Abstract
While certain markers of inflammation and hemostasis are elevated in persons at risk for future cardiovascular events, data assessing the relation between inflammatory and hemostatic markers of vascular risk and race/ethnicity are limited. Thus, in a cross sectional analysis of the Women’s Health Study (WHS), baseline soluble intercellular adhesion molecule-1 (ICAM-1), homocysteine and fibrinogen levels were measured among 23,687 women without a history of cardiovascular disease (CVD). Among 22,677 white, 242 Hispanic, 428 black and 340 Asian women, the distribution of median ICAM-1 levels was significantly lower among black (311.9 ng/ml, interquartile range (IQR) 220.1 – 380.0) and Asian (312.7 ng/ml, IQR 267.3 – 362.3) women than among white (343.1, IQR 301.9 – 394.9) and Hispanic (351.9 ng/ml, IQR 305.9 – 404.2) women (p value < 0.001). While homocysteine levels were marginally lower among Asian women (p=0.05), fibrinogen concentrations were higher among black women than their counterparts. After control for body mass index (BMI), hypertension, diabetes, smoking, alcohol use, family history of myocardial infarction, education, hormone use and lipids, ICAM-1 concentration remained significantly lower among black and Asian women. Meanwhile, homocysteine levels were lower in Asian women and fibrinogen levels remained higher in black women than their counterparts. In conclusion, this cross-sectional analysis revealed that baseline fibrinogen, ICAM-1 and homocysteine levels vary by self-reported race/ethnicity.
Keywords: cellular adhesion molecule, homocysteine, fibrinogen, race, ethnicity
Much of the data related to the distribution and utility of novel markers of vascular risk were obtained in primarily white populations and data from non-white populations remain limited. In this regard, we have previously shown within the Women’s Health Study (WHS) that C-reactive protein (CRP) levels are higher among African American women than other self-described race/ethnic groups despite adjustment for important confounders of CRP levels, data corroborated in the Dallas Heart Study (1–2). These data may have clinical relevance because while the self-designation of race/ethnicity is not a proxy for genetic heritability, data indicate that in the USA, race/ethnic self-designation tracks with cardiovascular outcome. Since hormone replacement therapy (HRT) is known to affect cardiovascular disease (CVD) risk, also of interest is the effect of HRT on the levels of novel CVD risk factors. Consequently, in this analysis we sought to examine the distribution of ICAM-1, fibrinogen and homocysteine concentrations by self-reported race/ethnicity among women of different race/ethnic groups participating in the WHS.
METHODS
Baseline blood samples were collected from 28, 345 initially apparently healthy postmenopausal women participating in the WHS (3), a randomized, placebo controlled, double-blinded trial of aspirin and vitamin E in the primary prevention of cancer and cardiovascular disease. Data for age, weight, height, blood pressure, smoking status, diabetic status, estrogen use, alcohol consumption, exercise frequency, family history of myocardial infarction, education, self-identified race/ethnicity were available for 23, 687 participants and form the basis of this analysis. Race/ethnicity, height and weight were self-reported. Race/ethnicity was reported in one of six categories (white, Hispanic, black, Asian/Pacific Islander, American Indian/Alaskan native, other) but only women who identified themselves as white, Hispanic, black or Asian/Pacific Islander (hereafter referred to as Asian) will be included in this analysis since the sample size of the other two categories were small.
Baseline blood samples were assayed for ICAM-1, fibrinogen, homocysteine and lipid levels. ICAM-1 concentrations were determined using an ELISA assay (R & D Systems, Minneapolis MN). Fibrinogen concentration was determined using a Roche Diagnostics (Indianapolis, IN) immunoturbidimetric assay utilizing reagents and calibrators from Kamiya Biomedical Company (Seattle, WA), and homocysteine was measured using an enzymatic assay with reagents and calibrators from Catch Inc. (Seattle, WA). Lipid levels were measured in a certified Centers for Disease Control and Prevention standardized laboratory.
