (Fig. 1A) revealed little individual variation in each group of five or six mice. As depicted, there was an interval of 15 days per order of magnitude increase in infectivity, giving FU an effective doubling time (ti) of only 4.5 days (the rate of agent replication minus the rate of agent clearance, see Fig. 1A). Endpoint titrations additionally yielded a titer of 1.7 × 109 infectious units (IU) per g of FU brain in moribund mice. Rather large doses of FU were chosen for challenge as 10−4 and 10−5 dilutions contained 5,000 and 500 IU, respectively. Endpoint titrations of SY were remarkably different, revealing about four orders of magnitude less infectivity than FU at terminal disease (3 × 105 IU/g), and the ti of SY was extended to ∼30 days. Relatively few infectious particles were inoculated to test interference (90 IU in the 1:100 diluted “vaccine” inoculum).
Figure 1.
(A) Interval to clinical disease of FU homogenates (10−1) in serial 1:10 dilutions. Day 1 is the inoculation day. Lines indicate the best fit in each experimental group, where the incubation in every inoculated mouse is shown by a single point. As derived (31), the effective doubling time ti = [ln 2/ln(titer end/titer start)] × the interval in days. Thus, for example, the 45-day interval for a 1:1,000 dilution of FU yields a ti of 4.5 days. (B) Predicted times for FU clinical and terminal disease at 10−4 (diamonds with solid and dotted lines, respectively) and at 10−5 (large and small open circles, respectively). Horizontal bars at right show the range of all control mice inoculated with SY only at 10−2 (light for clinical signs and dark for terminal illness). SY vaccinated mice challenged with a dilution of 10−4 (5,000 IU) of FU at clinical and terminal disease (large and small solid triangles respectively) and with FU diluted 10−5 (large and small open triangles) are within the range of SY controls, with the best-fit lines for the SY + FU 10−4 mice at clinical (solid line) and more terminal (dotted line) illness shown. Although some superinfected mice were sacrificed earlier during the clinical phase, the median distance between solid and dashed lines in superinfected mice was significantly longer (P < 0.001) than in FU controls.
To verify there was no loss in titer with storage or thawing of the FU vial, just before the challenge experiment a group of 11 more mice were again inoculated with FU at a dilution of 1:100. These concurrently incubating control mice showed full titer, yielding a comparable but slightly shorter incubation time of 122 ± 1.5 days versus 129.6 ± 1 days in the previous 10−2 titration (Fig. 1A, shorter incubation indicates higher titer). To test vaccination effects, SY was inoculated and established for 80 days before challenge with FU. The same SY material was also inoculated by itself as a parallel control on the same day. The 80-day seeding interval before challenge with FU was selected to allow for an eclipse phase of ∼30 days (31), plus approximately two doublings of SY. This time also allowed for complete resolution of traumatic damage from the first inoculation. Because previous studies have shown that ∼95% of inoculated agent is rapidly cleared from the brain after inoculation (31), probably fewer than 50 IU of SY were present at 80 days when higher doses of the FU challenge agent were inoculated. Fig. 1B shows that this low amount of SY was able to prevent superinfection by FU, as assessed by both incubation time and clinical symptoms.
The predicted times for clinical signs and terminal disease using the higher challenge dose of FU (diluted 10−4) are shown in Fig. 1B. Expected FU incubation times are moved to the right 80 days because this is equivalent to day 1 for FU. For reference, the SY controls are also shown. All 18 mice challenged with either dose of FU had only the incubation characteristics of SY, showing clinical symptoms at >360 days. The 10-fold higher challenge dose of FU was indistinguishable from the lower FU dose, further substantiating the obvious dominance of the SY agent. Additionally, even though a few clinically ill superinfected animals were killed before they became completely moribund, the prolonged clinical phase characteristic of SY is readily apparent. This interval closely matched the 35-day clinical phase in parallel SY controls, and was ∼3 times longer than the interval seen in FU infections (33 ± 4 versus 12.5 ± 1.4 days). Half of the superinfected mice also exhibited repetitive scratching, a feature not seen with FU infection but in reasonable accord with the percentage of scratching mice in SY controls. Statistically, the ability of SY to block FU infection was highly significant showing a clear difference from FU at either of the two dilutions (P < 0.0001; t test). Moreover, each group of superinfected mice was indistinguishable from the control SY mice in overall incubation time (P > 0.88 for both clinical and terminal disease).
