Abstract
Spread of neuroadapted Sindbis virus (NSV) to motor neurons (MN) of the spinal cord (SC) causes severe hind limb weakness in C57BL/6 mice and models the paralysis that can accompany alphavirus and flavivirus encephalomyelitis in humans. The fate of spinal MN dictates the severity of NSV-induced paralysis, and recent data suggest that MN damage can occur indirectly via the actions of activated microglial cells. Because the opioid receptor antagonist, naloxone (NAL), blocks microglial-mediated neurodegeneration in other models, we examined its effects during NSV infection. Drug treatment prevented paralysis and enhanced the survival of MN without altering NSV tropism, replication, or clearance from SC tissue. Further studies showed that NAL most effectively inhibited paralysis in a 72-hour window after NSV challenge, suggesting that the drug inhibits an early event in SC pathogenesis. Histochemical studies demonstrated that NAL blocked early microglial activation in SC tissue sections, and protein assays showed that the early induction of pathogenic IL-1β was blunted in SC homogenates. Finally, loss of glutamate transporter-1 (GLT-1) expression in SC, an astrocyte glutamate reuptake protein responsible for lowering toxic extracellular levels of glutamate and preventing MN damage, was reversed by NAL treatment. This GLT-1 loss proved to be highly IL-1β-dependent. Taken together, these data suggest that NAL is neuroprotective in the SC by inhibiting microglial activation that, in turn, maintains normal astrocyte glutamate homeostasis. We propose that drugs targeting such microglial responses may have therapeutic benefit in humans with related viral infections.
Keywords: Neuroprotection, viral encephalitis, motor neurons, microglial activation, IL-1β, Glutamate transporter-1
Introduction
Alphaviruses and flaviviruses are important causes of fatal encephalitis in humans worldwide. Most of these infections are transmitted to mammalian hosts via the bite of infected mosquito vectors. One of these pathogens, West Nile virus (WNV), is newly arrived in the Western hemisphere where it has caused hundreds of fatal human encephalitis cases over the past few years (Granwehr et al., 2004). Neurological disease caused by this pathogen occurs with a wide array of clinical features that sometimes delays a specific diagnosis. Even when recognized promptly, however, antiviral therapies have proven ineffective and treatment is supportive. The focal occurrence of WNV epidemics thus far makes the development and widespread use of an effective vaccine an uncertain prospect for the near future. Therefore, unresolved issues related to WNV infection raise many public health concerns (Hayes et al., 2005).
Several related alphaviruses also cause epidemic, mosquito-borne encephalitis in humans, and a few of these viruses have been adapted for study in convenient animal models. One of these pathogens, Sindbis virus (SV), causes an acute encephalomyelitis in adult mice that closely reproduces many features of human alphavirus and flavivirus infections. A neuroadapted strain of SV (NSV) produces a lethal disease in susceptible hosts that evolves over a period of 7-10 days (Jackson et al., 1988). Following direct intracerebral inoculation, NSV spreads rapidly from the brain to the spinal cord (SC), where it causes a progressive lower motor neuron (MN) paralysis similar to WNV (Havert et al., 2000; Jackson et al., 1987; Kelley et al., 2003). Like other alphaviruses and flaviviruses, NSV selectively targets neurons with minimal infection of glial cells (Johnson et al., 1972). Neuronal fate determines outcome, with the neurovirulence of each SV strain directly related to the extent of neuronal destruction it produces (Lewis et al., 1996). As an added layer of complexity, different neuronal populations undergo different types of cell death following NSV infection (Havert et al., 2000; Lewis et al., 1996), and many non-infected neurons are also damaged via bystander mechanisms, believed to be glutamate-mediated excitotoxicity (Nargi-Aizenman and Griffin, 2001). This bystander injury has led to the suggestion that host immune responses somehow contribute to NSV pathogenesis (Liang et al., 1999; Kimura and Griffin, 2000; Kimura and Griffin, 2003), although the degree to which neuronal damage occurs via host-derived rather than direct viral-induced mechanisms remains poorly understood.
Microglia are the principal endogenous immune cells of the central nervous system (CNS) (Lawson et al., 1990), and these cells become activated in response to injury, infection, or inflammation (Gehrmann et al., 1995; Perry and Gordon, 1991). Upon activation, microglia proliferate, change morphology, and assume many macrophage-like functions. When neurons die, microglia contribute to the removal of cellular debris (Streit, 1996; Streit et al., 1988). Activated microglia may also actively contribute to neuronal cell death, since therapies that inhibit microglial activation result in increased neuronal survival in numerous experimental models (Rogove and Tsirka, 1998; Thanos et al., 1993; Yrjanheikki et al., 1998). Activated microglia release cytotoxic substances such as IL-1β, TNFα, superoxide anion, and nitric oxide, all of which may contribute to neuronal cell death in vitro and in vivo (Bronstein et al., 1995; Liu et al., 2000; Takeuchi et al., 1998). Little, however, is known about the role of activated microglia in the pathogenesis of alphavirus and flavivirus encephalomyelitis.
The classic opioid receptor antagonist, naloxone (NAL), has been shown to block the production of inflammatory mediators by activated microglial cells in vitro (Liu et al., 2000; Chang et al., 2000; Liu et al., 2002), and to attenuate neurological deficits in animal models of stroke, SC injury, and Parkinson's disease in vivo (Faden et al., 1981; Hosobuchi et al., 1982; Liao et al., 2003; Lu et al., 2000). Indeed, an inflammatory basis for at least some of the neuronal injury that occurs in these diverse disorders has long been hypothesized to derive from microglial responses that consistently accompany them. Products of activated microglia are also implicated in the pathogenesis of various CNS viral infections, particularly those caused by HIV and HSV (Glass and Wesselingh, 2001; Marques et al., 2006; Wesselingh and Thompson, 2001). As previous studies from our group have shown evidence of widespread microglial activation in the CNS of SV-infected mice (Tyor et al., 1990), we investigated the effects of NAL in the more virulent NSV encephalomyelitis model. Here, we report that the drug prevents paralysis and limits MN destruction in a measurable therapeutic window, and that its mechanism of action appears to involve the blockade of local microglial activation and IL-1β production in the SC. This leads to normalized glutamate transporter expression and suggests that glutamate-mediated excitotoxicity is being prevented in NSV-induced encephalomyelitis. Taken together, these studies demonstrate that neuroprotective agents targeting such detrimental host immune responses have therapeutic potential in these otherwise untreatable infections.
Materials and Methods
Animal manipulations
All animal procedures had received prior approval from the Johns Hopkins institutional animal care and use committee and were performed under isoflurane anesthesia (Abbott Laboratories, North Chicago, IL). Five to 6 week-old C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Age-matched B6.129F1 (IL-1β+/+) and IL-1β-deficient (IL-1β−/−) mice were purchased from Taconic (Hudson, NY). To induce encephalomyelitis, all animals received intracerebral inoculations of 1000 plaque-forming units (PFU) of NSV in a volume of 20 μl of PBS into the right cerebral hemisphere. Mice were monitored daily for signs of disease in accordance with approved animal protocols. Twenty animals per group were used for all experiments unless otherwise noted. For those experiments where tissue samples were not being collected for ex vivo analysis, each animal was scored daily into one of the following categories: 0) normal or minimally affected, 1) mild paralysis (some weakness of one or both hind limbs), 2) moderate paralysis (weakness of one hind limb, paralysis of the other hind limb), 3) severe paralysis (complete paralysis of both hind limbs), or 4) dead. Many animals were treated with NAL at 20, 50 or 100 mg/kg (naloxone hydrochloride (−) isomer, Sigma Chemical Company, St. Louis, MO, catalogue number N7758) or saline given as a daily intraperitoneal injection starting at different times after viral challenge. The significance of clinical differences between groups was calculated by Kaplan-Meier analysis where noted (GraphPad Prism version 4.0, GraphPad Software Inc, San Diego, CA).