The significance of any differences in means or proportions of baseline cardiovascular risk factors was assessed by analysis of variance or chi square statistic respectively. Median ICAM-1, fibrinogen and homocysteine levels are reported for each race/ethnic group and the Kruskall-Wallis test evaluated any difference in their individual distributions. Since the distributions of ICAM-1, fibrinogen and homocysteine levels are skewed, log-transformed levels were used in regression analyses. We have also performed one way ANOVA followed by the student newman keuls test for multiple comparisons (p =0.05 level) utilizing log normalized levels of the different biomarkers.
Multivariate linear regression analyses were used to evaluate the relation between log-normalized levels of ICAM-1, fibrinogen, homocysteine and race/ethnic group. We first assessed the age-adjusted relationship between race/ethnic categories and log-normalized levels of ICAM-1, fibrinogen and homocysteine separately. Thereafter, we evaluated the individual contribution of various CVD risk factors to the plasma concentration of each vascular marker by constructing additional models that independently predicted log-ICAM-1/fibrinogen/homocysteine. These models included age, the potential confounder of interest and indicator variables for the different race/ethnic groups. White women served as the reference category. Fully-adjusted models included age, smoking status, diabetic status, hypertension history, exercise, HRT use, history of myocardial infarction in the mother/father < 60 years old, education level, lipid parameters (low density lipoprotein (LDL-C), high density lipoprotein (HDL-C)), body mass index and race/ethnic category.
RESULTS
There were 22, 677 white, 242 Hispanic, 428 black and 340 Asian participants. Overall, the mean age of the 23, 687 women at baseline was 54.2 ± 7.1 years. In general, Asian women were younger, had the lowest body mass index, were less likely to be smokers and to use alcohol than their counterparts (Table 1). Black women were more likely to have a history of hypertension, diabetes mellitus and had the highest body mass index compared to the women of other race/ethnic categories. The distribution of total cholesterol (p=0.6) and LDL-C (p=0.2) levels were not significantly different between women of the various race/ethnic groups. However, Hispanic women were more likely to have lower HDL-C than other women.
Table 1.
Baseline Characteristics According to Race/Ethnic Group
| Variable | White (N=22, 677) | Hispanic (N= 242) | Black (N= 428) | Asian (N= 340) | Overall p value |
|---|---|---|---|---|---|