Histological examination of each half brain showed all 18 superinfected mice had a pattern of mild cortical and hippocampal vacuolization at terminal disease that was typical for SY alone as depicted (14). As in SY controls, more prominent vacuolization was typically found in the head of the caudate nucleus and focally in the medial geniculate body. None of the superinfected mice had the fulminant vacuolar changes in the cortex that were characteristic of FU infection. Fig. 2 shows representative cortical sections from an FU control (Fig. 2A) compared with a SY mouse challenged with 5,000 IU of FU (fig. 2B). These micrographs also show PrP-res in parallel stained samples, and only FU had multiple punctate deposits of PrP-res associated with vacuoles. Small punctate or diffuse deposits of PrP-res were not observed in superinfected brains except around the inoculation site and in a small focal region of the geniculate body.
Figure 2.
Typical sections of cerebral cortex from an FU mouse (A) and an SY mouse (B) challenged with 5,000 IU of FU, stained, and developed in parallel for PrP-res (red) as described (32). (A) Extreme vacuolization of the cerebral cortex and a diffuse red blush of PrP-res and punctate PrP-res deposits characteristic for FU (arrows) are shown. (B) Representative region of SY cortex at identical magnification with markedly fewer vacuoles (arrows). As in SY control cortex, no background blush of PrP-res or punctate deposits are seen. Section was hematoxylin-counterstained (blue) to show nuclei. Both sections are from the formalin-fixed inoculated half brain and other side was used for Western blotting (Fig. 3).
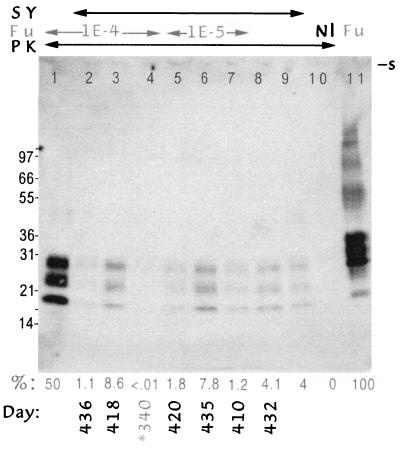
Further confirmation that superinfected animals had comparably low levels of PrP-res was provided by Western blotting and quantitative chemiluminescence as described (31, 32). To avoid assay of PrP-res from residual inoculum, the uninoculated half of the brain was homogenized for this assay. Fig. 3 shows equal homogenate loads from representative brains, and the calculated percent of PrP-res (of total brain PrP) is indicated. Superinfected samples for this limited demonstration blot included brains with the lowest as well as the most abundant PrP-res. For reference, Fig. 3, lane 10, shows no PrP-res bands in an uninfected brain and lane 11 shows the higher Mr bands in an undigested sample used to quantitate the percent of PrP-res by densitometry. An important feature to note is the identical size and pattern of PrP-res bands in brains infected by these very different strains (Fig. 3, compare the pool of four FU brains in lane 1 with individual and pooled SY control brains in lanes 8 and 9). Because both SY and FU were passaged in the same CD-1 mice before this experiment and because PrP-res band profiles were identical for both of these distinct agents, it is unlikely that changes in this host-encoded molecule are responsible for the observed strain differences. All end-stage superinfected brains had one-fifth to one-fortieth as much PrP-res as found in FU controls, with an average value of 4.1%, the same as in SY controls. The ∼2-fold higher level of PrP-res in 2 of 18 superinfected brains is probably because of the longer than average clinical phase of disease in these two mice (45 and 61 days, Fig. 3, lanes 3 and 6). These PrP results further substantiated the strong interference effect, although complete elimination of FU from all superinfected brains cannot be judged until further transmission experiments are completed. Finally, there was only a 10-fold difference in PrP-res between FU and SY, whereas the titers for FU and SY showed infectivity differences of ∼10,000-fold, reinforcing the lack of correlation between PrP-res and infectivity as described (9–11, 20, 28–32). The interference effect also did not depend on any unusual or spontaneous host genetic mutation, because superinfection was blocked in every mouse.
Figure 3.
Chemiluminesent detection of PrP in brain homogenates (equal sample loads) from control and superinfected mice. The arrows at the top indicate the mouse samples from SY or FU infection or both. The PK arrow indicates that samples 1–10 (but not 11) were digested with proteinase K. Nl is from an uninfected mouse showing no PrP-res. Lane 11 shows a pool of four FU brains used to quantitate total PrP (the 100% reference) and taken from the same vial used for inoculation; undigested samples of SY showed comparable levels of total PrP (data not shown). The percent of total PrP-res and the days when each individual mouse was sacrificed are indicated below each lane. The FU control inoculum shows more PrP-res (lane 1) than any terminal interference mouse (lanes 2, 3, and 5–7). PrP-res is similarly lower in a representative parallel SY control mouse from this passage (lane 8) and in a pool of four brains from the subsequent SY serial passage (lane 9). The average PrP-res of individual superinfected brains was 4.1%, which is in good accord with the SY controls. Lane 4 shows the very minimal PrP-res levels in a random superinfected mouse late in incubation (340 days) but just before the onset of clinical symptoms. For quantitation, homogenates were digested for maximal PrP-res detection as described (31), and films in the linear range were analyzed by 16-bit densitometry on Western blots as described with both densitometry and autoradiographic standards (32). No residual PrP is seen at the slot (s) and molecular weight markers are indicated at left in kDa.