Tissue viral titrations
To measure the amount of infectious virus present in lumbar SC tissues, animals were perfused with PBS and cords were extracted, weighed, snap frozen on dry ice, and stored at −80°C until viral titration assays were performed. At the time of these titrations, 10% (w/v) homogenates of each sample were prepared in MEM supplemented with 2% fetal bovine serum, and serial 10-fold dilutions of each homogenate were assayed for plaque formation on monolayers of BHK-21 cells, according to standard protocols used in our laboratory (Kerr et al., 2002). The results presented are the mean ± standard error of the mean (SEM) of the log10 of viral plaque-forming units per gram of tissue derived from 3 animals at each time point.
Histology
Animals designated for histological analyses were sequentially perfused with chilled PBS and 4% paraformaldehyde in PBS via a transcardial approach. All tissues were then post-fixed in 4% paraformaldehyde overnight at 4°C, after which they were embedded in paraffin for sectioning. All lumbar spinal cord sections were taken at the same level to ensure the cross-sectional area being compared between animals was similar. To assess the fate of lumbar MNs, the entire lumbar spinal column (SC plus adjacent nerve roots) was decalcified (Immunocal, Decal Corporation, Tallman, NY) and embedded in paraffin, after which sections were stained using our modified Bielschowsky silver staining method to label neurofilament proteins present in each nerve axon (Havert et al., 2000; Kerr et al., 2002; Nargi-Aizenman et al., 2004). MN axons were identified and counted in each lumbar ventral nerve root, a site where only MN axons travel after leaving the SC. Axonal density (the number of intact axons per cross-sectional area of each root) was determined for the right and left L4 and L5 ventral nerve root (i.e. four roots per animal) from triplicate mice, with or without NAL treatment (50 mg/kg/day) on days 0, 5, and 7 post-infection. These later stages of disease were studied because the loss of MN axons is delayed following destruction of the cell body in the SC. Data are presented as the mean ± SEM of the percent axonal loss in NSV-infected animals compared to uninfected controls. Cross-sectional area of each nerve root was calculated using ImageJ software, version 1.33u (National Institutes of Health (NIH), Bethesda, MD). Differences in the density of remaining nerve axons were analyzed using Student's t-test. Significant differences (noted with an *) were defined as having a p value <0.05.
Immunohistochemistry
For all immunoperoxidase staining, SC sections were deparaffinized, rehydrated, treated with 1% hydrogen peroxide in ice-cold methanol for 30 minutes to block endogenous peroxidase, and rinsed in TBS. Sections were blocked with 1% normal serum matching the animal in which the secondary antibody was raised for 1 hour at room temperature. Primary antibody was applied to each section, diluted in TBS containing 0.5% non-fat skim milk plus 1% normal serum overnight at 4°C. The following primary antibodies and stains were utilized: polyclonal anti-SV (1:100, Kimura and Griffin, 2003) which recognizes viral structural protein antigens, anti-CD45 (Chemicon International Inc, Temecula, CA; CBL1326, 1:80) which recognizes all haematopoietic cells, anti-CD3 (Dako, Glostrup, Denmark; A0452, 1:100) which stains T cells, and biotinylated lycopersicon esculentum (tomato) lectin (Sigma, L0651, 5 μg/ml) which stains activated microglia (Acarin et al., 1994). Secondary antibodies were conjugated to biotin and used at 1:200. For tomato lectin staining, TBS plus 0.1% triton was used for all washes and all serum was removed from the staining process, since lectins are known to bind non-specifically to these proteins (Acarin et al., 1994). Following several washes to remove all unbound secondary antibody, sections were sequentially incubated with avidin-biotin complex solution (Vector Laboratories, Burlingame, CA) in TBS, and then treated with 0.5 mg/ml diaminobenzidine (Polysciences, Warrington, PA) in TBS containing 0.01% hydrogen peroxide. All sections were counterstained with hematoxylin (Fisher Scientific, Fair Lawn, NJ), dehydrated in graded alcohol washes, mounted with glass coverslips in Permount (Fisher), and directly visualized using light microscopy for photography using Spot advanced software version 4.0.1 (Diagnostic Instrument Inc, Sterling Heights, MI). The number of lectin-positive microglial cells per cross section of SC was counted from triplicate animals at days 1, 2, and 3 post-infection with or without NAL treatment (50 mg/kg/day). Differences in the number of lectin-positive microglial cells between groups were analyzed using Student's t-test, with significance again defined as having a p value <0.05 (noted with an *).
Immunoblotting of tissue extracts
Lumbar SC tissues were sonicated in lysis buffer (10 mM Tris, 1% SDS, 1 mM Sodium Orthovanadate, pH 7.6), following the addition of a protease inhibitor cocktail (Sigma). Protein concentrations were determined using a standard protein assay (Bio-Rad, Hercules, CA). A total of 5μg of each sample was separated on 12% SDS-PAGE gels (Bioexpress, Kaysville, UT). After electrophoresis, proteins were transferred to a nitrocellulose membrane (Hybond-C extra, Amersham Biosciences UK Limited, Buckinghamshire, England) using an electrophoretic transfer system (Bio-Rad) in 20% methanol transfer buffer. Membranes were blocked for 1 hour in 5% non-fat skim milk diluted in PBS containing 0.05% Tween-20, and incubated overnight with primary antibody diluted in 1% nonfat skim milk. Primary antibodies used for the quantification of SC protein expression included: anti-GLT-1 (Sigma, E-1401, 1:200) and anti-actin (Chemicon, MAB1501, 1:10,000). After washing, membranes were incubated for 1 hour at room temperature with secondary antibodies (1:2000 for anti-rabbit (NA934V, Amerham) and 1:10,000 for anti-mouse (NA931V, Amersham), washed, developed with Supersignal West Pico chemiluminescent substrate (Pierce, Rockford, IL), and visualized using a Fuji Luminsecent Image Analyzer (LAS-1000plus camera, Fuji Photo Film Co., Ltd., Tokyo, Japan). The intensity of each band was determined using ImageJ software (NIH). Each GLT-1 blot was stripped and reprobed for actin expression. Protein expression in infected SC tissue was compared to levels in uninfected controls, all of which were normalized to actin expression.
Tissue cytokine assays
IL-1β and TNFα levels in SC lysates were determined using ELISA kits following the manufacturers' instructions (KMC0011 and KMC3011, respectively, Biosource, Camarillo, CA). Each lysate was made in PBS and standardized such that 50μg of total protein was applied to each ELISA plate. Cytokine levels in test samples were calculated based on direct comparisons to standards provided with the kit.