| Age (years) | 54.2 ± 7.1 | 53.5 ± 6.5 | 53.5 ± 6.1 | 52.9 ± 5.5 | <0.001 |
| Smokers | |||||
| Never | 51.3% | 65.3% | 50.9% | 84.4% | |
| Past | 37.3% | 24.4% | 32.7% | 12.4% | |
| Current | 11.3% | 10.3% | 16.4% | 3.2% | <0.001 |
| Body Mass Index (kg/m2) | 25.9 ± 4.9 | 26.1 ± 4.9 | 28.6 ± 5.6 | 23.4 ± 3.3 | <0.001 |
| Hypertension | 24.2% | 24.0% | 44.9% | 25.3% | <0.001 |
| Diabetes | 2.6% | 5.8% | 9.1% | 3.2% | <0.001 |
| Family History of Myocardial Infraction | 13.0% | 7.9% | 10.1% | 7.4% | <0.001 |
| Hormone Replacement Therapy | |||||
| Never | 46.1% | 47.9% | 51.2% | 52.1% | |
| Past | 9.9% | 12.0% | 10.7% | 9.4% | |
| Current | 43.9% | 40.1% | 38.1% | 38.5% | 0.05 |
| Alcohol use | |||||
| Rare/never | 43.1% | 51.7% | 56.3% | 74.4% | |
| 1–3/month | 13.3% | 12.0% | 17.0% | 7.4% | |
| 1–6/week | 32.8% | 29.8% | 22.0% | 14.7% | |
| 1+/day | 10.8% | 6.6% | 4.7% | 3.5% | <0.001 |
| Exercise | |||||
| Rare/never | 37.0% | 45.9% | 36.5% | 40.0% | |
| <1 X/week | 19.5% | 15.3% | 19.9% | 16.5% | |
| 1–3 X/week | 32.0% | 28.9% | 34.4% | 32.1% | |
| 4 X/week | 11.4% | 9.9% | 9.4% | 11.5% | 0.2 |
| Cholesterol (mg/dL) | |||||
| Total | 211.3 ± 41.6 | 212.0 ± 42.6 | 210.6 ± 44.4 | 208.3 ± 35.1 | 0.6 |
| Low Density Lipoprotein | 123.7 ± 34.1 | 125.2 ± 33.7 | 126.3 ± 39.3 | 121.4 ± 29.7 | 0.2 |
| High Density Lipoprotein | 53.9 ± 15.0 | 50.9 ± 14.8 | 53.9 ± 15.7 | 54.7 ± 14.2 | 0.01 |
| Apolipoprotein B/A1 Ratio | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.2 | <0.001 |
Values are represented as means ± SD;
Total= Total cholesterol; Apolipoprotein B/A1 ratio= Apolipoprotein B to apolipoprotein A1 ratio
Figure 1 shows the median ICAM-1 levels by race/ethnic group. Overall, ICAM-1 levels were lower among black (311.9 ng/ml, IQR 220.1, 380.0) and Asian (312.7 ng/ml, IQR 267.3, 362.3) women than among white (343.1 ng/ml, IQR 301.9, 394.9) and Hispanic (351.9 ng/ml, IQR 305.9, 404.2) women [p overall < 0.001]. In fully adjusted multivariate regression analyses, ICAM-1 levels among black and Asian women remained 20.2% and 8.8% lower respectively than the ICAM-1 levels of their white counterparts. As expected, multiple comparison testing for differences in ICAM-1 levels between the various race/ethnic groups indicate that the ICAM-1 levels of black and Asian women were significantly different from each other and from those of white and Hispanic women at the 0.05 significance level. There was no significant difference between baseline ICAM-1 levels of white and Hispanic women (Table 2).
Figure 1a–c.
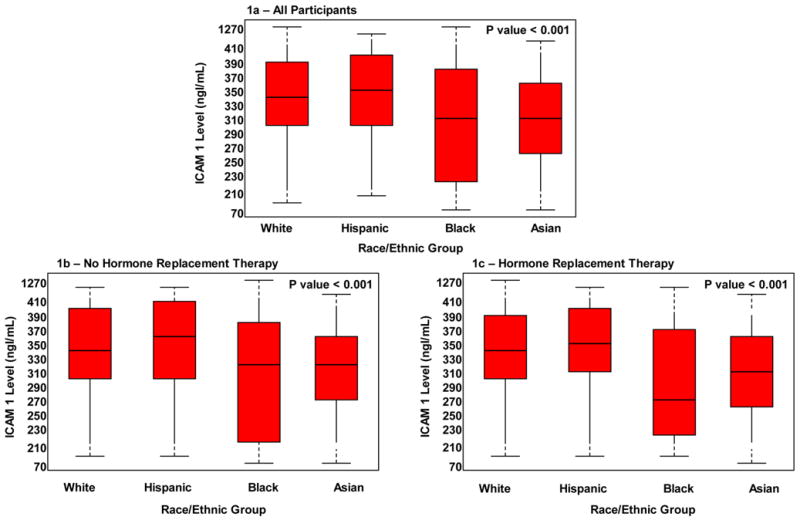
Median Levels of soluble-Intercellular Adhesion Molecule-1 According to Race/Ethnic Group
Table 2.