There was no evidence that a limited availability of host PrP was responsible for blockage of FU replication. More than 90% of this protein showed no signs of an altered conformation as determined by its protease resistance, and this amount of normal host PrP is in vast excess of that needed to act as a viral receptor. Moreover, there was negligible PrP-res during the period when FU should have invaded cells. Even at late stages of infection, just before the appearance of SY symptoms, PrP-res was barely detectable. As shown in Fig. 3, lane 4, a random mouse killed at 340 days, to determine PrP resistance and neuropathology, had ≤0.01% PrP-res. This mouse also showed only questionable vacuolization and no apparent cellular loss, further emphasizing the availability of many other cellular sites for FU replication. In fact, the vast majority of PrP was unaltered at times when FU, if unimpeded, should have replicated by 17 orders of magnitude. Certainly even less, or possibly no, PrP-res was present at 80 days, the time of FU challenge. Thus, if PrP is a host molecule essential for the life cycle of these agents, then any change in PrP described thus far is neither limiting nor sufficient to explain the early interference effect. Additionally, the data above do not support the original proposal that competition for a “mulitimeric” product of sinc (the gene locus for PrP) limits the “replication sites” available for scrapie superinfection (4, 5). Indeed, given the low numbers of infectious SY particles present at the time of challenge, it is unlikely that any host-encoded protein in the brain is limited via interaction with the infectious particle, even if one discounts initial agent clearance (yielding maximally 260 IU of SY at 80 days).
The present interference results using CJD strains are more dramatic than found in the original intracerebral scrapie study where a smaller ∼30-day delay was observed (4). Moreover, the present results additionally demonstrate that a much higher dose of challenge agent (5,000 as compared with 100 IU in scrapie) can be blocked by lower doses of a “vaccine” inoculum given only once. Thus these data further substantiate the vaccination approach and broaden the centrality of independent agent strains. The more robust CJD interference effects observed above may be based on the geographic origins and different passage histories of our CJD strains because passage in different species can propel the evolution of unique agent strains (32). Furthermore, it is remarkable that an agent linked to an unusual human PrP amino acid change can be blocked by one derived from a person without this genomic alteration. Additionally, the propagation of agent-specified characteristics in the distant murine species with even more variant PrP sequences, and the lack of any demonstrable strain-specific PrP-res bands in this species, indicate agent properties independent of PrP. Different strains must encode distinct properties of virulence yet at the same time preserve common molecular features that can be recognized by the host.
The most parsimonious explanation for interference is the assumption that these agents contain a viral genome that specifies strains. Indeed, when one reviews all the reproducible data in this field, there are no biological, physical, or molecular data that exclude a viral agent (14, 21, 33). Because an invading infectious agent prevents superinfection, the attenuated agent itself, or the response that it elicits, must be responsible for interference. The attenuated strain SY may produce many defective interfering particles (without core nucleic acid) or viral products that are capable of blocking the cellular sites needed for completion of its own life cycle. Such products, or the defective particle itself, could inhibit the second challenge agent. This type of mechanism is well described for some viruses. In this case significant production of viral protein(s) that would not partition with infectious particles during fractionation and the accumulation of specific viral protein(s) at early stages of infection would be expected. An alternative but not mutually exclusive mechanism for interference centers on host recognition of one or more foreign features common to both strains. This host response could involve cryptic inflammatory, immune, or intracellular pathways. Experiments with prolonged rat CJD infections indicate that host inflammatory responses to infection can occur well before the onset of clinical disease (32) and lymphocytes can infiltrate the brain early in scrapie (34). Indeed, attenuated agents that produce prolonged disease may be those that are less capable of escaping typical host immune mechanisms, and a number of factors could modulate host antibody effects to some strains (33). There have been very few antibody studies in scrapie and CJD, and most of these were done many years ago (e.g., ref. 35). All investigators have used very crude preparations for inoculation and similarly crude material (with many proteins and very small amounts of agent) to monitor specific immunoglobins. Given the current data, a reevaluation of agent-specific antibodies seems reasonable.