Results
NAL prevents hind limb paralysis in murine NSV encephalomyelitis
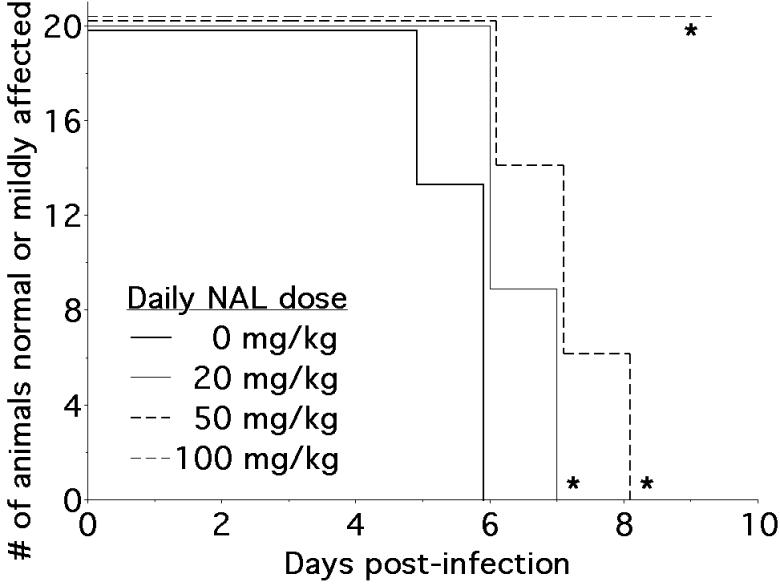
To assess the effects of NAL on the clinical course of NSV infection, mice were treated daily with 20, 50, or 100 mg/kg/day of the drug over the first week of infection. The development and magnitude of paralysis following virus challenge was compared between drug-treated animals and saline-treated controls. NAL had a pronounced affect on the development of hind limb paralysis, where drug-treated mice showed either a significant delay in the onset of paralysis, less severe overall disease, or both compared to untreated controls (Fig. 1). Thus, all saline-treated animals showed moderate or severe paralysis by day 6 post-infection. For animals treated with 20 or 50 mg/kg/day of NAL, the onset of paralysis was not seen until day 7 and day 8, respectively. At a dose of 100 mg/kg/day, none of the animals showed any signs of paralysis before death. One quarter of mice given NAL at a dose of 50 mg/kg/day survived infection that was uniformly fatal in the control group (survival curves not shown). Animals treated with NAL at a dose of 100 mg/kg/day became agitated, aggressive, and difficult to assess, so all subsequent experiments were done at a dose of 50 mg/kg/day.
Figure 1.
NAL treatment attenuates hind limb paralysis in NSV-infected mice. Hind limb paralysis was assessed in 5-6 week-old mice (n=20 per group) treated with different daily doses of NAL (20 mg/kg, 50 mg/kg, or 100 mg/kg) compared to a saline vehicle control for 7 days following NSV challenge. Each NAL-treated group showed a statistically significant difference in the onset of more severe paralysis compared to the control group (*p < 0.02).
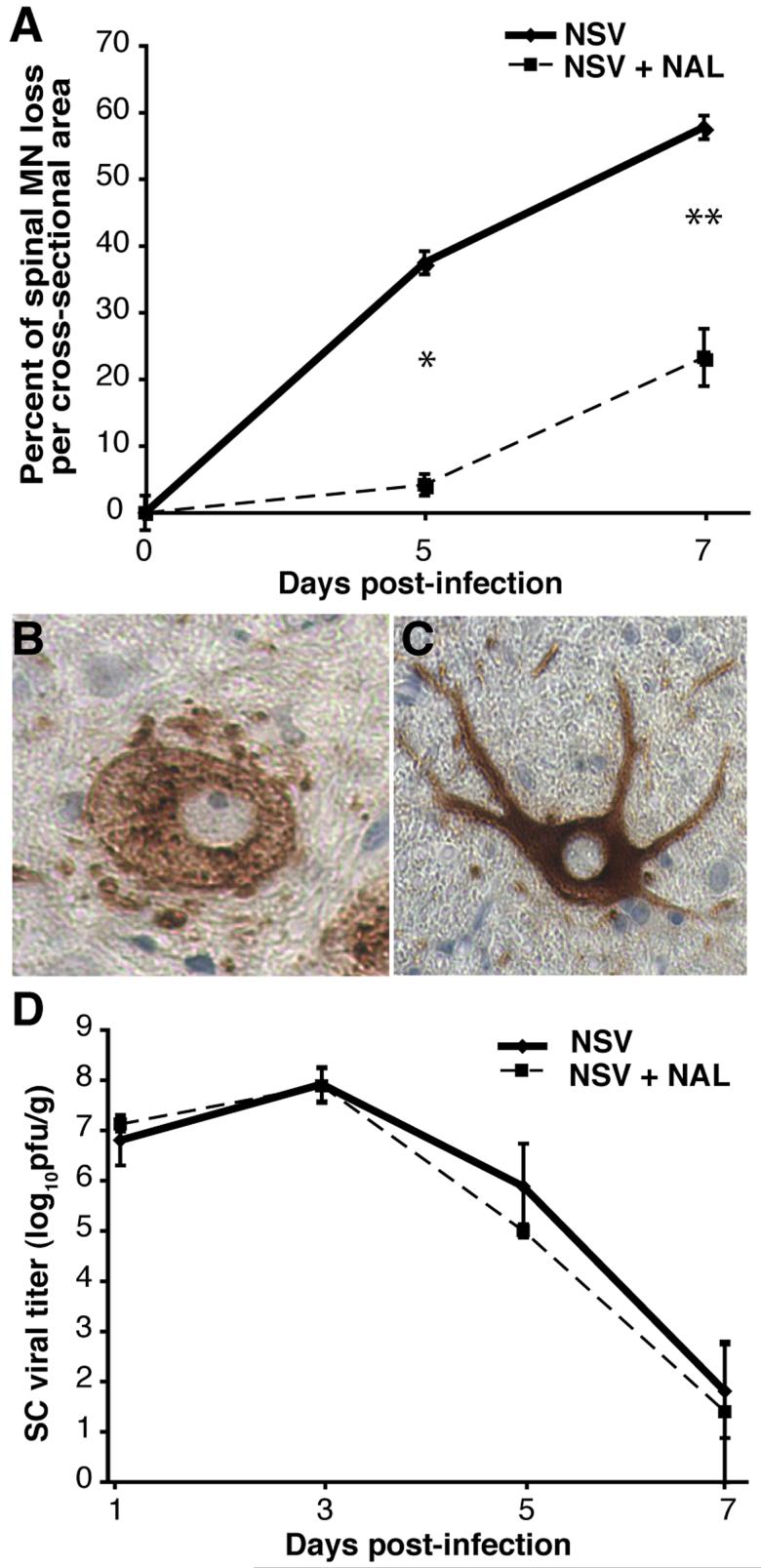
NAL reduces the degeneration of spinal MN without altering NSV tropism, replication or clearance from the SC of NSV-infected mice
To determine the effect of NAL on MN survival following NSV infection, the degeneration of spinal MN axons was quantified in lumbar ventral nerve roots. This is a convenient and validated measure of the injury to the MN cell bodies themselves (Havert et al., 2000; Kerr et al., 2002; Nargi-Aizenman et al., 2004). We found that MN loss was significantly reduced at day 5 and day 7 post-infection in NAL-treated animals (50 mg/kg/day) compared to controls (Fig. 2A). To confirm that NAL did not simply alter viral tropism in the SC, immunohistochemistry for viral antigens was performed on tissue sections from animals with or without drug treatment. In both cases, equivalent cytoplasmic staining of MNs was seen (Fig. 2B, C). Furthermore, the infected MN of animals treated with NAL retained the typical angular morphology of healthy cells (Fig. 2C), whereas infected MNs from saline-treated animals appeared swollen and abnormal (Fig. 2B). Finally, to assess the effect of NAL on NSV replication and clearance from the SC, levels of infectious virus were measured in tissue homogenates by plaque titration assay. Peak SC viral titers were seen on day 3 post-infection in both groups, with similar rates of viral clearance over time (Fig. 2D). As a result, we conclude that NAL exerts a purely neuroprotective effect in the SC of NSV-infected mice, without altering virus tropism, replication, or clearance from that site.