Percent Differences and Associated 95% Confidence Intervals for the Various Biomarkers According to Race/Ethnic Group: All Participants
| Biomarker | Model | Hispanic (n=242) | Black (n=428) | Asian (n=340) |
|---|---|---|---|---|
| Log soluble- Intercellular Adhesion Molecule-1 | Unadjusted | 1.2% | −16.2% | −13.1% |
| P value | 0.4 | <0.001 | <0.001 | |
| Adjusted for Age | 1.5% | −15.9% | −12.6% | |
| P value | 0.3 | <0.001 | <0.001 | |
| Fully Adjusted* | 0.4% | −20.2% | −8.8% | |
| P value | 0.8 | <0.001 | <0.001 | |
|
| ||||
| Log Homocysteine | Unadjusted | 1.6% | 1.0% | −7.5% |
| P value | 0.4 | 0.5 | <0.001 | |
| Adjusted for Age | 1.9% | 1.3% | −7.2% | |
| P value | 0.4 | 0.4 | <0.001 | |
| Fully Adjusted* | 2.3% | −0.5% | −3.4% | |
| P value | 0.3 | 0.7 | 0.05 | |
|
| ||||
| Log Fibrinogen | Unadjusted | 3.3% | 12.1% | −1.6% |
| P value | 0.02 | <0.001 | 0.2 | |
| Adjusted for Age | 3.7% | 12.5% | −0.9% | |
| P value | 0.01 | <0.001 | 0.5 | |
| Fully Adjusted* | 2.3% | 7.7% | 1.0% | |
| P value | 0.09 | <0.001 | 0.4 | |
Includes age, body mass index, physical activity, history of hypertension, smoking status (coded as past smoking and current smoking), diabetic status, alcohol use, family history of MI, estrogen use (coded as past and current hormone use), education level (years), exercise, LDL-C, HDL-C Referent population – White (n=22,677)
Among Asian women, alcohol use (−13.1%, p <0.001) and among black women, body mass index (−17.3%; p <0.001) resulted in the largest attenuations in ICAM-1 concentrations. Overall, black race and a history of current smoking were the strongest determinants of baseline ICAM-1 l concentrations. There were no significant differences in median ICAM-1 levels between HRT users (341.3 ng/ml, 300.8, 391.1) and non-users (343.4 ng/ml, IQR 300.8, 396.5) [p = 0.09].
Figure 2 shows the distribution of homocysteine levels by race/ethnicity. Homocysteine levels were lower among Asian women than those of black, white and Hispanic women (Table 2). However, median homocysteine levels were higher among women not taking HRT (HRT: 10.2 umol/L, IQR 8.5, 12.3 versus no HRT: 10.7 umol/L, IQR 8.8, 13,2; p <0.001).
Figure 2a–c.
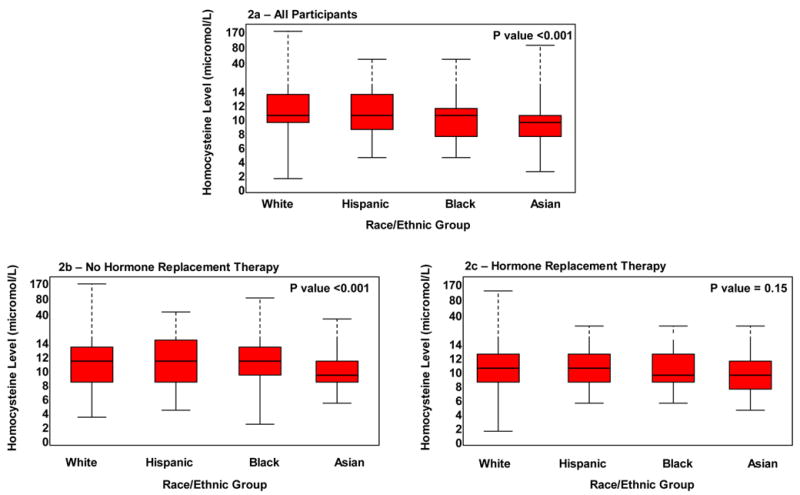
Median Levels of Homocysteine According to Race/Ethnic Group
Black women were noted to have the highest fibrinogen levels (395.7 mg/dL, IQR 341.4, 454.5) [Figure 3]. Fibrinogen levels of Asian and white women were similar, whereas those of black and Hispanic women were different from each other and from the fibrinogen levels of white and Asian women (p = 0.05). Similar to the case for ICAM-1, multivariate models demonstrated that black race and current smoking were the strongest determinants of fibrinogen levels. Finally, fibrinogen levels were higher among non-HRT users compared to HRT users (HRT: 341.5 mg/dL, IQR 301.5, 388.1 versus no HRT: 358.2 mg/dL, IQR 312.0, 412.6; p <0.001).
Figure 3a–c.