Interference might also be based on intracellular mechanisms that antecede the evolutionary development of more sophisticated immune responses. Such mechanisms may include lysosomal compartmentalization (31) or a variety of chemokines and intracellular products. A nonspecific response to injected homogenates is excluded because interference effects were not reproduced by previous inoculation with noninfectious brain homogenates. Additionally, interference required administration of the slow scrapie strain at least 35 days before superinfection (4), an observation that implicates host recognition of the foreign agent and, possibly, agent processing or complementation by an endogenous virus (36, 37). Finally, host recognition may be responsible for significant agent clearance, making the doubling time of attenuated agents appear deceptively long and obscuring a faster and more conventional viral replication time.
Further experiments using several peripheral routes of infection including oral administration and inoculation of lower doses of attenuated strains should be informative. There are also established laboratory strains that when passaged in a different species show little pathogenicity for their host of origin. Because low doses of the mouse-passaged SY agent showed a strong blocking effect, an agent selected in a different species might be sufficient to supress infection in the original host from which it derived. Thus infectious agents causing negligible disease or very prolonged incubation in mice, such as the 263K hamster-passaged scrapie strain, may prevent superinfection by more virulent mouse-passaged agents, including those isolated from BSE. Similarly, several CJD agents that have been propagated and selected in this laboratory may block more virulent strains of CJD in primates if they retain sufficient invasiveness to be recognized by the host but have little or no pathogenicity. In contrast, a killed vaccine may not retain this effect. Indeed, only treatment of a “slow” scrapie strain with 5 Mrads of ionizing radiation or 12 M urea completely abrogated an interference effect, whereas other treatments did not (6). However, partial denaturation of reasonably purified infectious preparations may further vaccine development if such treatments expose additional agent components that are correctly delivered to and recognized by the host. Chemical modifications of purified infectious preparations may also generate more completely attenuated structures that can propagate and preserve their interfering properties, and clearly the elucidation of intrinsic agent molecules should further the development of recombinant vaccines. The present results suggest reasons for a renewed interest in a live vaccination approach to prevent a spectrum of slow viral infections including HIV. Nevertheless, it is important to emphasize that with CJD and other agents of this class, it is premature to undertake any experiments outside a controlled laboratory setting without strong evidence showing an attenuated agent is incapable of causing late-onset neurodegenerative disease.
Acknowledgments
I am indebted to William Fritch for excellent help with the animal work, to Z. Y. Lu for assisting with blots, and to Dr. Nicholas Avgeropoulos for suggestions on the manuscript. Paul Brown kindly provided mouse-passaged FU brain. This work was supported by National Institutes of Health Grants NS12674 and NS34569.
ABBREVIATIONS
- CJD
Creutzfeldt–Jakob disease
- BSE
bovine spongiform encephalopathy
- PrP
prion protein
- ti
effective doubling time
- PrP-res
protease-resistant PrP
- IU
infectious unit(s)
References
- 1.Halsband R. J Hist Med Allied Sci. 1953;8:390–405. doi: 10.1093/jhmas/viii.october.390. [DOI] [PubMed] [Google Scholar]
- 2.Sabin A B. J Biol Stand. 1973;1:115. [Google Scholar]
- 3.Oldstone M B A. J Virol. 1991;65:6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson A, Fraser H, Meikle V, Outram G. Nat New Biol. 1972;237:244–245. doi: 10.1038/newbio237244a0. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson A G, Fraser H, McConnell I, Outram G W, Sales D I, Taylor D M. Nature (London) 1975;253:556. doi: 10.1038/253556a0. [DOI] [PubMed] [Google Scholar]
- 6.Kimberlin R H, Walker C A. Intervirology. 1985;23:74–81. doi: 10.1159/000149588. [DOI] [PubMed] [Google Scholar]