Figure 2.
NAL treatment (50 mg/kg/day) increases MN survival in NSV-infected mice without altering virus tropism, replication, or clearance from the SC. (A) The loss of MN axons in lumbar ventral nerve roots among NAL-treated animals (broken line) was significantly reduced compared to untreated controls (solid line) at two time points examined (*p < 0.05, **p < 0.01). (B, C) Immunostaining for viral antigens showed selective staining of motor neurons in both saline-treated (B) and NAL-treated animals (C) in lumbar SC sections. Magnification 400x. (D) Replication and clearance of NSV from the lumbar SC of NAL-treated animals (broken line) was unchanged compared to saline-treated controls (solid line). Each point represents the mean ± SEM of the log10 PFU per gram of tissue from 3 animals. Viral growth curves were not statistically different from each other at any time point.
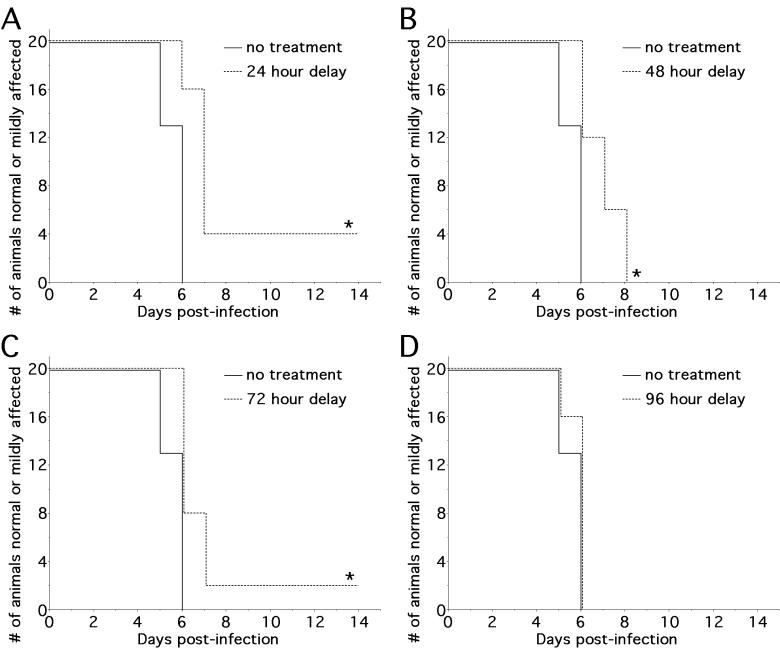
NAL treatment interrupts an early event in SC pathogenesis
To characterize the therapeutic window of NAL during NSV infection, parallel groups of animals were treated with a dose of 50 mg/kg/day starting at 24-hour intervals following viral challenge and then observed for disease manifestations over time. All NAL-treated animals had a significant delay in the onset of paralysis, as well as a milder overall disease, regardless of whether the drug was administered on the day of viral inoculation or delayed for up to three days (Fig. 3A-C). The therapeutic benefit of NAL was diminished, however, if treatment was delayed by four days (Fig. 3D). With early treatment, 25% of animals treated from onset (data not shown) and 20% of animals treated from day 1 did not develop paralysis at all (Fig. 3A). In contrast, all animals treated with NAL starting from day 2 post-infection developed paralysis following NSV infection, albeit with delayed kinetics (Fig. 3B). We conclude that NAL treatment is most effective when given within 24 hours of viral challenge.
Figure 3.
NAL treatment (50 mg/kg/day) alters an early event in NSV pathogenesis in the SC. Hind limb paralysis in 5-6 week-old mice (n=20 per group) was assessed with NAL treatment starting at 24 hours intervals after viral challenge and continued until day 7 post-infection. (A-C) Three dosing regimens (A, 24 hour delay; B, 48 hour delay; C, 72 hour delay) resulted in a statistically significant delay in the onset of more severe paralysis (*p < 0.02). (D) Delay beyond 72 hours post-infection did not alter the onset or severity of paralysis compared to untreated controls.
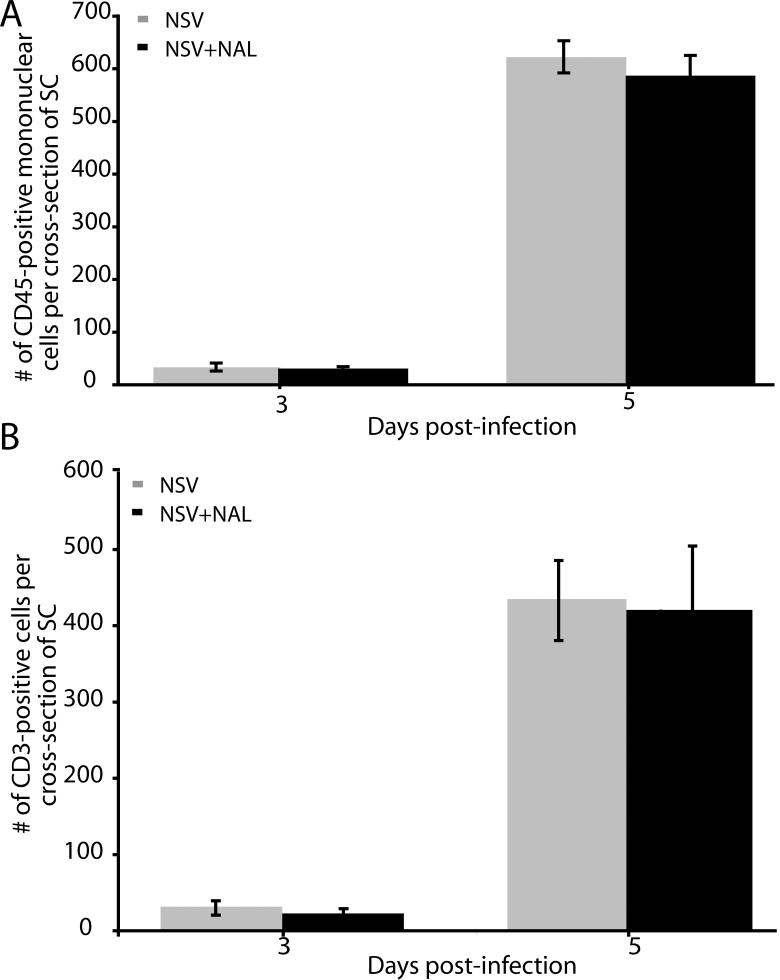
NAL treatment does not alter the overall inflammatory response in the SC of NSV-infected mice
Since NAL treatment protected NSV-infected animals and their vulnerable spinal MN without affecting SC virus replication or spread, we investigated its effects on the host response to infection. To determine whether NAL treatment (50 mg/kg/day) influenced the overall development of SC inflammation, anti-CD45 immunohistochemistry was performed to quantify all infiltrating mononuclear cells. These assays showed that the number of recruited immune cells was not affected by NAL treatment (Fig. 4A). Similar results were obtained with anti-CD3 immunohistochemistry, where the same time course and magnitude of T cell infiltration was identified with or without NAL treatment (Fig. 4B). We conclude that the drug has no effect on the adaptive immune response in the SC during NSV infection.