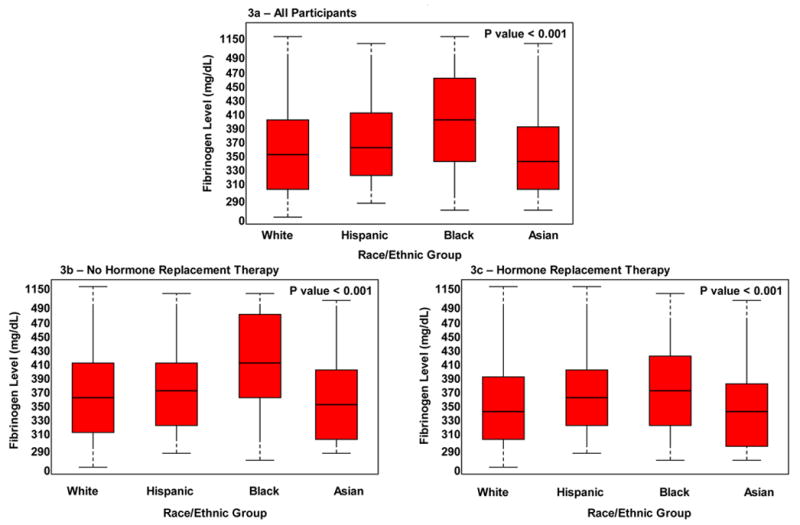
Median Levels of Fibrinogen According to Race/Ethnic Group
DISCUSSION
These cross-sectional data demonstrate that baseline plasma concentrations of ICAM-1, fibrinogen and homocysteine vary by self reported race/ethnicity. Prior data examining ICAM-1 levels among different race/ethnic groups are scant. Our results support European data from Miller et al (4) who demonstrated that Afro-Caribbean and West Africans living in England had significantly lower ICAM-1 levels than whites or South Asians. Likewise, results from a case control evaluation among Atherosclerosis Risk in Communities Study (ARIC) participants that assessed the relationship between ICAM-1 levels and CVD indicate that among controls, ICAM-1 levels were also lower in blacks than in whites (5). The lower ICAM-1 levels noted in both European and American blacks have inconsistent relations with CVD risk for these groups in the respective countries. For example, in England CVD risk is lower among blacks than whites, while in the US, CVD risk is higher among blacks compared to whites. This observed dichotomy between ICAM-1 levels among blacks and differing CVD population risk suggests that intrinsic biology likely heavily dictates the observed differences in ICAM-1 levels by race/ethnic identification.
Our finding of higher fibrinogen levels among black women compared to women in the other race/ethnic groups concurs with published data related to fibrinogen in this regard. Previous studies indicate that fibrinogen levels are higher among blacks than whites and Asian sub-populations. For example, research from the Coronary Artery Risk Development in Young Adults (CARDIA), Cardiovascular Health Study (CHS), and ARIC all demonstrate that fibrinogen levels were significantly higher among blacks and women than among whites and men (6–8). By contrast, among Afro-Caribbean and West-African blacks residing in London, fibrinogen levels were noted to be lower than those of whites and South Asians (9). It is plausible that both environmental and genetic factors influence fibrinogen levels, although the degree of contribution of each factor is unknown. Of interest is the finding of a low prevalence of the C148 → T allele in the black population in England, a polymorphism that is associated with higher fibrinogen levels. Meanwhile, among various Asian groups, fibrinogen levels seem to differ. Japanese men appear to have lower fibrinogen levels than Americans (10), a finding that correlates with lower rates of coronary heart disease mortality observed in this population. Still, in South Asian populations, studies related to baseline fibrinogen levels are conflicting. Whereas some research show fibrinogen levels to be higher among South Asians than in whites, particularly among Hindus, a finding that might be in part be related to the prevalence of the metabolic syndrome and vegetarianism in this population (11–12). Other data show that fibrinogen levels are similar among South Asians and whites (13–14).