- 7.Prusiner S B. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 8.Collinge J, Sidle K, Meads J, Ironside J, Hill A. Nature (London) 1996;383:685–667. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 9.Sklaviadis T L, Manuelidis E E, Manuelidis L. J Virol. 1989;63:1212–1222. doi: 10.1128/jvi.63.3.1212-1222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akowitz A, Sklaviadis T, Manuelidis E E, Manuelidis L. Microb Pathog. 1990;9:33–45. doi: 10.1016/0882-4010(90)90038-r. [DOI] [PubMed] [Google Scholar]
- 11.Riesner D, Kellings K, Post K, Wille H, Serban H, Groth D, Baldwin M A, Prusiner S B. J Virol. 1996;70:1714–1722. doi: 10.1128/jvi.70.3.1714-1722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuelidis L, Sklaviadis T, Akowitz A, Fritch W. Proc Natl Acad Sci USA. 1995;92:5124–5128. doi: 10.1073/pnas.92.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozel M, Baldauf E, Beekes M, Diringer H. In: Transmisssible Subacute Spongiform Encephalopathies: Prion Diseases. Court L, Dodet B, editors. Paris: Elsevier; 1996. pp. 369–373. [Google Scholar]
- 14.Manuelidis L. In: Transmisssible Subacute Spongiform Encephalopathies: Prion Diseases. Court L, Dodet B, editors. Paris: Elsevier; 1996. pp. 375–387. [Google Scholar]
- 15.Aguzzi A, Weissmann C. Nature (London) 1997;389:795–798. doi: 10.1038/39758. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao K K, Groth D, Yang S-L, Serban H, Rapp D, Foster D, Scott M, Torchia M, DeArmond S J, Prusiner S B. Ann NY Acad Sci. 1994;724:241–245. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manuelidis L. Ann N Y Acad Sci. 1994;724:259–281. doi: 10.1111/j.1749-6632.1994.tb38916.x. [DOI] [PubMed] [Google Scholar]
- 18.Carp R, Kascsak R, Rubenstein R, Merz P. Trends Neurosci. 1994;17:148–149. doi: 10.1016/0166-2236(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Race R, Priola S, Bessen R, Ernst D, Dockter J, Rall G, Mucke L, Chesebro B, Oldstone M. Neuron. 1995;15:1183–1191. doi: 10.1016/0896-6273(95)90105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuelidis L, Sklaviadis T, Manuelidis E E. EMBO J. 1987;6:341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuelidis L. Transfusion. 1994;34:915–928. doi: 10.1046/j.1537-2995.1994.341095026981.x. [DOI] [PubMed] [Google Scholar]
- 22.Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Auget M, Weissmann C. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Katamine S, Shigematsu K, Nakatani A, Moriuchi R, Nishida N, Kurokawa K, Nakaoke R, Sato H, Jishage K, et al. J Virol. 1995;69:7586–7592. doi: 10.1128/jvi.69.12.7586-7592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manuelidis L, Manuelidis E E. In: Encyclopedia of Virology. Webster R, editor. San Diego: Academic; 1994. pp. 1361–1369. [Google Scholar]
- 25.Will R, Ironside J, Zeidler M, Cousens S, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 26.Manuelidis E E, Gorgacz E J, Manuelidis L. Nature (London) 1978;271:778–779. doi: 10.1038/271778a0. [DOI] [PubMed] [Google Scholar]
- 27.Tateishi J, Nagara H, Hikita K, Sato Y. Ann Neurol. 1984;15:278–280. doi: 10.1002/ana.410150313. [DOI] [PubMed] [Google Scholar]
- 28.Manuelidis L, Sklaviadis T, Manuelidis E. In: Unconventional Virus Diseases of the Central Nervous System, Masson. Court L E A, editor. Bretagne: Abbaye de Melleray; 1989. pp. 489–507. [Google Scholar]
- 29.Xi Y G, Ingrosso A, Ladogana A, Masullo C, Pocchiari M. Nature (London) 1992;356:598–601. doi: 10.1038/356598a0. [DOI] [PubMed] [Google Scholar]
- 30.Wille H, Zhang G-H, Baldwin M A, Cohen F E, Prusiner S B. J Mol Biol. 1996;259:608–621. doi: 10.1006/jmbi.1996.0343. [DOI] [PubMed] [Google Scholar]
- 31.Manuelidis L, Fritch W. Virology. 1996;215:46–59. doi: 10.1006/viro.1996.0033. [DOI] [PubMed] [Google Scholar]
- 32.Manuelidis L, Fritch W, Xi Y G. Science. 1997;277:94–98. doi: 10.1126/science.277.5322.94. [DOI] [PubMed] [Google Scholar]
- 33.Manuelidis, L. (1998) Annales de L’Institute Pasteur, in press.
- 34.Betmouni S, Perry V, Gordon J. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- 35.Eklund C M, Kennedy R C, Hadlow W J. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Murdoch G, Sklaviadis T, Manuelidis E E, Manuelidis L. J Virol. 1990;64:1477–1486. doi: 10.1128/jvi.64.4.1477-1486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akowitz A, Sklaviadis T, Manuelidis L. Nucleic Acids Res. 1994;22:1101–1107. doi: 10.1093/nar/22.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]