Figure 4.
NAL treatment (50 mg/kg/day) does not affect immune cell infiltration into the SC of NSV-infected mice. (A) No difference in the number of CD45-positive mononuclear cells were found in the SC between NAL-treated animals (black bars) and saline-treated controls (grey bars). (B) Similarly, the numbers of CD3-positive T cells in SC tissue sections was the same between the two groups. Each bar represents the mean ± SEM of positive cells per cross section of lumbar SC from 3 animals at each time point.
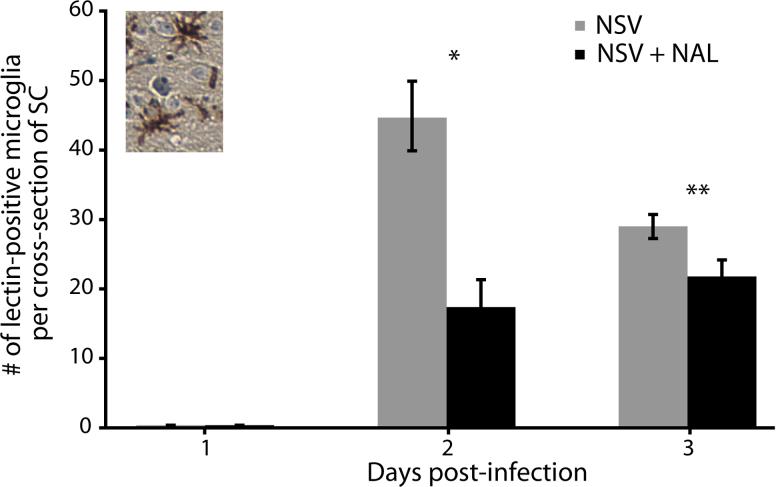
NAL treatment blocks early microglial activation in the SC of NSV-infected mice
NAL has been shown to block microglial activation and microglial-induced neurodegeneration in an animal model of Parkinson's disease (Liu et al., 2000; Lu et al., 2000). To assess the effect of NAL on microglial activation during NSV infection, immunostaining with a microglial-specific lectin was performed on SC sections (Acarin et al., 1994). The number of lectin-positive cells, identified by their morphology (Fig. 5, inset), was counted with or without drug treatment (50 mg/kg/day). While labeled microglial cells were not identified in SC 24 hours after viral challenge, their numbers increased thereafter and there was a clear reduction of staining with NAL treatment (Fig. 5). This microglial response precedes the infiltration of circulating immune cells that peaks on day 5 post-infection (Fig. 4).
Figure 5.
NAL treatment (50 mg/kg/day) reduces the total number of lectin-positive microglial cells found in the lumber SC of NSV-infected mice. Labeled microglia were counted in SC sections from animals with or without NAL treatment at days 1-3 post-infection. Inset photo shows the representative morphology of labeled microglial cells being counted. Each bar represents the mean ± SEM of the number of lectin-positive microglia per cross-section of SC from 6 animals at each time point. NAL treatment significantly reduced the number of labeled microglia detected at day 2 (*p < 0.01) and day 3 (**p < 0.03) post-infection.
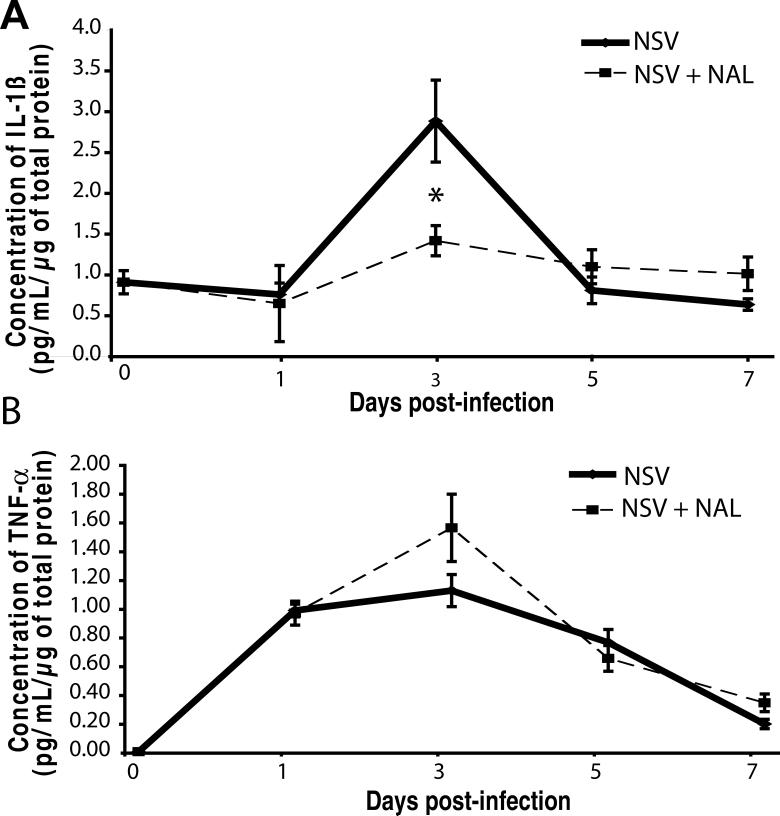
NAL selectively blunts the local induction of IL-1β in the SC of NSV-infected mice
The pro-inflammatory cytokines, IL-1β and TNFα, are both produced by activated microglia, and IL-1β is centrally involved in NSV pathogenesis (Liang et al., 1999). To determine whether NAL treatment alters the induction of these mediators in the SC during NSV infection, cytokine levels were measured in tissue homogenates by ELISA. Animals treated with saline showed peak IL-1β levels of 2.8 pg/mL/μg of total protein at day 3 post-infection, followed by a rapid decline thereafter (Fig. 6A). In contrast, drug-treated animals (50 mg/kg/day) showed peak IL-1β of only 1.4 pg/mL/μg of total protein, that was significantly lower than controls (Fig. 6A). Conversely, NAL-treated animals actually had higher SC levels of TNFα (1.4 pg/mL/μg of total protein) compared to controls (1.1 pg/mL/μg of total protein) at day 3 post-infection (Fig. 6B). These data show that NAL exerts a selective immunoregulatory effect in the SC during NSV infection, through what we suggest is its action on tissue microglial cells.
Figure 6.
NAL treatment (50 mg/kg/day) selectively reduces the local induction of IL-1β in the SC of NSV-infected mice. ELISA was used to measure levels of two pro-inflammatory cytokines, IL-1β and TNF-α, in the lumbar SC homogenates from NSV-infected animals with or without NAL treatment. Each point represents the mean ± SEM of the concentration of IL-1β or TNF-α (pg/mL/μg of total protein) from 3 animals at each time point. NAL treatment (broken line) significantly dampened the local induction of IL-1β, but not TNF-α, compared to saline-treated animals (solid line) on day 3 post-infection (*p <0.05).