These data demonstrate that homocysteine levels were lower among Asian women than among women who belonged to the other race/ethnic groups. In data obtained prior to folate supplementation from the National Health and Nutrition Examination Survey III, whites were noted to have the highest homocysteine levels, followed by Mexican Americans and blacks, a finding that might be due to the higher prevalence of the MTHFR 677C → T point mutation that causes decreased activity of the noted enzyme among whites (15). However, both pre and post folate fortification data from the Northern Manhattan Study show that while blacks had the highest homocysteine levels followed by whites and Hispanics, only among whites and Hispanics were these levels significantly predictive of vascular outcomes and stroke (16). In contrast to our finding, research related to homocysteine levels among South Asians residing in England as well as in Canada show that they have higher homocysteine levels than their white and black counterparts (17–18). As was the case for fibrinogen levels, Hindu ethnicity and vegetarianism heavily influenced the higher homocysteine levels observed in these populations. In this study, we are unable to assess within ethnic group heterogeneity.
This study also adds information regarding the effect of HRT on ICAM-1, fibrinogen and homocysteine levels. Several reports demonstrate that oral HRT decreases ICAM-1 plasma concentrations. However, in this large cohort of apparently healthy women, there were no significant differences in ICAM-1 concentrations based on HRT status. One limitation of published reports that might have affected results is the short follow-up time related to measurement of ICAM-1 levels in relation to HRT use. HRT resulted in statistically significant decreases in homocysteine and fibrinogen levels among participants, a finding that is consistent with most published reports related to oral HRT use (19–21). Of note, transdermal HRT does not appear to significantly affect fibrinogen levels (19, 22), whereas raloxifene seems to lower fibrinogen levels (20, 23). Finally, a majority of reports regarding the effect of HRT on homocysteine concentrations indicate that both oral HRT and raloxifene decrease homocysteine levels (24–25).
Footnotes
Grant Support:
Dr. Albert is supported by an award from the Robert Wood Johnson Foundation, New Jersey. Both Drs. Albert and Ridker are supported by a grant from the Donald W. Reynolds Foundation, Las Vegas NV.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albert MA, Glynn RJ, Buring J, Ridker P. C-reactive protein levels among women of various race/ethnic groups living in the United States. Am J Cardiol. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 2.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–3004. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 4.Miller MA, Giuseppe A, Kerry SM, Strazzullo P, Cook DG, Cappuccio FP. Ethnic differences in circulating soluble adhesion molecules: the Wandsworth Heart and Stroke Study. Clin Sci. 2003;104:591–598. doi: 10.1042/CS20020333. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1 and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 6.Green D, Rulth KJ, Folsom AR, Liu K. Hemostatic factors in the Coronary Artery Risk Development In Young Adults (CARDIA) Study. Arterioscler Thromb. 1994;14:686–693. doi: 10.1161/01.atv.14.5.686. [DOI] [PubMed] [Google Scholar]
- 7.Tracy RP, Bovill EG, Fried LP, Heiss G, Lee MH, Polak JF, Psaty BM, Savage PJ. The distribution of coagulation factors VII and VIII and fibrinogen in adults over 65 years. Results from the Cardiovascular Health Study. Ann Epidemiol. 1992;2:509–519. doi: 10.1016/1047-2797(92)90100-5. [DOI] [PubMed] [Google Scholar]
- 8.Nabulsi AA, Folsom AR, Heiss G, Weir SS, Chambless LE, Watson RL, Eckfeldt JH. Fasting hyperinsulinemia and cardiovascular disease risk factors in non-diabetic adults: stronger associations in lean versus obese subjects. Atheroslerosis Risk in Communities Study Investigators. Metabolism. 1995;44:914–922. doi: 10.1016/0026-0495(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 9.Cook DG, Cappuccio FP, Atkinson RW, wicks PD, Chitolie A, Nakandakare ER, Sagnella GA, Humphries SE. Ethnic differences in fibrinogen levels: the role of environmental factors and the beta-fibrinogen gene. Am J Epidemiol. 2001;153:799–806. doi: 10.1093/aje/153.8.799. [DOI] [PubMed] [Google Scholar]