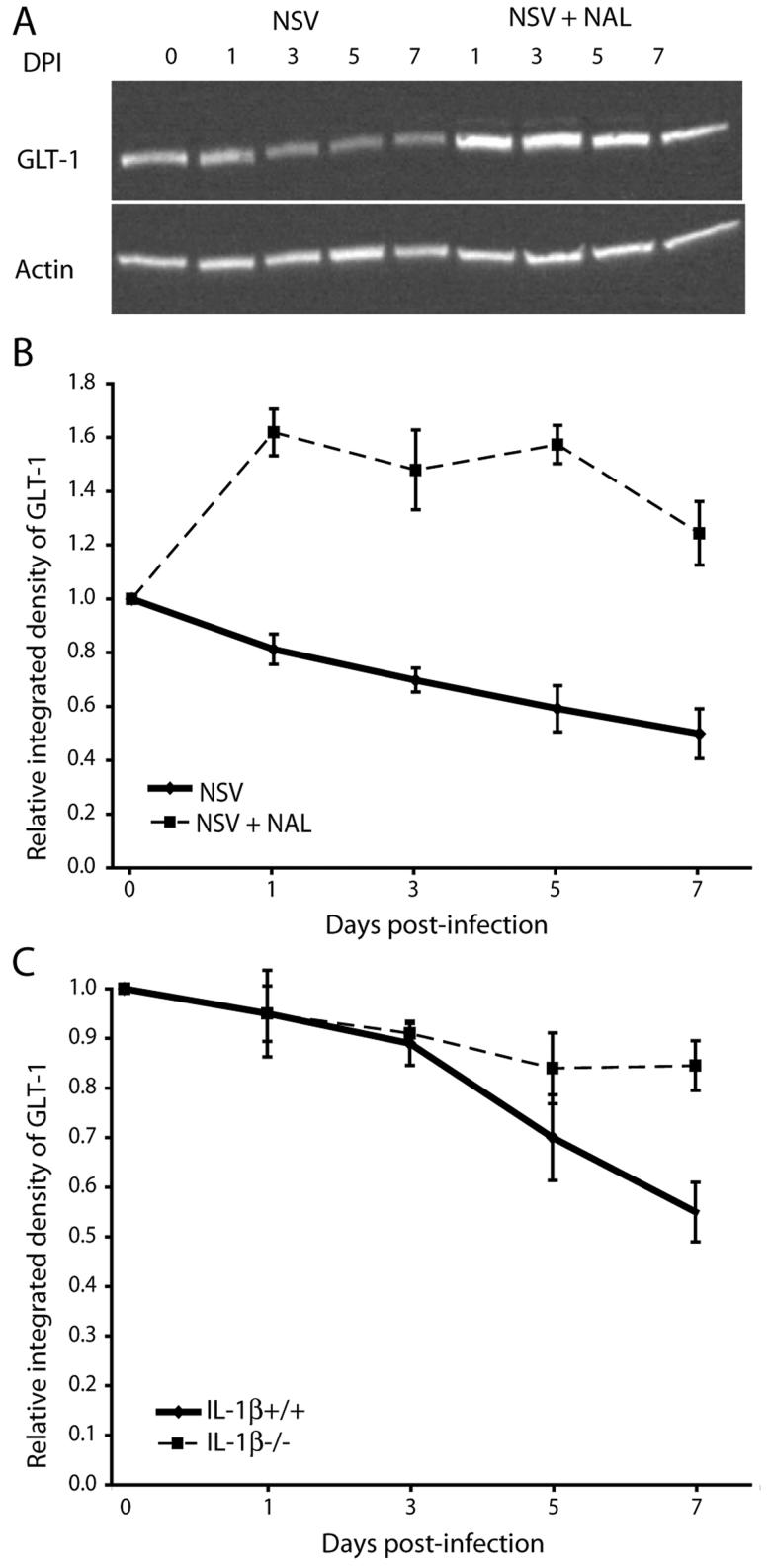
NAL limits the IL-1β-dependent loss of the astroglial glutamate transporter, GLT-1, in the SC of NSV-infected mice
Glutamate transporter-1 (GLT-1) is expressed primarily on astrocytes and is the principal glutamate reuptake mechanism throughout most regions of the CNS, including the SC (Rothstein et al., 1996). Decreased astrocyte-mediated glutamate transport contributes to excitotoxic MN injury in several human disease states (Rothstein et al., 1995; Werner et al., 2001), and it is a fundamental mechanism of paralysis and MN degeneration in the SC of NSV-infected mice (Darman et al., 2004). To determine if NAL-mediated protection against NSV-induced paralysis and MN injury was associated with altered SC GLT-1 expression, homogenates from NSV-infected mice with or without NAL treatment (50 mg/kg/day) were analyzed by Western blot. Quantitative analysis showed that NAL significantly increased GLT-1 expression in the SC at all days post-infection compared to uninfected controls (Fig. 7A, B). In contrast, saline-treated animals showed more than a 40% reduction in GLT-1 expression by day 7 post-infection (Fig. 7A, B). To then determine how IL-1β influenced GLT-1 expression during NSV infection, similar Western blots were performed on SC tissues from IL-1β+/+ and IL-1β−/− mice. These assays showed that GLT-1 loss was highly dependent on the presence of IL-1β, since it was nearly abolished in IL-1β−/− animals (Fig. 7C). As IL-1β−/− mice are known to be highly resistant to NSV-induced paralysis and death (Liang et al., 1999), and because NAL blunts the pre-symptomatic induction of IL-1β in the SC of NSV-infected animals (Fig. 6A), we propose that drug treatment prevents paralysis by inhibiting microglial production of IL-1β which, in turn, prevents astrocytic GLT-1 loss, maintains local glutamate homeostasis, and reduces glutamate-mediated excitotoxic injury of MNs.
Figure 7.
NAL treatment (50 mg/kg/day) influences SC expression of the astrocyte glutamate transporter GLT-1 during NSV infection. (A) GLT-1 and actin expression in lumbar SC lysates from mice with or without NAL treatment at different days post-infection (DPI). (B) Quantification of GLT-1 expression relative to actin expression in NAL-treated animals (broken line) compared to saline-treated controls (solid line) normalized to expression levels in uninfected controls. Each point represents the mean ± SEM of the relative integrated density of GLT-1 for 3 animals. (C) Quantification of GLT-1 expression relative to actin expression in lumbar SC lysates from IL-1β−/− (broken line) and IL-1β+/+ (solid line) animals. Each point represents the mean ± SEM of the relative integrated density of GLT-1 calculated from 3 animals at each time point and normalized to actin expression.
Discussion
The pathogenesis of SV encephalitis in mice is complex, with virus and host factors both contributing to disease outcome. Although a unifying theme in this model is that neurovirulence relates directly to the extent of neuronal destruction produced by each viral strain (Lewis et al., 1996), different populations of infected cells die through multiple pathways (Havert et al., 2000; Kerr et al., 2002), and non-infected neurons are also damaged via bystander mechanisms such as glutamate-mediated excitotoxicity (Darman et al., 2004; Nargi-Aizenman and Griffin, 2001). Host responses have also been implicated in disease pathogenesis (Liang et al., 1999; Kimura and Griffin, 2000), and the possibility that a targeted anti-inflammatory strategy might protect vulnerable neuronal populations (regardless of whether they harbor virus or not) has been raised. Here, we report that not only does NAL prevent the paralysis and MN destruction that occurs following spread of NSV infection to the SC, but also that the inflammatory cytokine, IL-1β, is further implicated in this aspect of disease pathogenesis. The fact that mitigation of symptoms can be achieved without any direct effect on SC virus replication or spread clarifies the importance of host immune responses in this manifestation of disease. Furthermore, therapies targeting the immune response rather than the virus itself may actually be a more practical clinical approach, since most symptomatic patients have undetectable viral loads once they reach medical attention (Bowen and Calisher, 1976). As a result, antiviral agents may not be very effective at this stage of disease. Thus, a future goal is to extend these observations to other alphavirus and flavivirus infection models with an eye towards eventual testing in humans.
NAL has already been established as an experimental therapeutic in neurological disease. Early studies showed that the drug attenuated deficits in animal models of stroke and SC injury (Faden et al., 1981; Hosobuchi et al., 1982), and more recent investigations have demonstrated that it protects vulnerable dopaminergic neurons in an experimental Parkinson's disease model (Lu et al., 2000). Interestingly, studies in the Parkinson's disease model, in particular, have shown that both NAL stereoisomers are equally neuroprotective (Liu et al., 2000; Liu et al., 2002). In contrast, only (−)-naloxone isomer binds to and antagonizes opioid receptors, while the (+)-enantiomer is inert in this regard (Iijima et al., 1978; Marcoli et al., 1989). Further investigations have now fully dissociated the neuroprotective effect of NAL from its opioid receptor signaling (Liu and Hong, 2003). A search for alternative mechanisms of action has shown that NAL can directly block the production of inflammatory mediators by activated microglial cells (Liu et al., 2000; Chang et al., 2000). Furthermore, very recent studies find that the drug inhibits activity of the microglial enzyme, NADPH oxidase, known to induce oxidative tissue damage in models of injury or infection (Qin et al., 2004; Qin et al., 2005). Such a mechanism of action correlates with our preliminary findings showing that mice deficient in gp91phox, a critical component of the microglial NADPH oxidase enzyme complex, also do not develop paralysis following NSV challenge (N. Prow and D. Irani, unpublished observations). Inhibition of microglial NADPH oxidase in our model may reduce oxidative damage, microglial activation, and/or the production of pro-inflammatory cytokines such as IL-1β, leading to preservation of spinal MNs and effect the development of paralysis. Some of our current studies are focused on determining how NAL influences the activity of microglial NADPH oxidase, how the enzyme contributes to the pathogenesis of NSV in the SC, and ultimately whether this enzyme is a good therapeutic target in this disease.
A study by Liang, et al., was one of the first to implicate innate immune responses, and in particular a role for the inflammatory cytokine, IL-1β, in NSV pathogenesis (Liang et al., 1999). Although these investigators were not able to show that IL-1β-deficient mice had reduced levels of virus-induced neuronal cell death compared to strain-matched controls, they still concluded that endogenous IL-1β plays a major pathophysiological role in disease given the strong resistance of IL-1β deficient animals to both paralysis and death (Liang et al., 1999). In the context of our data showing that NAL reduced the local induction of IL-1β in the SC (Fig. 6A), we propose that IL-1β does not influence the cellular injury and death caused by direct viral infection of neurons (apoptotic), but that it is a central mediator of the indirect, bystander neuronal injury arising from activated microglial cells (non-apoptotic). Indeed, we have shown that much of the MN injury in the SC of NSV-infected animals is not apoptotic in nature (Havert et al., 2000; Kerr et al., 2002), and that it occurs through a non cell-autonomous mechanism involving glutamate-medicated excitotoxicity (Darman et al., 2004; Nargi-Aizenman et al., 2004). Furthermore, we propose that IL-1β is most likely produced by activated SC microglial cells in the NSV model since peak levels are detected after microglial activation (Fig. 4), but before peak astrocyte activation which occurs on day 5 post-infection (N. Prow and D. Irani, unpublished observations). We also have found that another unrelated drug (minocycline) known to inhibit microglial activation can block NSV-induced paralysis through actions upstream of this bystander injury cascade (Darman et al., 2004). The results presented here reinforce the importance of IL-1β as one means to activate this form of neuronal injury in the SC during experimental alphavirus encephalomyelitis. Thus, like NADPH oxidase, inflammatory mediators such as IL-1β become logical therapeutic targets in these infections.
Another pro-inflammatory cytokine, TNF-α, is known to be released from activated microglial cells (Bronstein et al., 1995) and is induced in CNS tissues following NSV infection (Wesselingh et al., 1994). Unlike IL-1β, however, we find that NAL treatment actually augments TNF-α levels induced in the SC with infection (Figure 6B). The significance of this data is not clearly understood; it could be that drug-induced induction of TNF-α somehow contributes to its neuroprotective effect, or that local changes in levels of this mediator are simply unrelated to disease outcome. In either case, it is possible to conclude that NAL exerts a selective immunoregulatory effect in the SC during NSV infection rather than non-specific inhibition of multiple inflammatory mediators.
Finally, regarding the downstream effector pathways of neuronal injury in the SC, several recent studies in the NSV model have shown that glutamate-mediated excitotoxicity is a central pathogenic event (Darman et al., 2004; Nargi-Aizenman et al., 2004). Thus, drugs that block the AMPA subtype of glutamate receptors prevent NSV-induced paralysis and MN destruction without having any inhibitory effect on virus replication in the SC (Nargi-Aizenman et al., 2004). Furthermore, expression of the main astrocyte glutamate reuptake protein, GLT-1, decreases in the SC of NSV-infected animals (Fig. 7), thereby predisposing local tissues to higher local extracellular glutamate levels and subsequent excitotoxic neuronal injury (Darman et al., 2004). Other groups have identified a similar loss of glutamate transporter expression in active multiple sclerosis (MS) lesions, a finding that correlates with local oligodendrocyte and axonal damage (Werner et al., 2001). Loss of glutamate transporters directly within inflammatory lesions implies a role for host responses in this process, but little is known about the mediators involved. Here, we show that NAL blunts the induction of pathogenic IL-1β in the SC just prior to the onset of paralysis (Fig. 6), and importantly, that IL-1β is a critical mediator of the NSV-induced loss of SC GLT-1 expression that leads to MN injury and paralysis (Fig. 7). To our knowledge, this is one of the clearest demonstrations that a specific inflammatory mediator is directly involved in disrupted glutamate homeostasis via an effect on glutamate transport. Furthermore, we show that NAL actually increases SC GLT-1 expression levels above pre-infection baseline (Fig. 7B). Thus, it may be that the drug has other actions aside from its effects on IL-1β that govern GLT-1 expression. Interestingly, several antimicrobial agents, including the β-lactams, have recently been shown to protect spinal MN by increasing glutamate transporter expression via gene activation (Rothstein et al., 2005).
In conclusion, we find that NAL confers potent protection against paralysis in a murine model of mosquito-borne viral encephalomyelitis, even when started in a defined window after viral challenge and despite having no effect on SC viral replication or spread. Further studies demonstrate the drug prevents virus-induced microglial activation, and that the diminished production of the pro-inflammatory mediator, IL-1β, appears to be a central mechanism underlying its protective effect. We propose drugs such as NAL that target these detrimental host responses arising from activated microglia could be of therapeutic benefit in human cases of encephalitis caused by other mosquito-borne viruses. We also suggest these host responses activate a cascade of neurodegenerative events that clarifies the pathogenesis of these life-threatening infections.