- 10.Iso H, Folsom AR, Sato S, Wu KK, Shimamoto T, Koike K, Iida M, Komachi Y. Plasma fibrinogen and its correlates in Japanese and US population samples. Arterioscler Thromb. 1993;13:783–790. doi: 10.1161/01.atv.13.6.783. [DOI] [PubMed] [Google Scholar]
- 11.Kain K, Blaxill JM, Catto AJ, Grant PJ, Carter AM. Increased fibrinogen levels among South Asians versus Whites in the United Kingdom are not explained by common polymorphisms. Am J Epidemiol. 2002;156:174–179. doi: 10.1093/aje/kwf017. [DOI] [PubMed] [Google Scholar]
- 12.Kain K, Catto AJ, Grant PJ. Impaired fibrinolysis and increased fibrinogen levels in South Asian subjects. Atherosclerosis. 2001;156:457–461. doi: 10.1016/s0021-9150(00)00684-5. [DOI] [PubMed] [Google Scholar]
- 13.McKeigue PM, Marmot MG, Syndercombe Court YD, Cottier DE, Rahman S, Riemersma RA. Diabetes, hyperinsulinemia, and coronary risk factors in Bangladeshis in east London. Br Heart J. 1988;60:390–396. doi: 10.1136/hrt.60.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischbacher C, Bopal R. Letter to the editior, re “Increased fibrinogen levels among South Asians versus Whites in the United Kingdom are not explained by common polymorphisms”. Am J Epidemiol. 2003;157:664–665. doi: 10.1093/aje/kwg032. [DOI] [PubMed] [Google Scholar]
- 15.Gangi V, Kafai MR. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2003;77:826–833. doi: 10.1093/ajcn/77.4.826. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 17.Cappuccio FP, Bell R, Perry IJ, Gilg J, Ueland PM, Refsum H, Sagnella GA, Jeffery S, Cook DG. Homocysteine levels in men and women of different ethnic and cultural background living in England. Atherosclerosis. 2002;164:95–102. doi: 10.1016/s0021-9150(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 18.Annand S, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 19.Tanner MZ, Ozpolat E, Taskiran C, Onan MA, Gursel T, Karabulut E, Gursoy R, Himmetoglu O. Effects of four different regiments of hormone replacement therapy on hemostatic parameters: a prospective randomized study. Maturitas. 2006;53:267–273. doi: 10.1016/j.maturitas.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Diaz AR, Jr, Melo RN, Gebara OC, D’Amico EA, Nussbacher A, Halbe HW, Pinotti JA. Effects of conjugated equine estrogens or raloxifene on lipid profile, coagulation and fibrinolysis factors in postmenopausal women. Climacteric. 2005;8:63–70. doi: 10.1080/13697130500042581. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Howard BV, Cowan LD, welty TK, Schaefer CF, Wild RA, Yeh J, Lee ET. Associations of postmenopausal hormone therapy with markers of hemostasis and inflammation and lipid profiles in diabetic and non-diabetic American Indian women: the Strong Heart Study. J Women Health. 2004;13:155–163. doi: 10.1089/154099904322966137. [DOI] [PubMed] [Google Scholar]
- 22.Yilmazer M, Fenkei J, Fenkci S, Sonmezer M, Aktepe O, Altindis M, Kurtay G. Hormone replacement therapy, C-reactive protein and fibrinogen in healthy post-menopausal women. Maturitas. 2003;46:245–253. doi: 10.1016/s0378-5122(03)00217-2. [DOI] [PubMed] [Google Scholar]
- 23.Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, Shah AS, Anderson PW. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- 24.Davison S, Davis SR. New markers for cardiovascular disease risk in women: Impact of endogenous estrogen status and exogenous postmenopausal hormone therapy. Clin Endo Metab. 2003;88:2470–2478. doi: 10.1210/jc.2002-021929. [DOI] [PubMed] [Google Scholar]
- 25.Gol M, Akan P, Dogan E, Karas C, Saygili U, Posaci C. Effects of estrogen, raloxifene and hormone replacement therapy on serum C-reactive protein and homocysteine levels. Maturitas. 2006;53:252–259. doi: 10.1016/j.maturitas.2005.05.006. [DOI] [PubMed] [Google Scholar]