Acknowledgements
This work was supported by grants from the Charles A. Dana Foundation (D. I.) and from the NIH (AI057505, D.I.). The authors acknowledge Dr. Diane Griffin for her helpful scientific input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acarin L, Vela JM, Gonzalez B, Castellano B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J Histochem Cytochem. 1994;42:1033–1041. doi: 10.1177/42.8.8027523. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS, McMillian MK. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res. 1995;704:112–116. doi: 10.1016/0006-8993(95)01189-7. [DOI] [PubMed] [Google Scholar]
- Bowen GS, Calisher CH. Virological and serological studies of Venezuelan Equine Encephalomyelitis in humans. J Clin Microbiol. 1976;4(1):22–27. doi: 10.1128/jcm.4.1.22-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RC, Rota C, Glover RE, Mason RP, Hong JS. A novel effect of an opioid receptor antagonist, naloxone, on the production of reactive oxygen species by microglia: a study by electron paramagnetic resonance spectroscopy. Brain Res. 2000;854:224–229. doi: 10.1016/s0006-8993(99)02267-2. [DOI] [PubMed] [Google Scholar]
- Darman J, Backovic S, Dike S, Maragakis NJ, Krishnan C, Rothstein JD, Irani DN, Kerr DA. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J Neurosci. 2004;24:7566–7575. doi: 10.1523/JNEUROSCI.2002-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Jacobs TP, Holaday JW. Opiate antagonist improves neurologic recovery after spinal injury. Science. 1981;211:493–494. doi: 10.1126/science.7455690. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;49:569–575. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL. Microglia in HIV-associated neurological diseases. Microsc Res Tech. 2001;54:95–105. doi: 10.1002/jemt.1124. [DOI] [PubMed] [Google Scholar]
- Granwehr BP, Lillibridge KM, Higgs S, Mason PW, Aronson JF, Campbell GA, Barrett AD. West Nile virus: where are we now? Lancet Infect Dis. 2004;4:547–56. doi: 10.1016/S1473-3099(04)01128-4. [DOI] [PubMed] [Google Scholar]
- Havert MB, Schofield B, Griffin DE, Irani DN. Activation of divergent neuronal cell death pathways in different target cell populations during neuroadapted Sindbis virus infection of mice. J Virol. 2000;74:5352–5356. doi: 10.1128/jvi.74.11.5352-5356.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi Y, Baskin DS, Woo SK. Reversal of induced ischemic neurologic deficit in gerbils by the opiate antagonist naloxone. Science. 1982;215:69–71. doi: 10.1126/science.6274019. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa J, Jacobson AE, Brossi A, Rice KC. Studies in the (+)-morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J Med Chem. 1978;21:398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Moench TR, Griffin DE, Johnson RT. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Invest. 1987;56:418–423. [PubMed] [Google Scholar]
- Jackson AC, Moench TR, Trapp BD, Griffin DE. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Invest. 1988;58:503–509. [PubMed] [Google Scholar]
- Johnson RT, McFarland HF, Levy SE. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972;125:257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- Kelley TW, Prayson RA, Isada CM. Spinal cord disease in West Nile virus infection. N Engl J Med. 2003;348:564–566. doi: 10.1056/NEJM200302063480618. [DOI] [PubMed] [Google Scholar]
- Kerr DA, Larsen T, Cook SH, Fannjiang YR, Choi E, Griffin DE, Hardwick JM, Irani DN. BCL-2 and BAX protect adult mice from lethal Sindbis virus infection but do not protect spinal cord motor neurons or prevent paralysis. J Virol. 2002;76:10393–10400. doi: 10.1128/JVI.76.20.10393-10400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Griffin DE. The role of CD8(+) T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J Virol. 2000;74:6117–6125. doi: 10.1128/jvi.74.13.6117-6125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Griffin DE. Extensive immune-mediated hippocampal damage in mice surviving infection with neuroadapted Sindbis virus. Virology. 2003;311:28–39. doi: 10.1016/s0042-6822(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lewis J, Wesselingh SL, Griffin DE, Hardwick JM. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Goldman JE, Jiang HH, Levine B. Resistance of interleukin-1beta-deficient mice to fatal Sindbis virus encephalitis. J Virol. 1999;73:2563–2567. doi: 10.1128/jvi.73.3.2563-2567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SL, Chen WY, Raung SL, Chen CJ. Neuroprotection of naloxone against ischemic injury in rats: role of mu receptor antagonism. Neurosci Lett. 2003;345:169–172. doi: 10.1016/s0304-3940(03)00540-8. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–617. [PubMed] [Google Scholar]
- Liu B, Hong JS. Neuroprotective effect of naloxone in inflammation-mediated dopaminergic neurodegeneration. Dissociation from the involvement of opioid receptors. Methods Mol Med. 2003;79:43–54. doi: 10.1385/1-59259-358-5:43. [DOI] [PubMed] [Google Scholar]
- Liu B, Jiang JW, Wilson BC, Du L, Yang SN, Wang JY, Wu GC, Cao XD, Hong JS. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J Pharmacol Exp Ther. 2000;295:125–132. [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1-42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002;302:1212–1219. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Lu X, Bing G, Hagg T. Naloxone prevents microglia-induced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience. 2000;97:285–291. doi: 10.1016/s0306-4522(00)00033-6. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Ricevuti G, Mazzone A, Pasotti D, Lecchini S, Frigo GM. A stereoselective blockade by naloxone of opioid and non-opioid-induced granulocyte activation. Int J Immunopharmacol. 1989;11:57–61. doi: 10.1016/0192-0561(89)90099-4. [DOI] [PubMed] [Google Scholar]
- Marques CP, Hu S, Sheng W, Lokensgard JR. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006;121(1):1–10. doi: 10.1016/j.virusres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nargi-Aizenman JL, Griffin DE. Sindbis virus-induced neuronal death is both necrotic and apoptotic and is ameliorated by N-methyl-D-aspartate receptor antagonists. J Virol. 2001;75:7114–7121. doi: 10.1128/JVI.75.15.7114-7121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargi-Aizenman JL, Havert MB, Zhang M, Irani DN, Rothstein JD, Griffin DE. Glutamate receptor antagonists protect from virus-induced neural degeneration. Ann Neurol. 2004;55:541–549. doi: 10.1002/ana.20033. [DOI] [PubMed] [Google Scholar]
- Perry VH, Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J. 2005;19:550–557. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen MA, Levey I, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Isobe KI, Miyaishi O, Sawada M, Fan ZH, Nakashima I, Kiuchi K. Microglial NO induces delayed neuronal death following acute injury in the striatum. Eur J Neurosci. 1998;10:1613–1620. doi: 10.1046/j.1460-9568.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal death and enhances axonal regeneration in vivo and in vitro. J. Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Stoll G, Griffin DE. The characterization of Ia expression during Sindbis virus encephalitis in normal and athymic nude mice. J Neuropathol Exp Neurol. 1990;49:21–30. doi: 10.1097/00005072-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Levine B, Fox RJ, Choi S, Griffin DE. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggest a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- Wesselingh SL, Thompson KA. Immunopathogenesis of HIV-associated dementia. Curr Opin Neurol. 2001;14:375–379. doi: 10.1097/00019052-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15,769–15,774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]