Abstract
Because of concerns about zoonotic transmission of monkeypox to humans and the bioterrorism threat posed by orthopoxviruses, there is renewed interest in probing cellular and molecular mechanisms of host defense to these pathogens. In particular, it is essential to understand viral–host interactions in the respiratory tract, which is the route of infection for smallpox and a likely route of transmission for monkeypox. In this study, we analyze functions of alveolar macrophages in poxvirus infection, using a recombinant vaccinia virus expressing firefly luciferase to quantify infection in mice and cell culture. Depletion of alveolar macrophages with liposomal clodronate worsens the overall severity of infection in mice, including greater replication and systemic dissemination of vaccinia as determined by bioluminescence imaging. Absence of alveolar macrophages increases total numbers of granulocytes and granulocytes/monocyte progenitor cells in the lungs during vaccinia infection, indicating that protective effects of alveolar macrophages may be mediated in part by reducing the host inflammation. Alveolar macrophages also limit vaccinia infection in respiratory epithelium, as shown by a co-culture model of cell lines derived from alveolar macrophages and lung epithelium. Collectively, these data demonstrate that alveolar macrophages are key determinants of host defense against local and systemic infection with poxviruses.
Keywords: Vaccinia, Macrophage, Imaging
The respiratory tract is a key route of spread for orthopoxviruses, including the viruses that cause smallpox and monkeypox (Fenner et al., 1988). In addition, the potential use of these viruses as agents of biological warfare raises the possibility for exposure to levels of aerosolized viruses that markedly exceed doses encountered during normal infection. Therefore, defining cellular and molecular determinants of the host response to poxviruses in the respiratory system is essential for understanding basic questions about host immunity to these pathogens and limiting the bioterrorism threat posed by poxviruses.
Host defense against poxviruses in the respiratory tract begins at the level of infected cells, which likely are epithelial cells. Infected cells initiate an intracellular anti-viral response to limit viral replication and secrete interferons and other anti-viral cytokines as part of the local innate immune response. Local immunity is amplified through macrophages and dendritic cells, which also migrate to draining lymph nodes to activate adaptive immunity. Ultimately, local innate immunity and systemic adaptive immunity combine to control acute infection and establish lasting immunity against subsequent poxvirus infection.
Alveolar macrophages are the predominant resident phagocytic cells in the lungs that respond to inhaled organisms in the respiratory tract, initiate local immune responses to pathogens, and maintain functional integrity of lung epithelium. Under basal conditions, alveolar macrophages appear to promote an immunosuppressive microenvironment in the lungs. These cells suppress activation of adaptive immunity by limiting access of antigens to dendritic cells and reducing functions of dendritic cells, T cells, and B cells (MacLean et al., 1996, Thepen et al., 1994). In response to bacterial pathogens, alveolar macrophages decrease the overall severity of disease, in part by reducing levels of pro-inflammatory cytokines and recruitment of leukocytes to the lungs (Broug-Holub et al., 1997, Knapp et al., 2003). However, functions of these cells in the context of viral infections remain poorly defined, particularly for respiratory infection with poxviruses.
We investigated alveolar macrophages in host defense to poxviruses, using a recombinant vaccinia virus that expresses firefly luciferase as a model for analyzing functions of these cells in local and systemic responses to infection. In mice, we found that depletion of alveolar macrophages increases local and systemic replication of vaccinia virus and overall severity of disease. Absence of alveolar macrophages increases recruitment of inflammatory cells to the lungs during vaccinia infection, which likely contributes to more severe disease in these animals. Alveolar macrophages also significantly limit infection of lung epithelial cells in an in vitro co-culture model system. Collectively, our results demonstrate functions of alveolar macrophages to limit viral replication and disease severity in response to infection with an orthopoxvirus.
Results
Depletion of alveolar macrophages with liposomal clodronate
Treatment of mice with liposomes containing the bisphosphonate compound clodronate is a well-established method for depleting alveolar macrophages, a cell population that comprises > 95% of cells in the normal airway. This technique does not damage respiratory epithelium or cause toxicity to neutrophils, another phagocytic cell type (van Rooijen, 1989) (Qian et al., 1994).
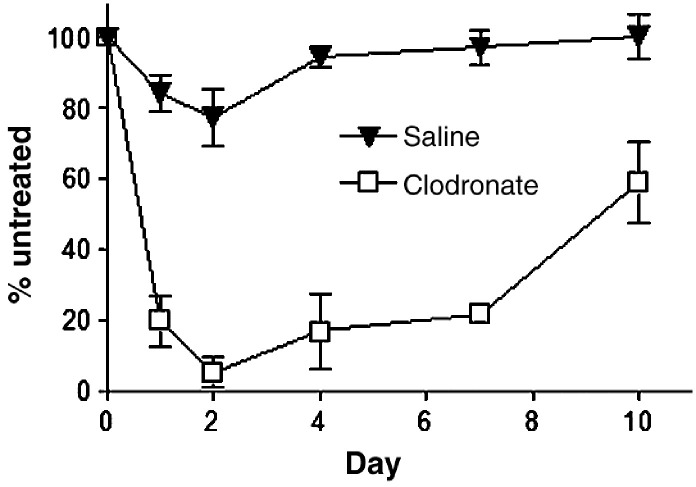
To establish the kinetics of macrophage depletion, we intratracheally injected 100 μl of liposomes containing clodronate or saline as a control (clodronate liposomes) into Sv129/Ev mice. Sv129/Ev mice have been used by our laboratory and others for studies of vaccinia pathogenesis (van den Broek et al., 1995) (Luker et al., 2005). Numbers of alveolar macrophages recovered by bronchoalveolar lavage were quantified at various times after treatment and compared with alveolar macrophages in untreated, strain-matched mice. In mice treated with clodronate liposomes, only ≈ 5% of alveolar macrophages remained on day 2, and numbers of alveolar macrophages were ≤ 25% of normal through 7 days (Fig. 1 ). By comparison, treatment with saline liposomes had only a minimal effect on numbers of alveolar macrophages. These data show that we are able to effectively deplete alveolar macrophages for most of the expected period of acute infection with vaccinia virus.
Fig. 1.
Depletion of alveolar macrophages with clodronate liposomes. 129 Ev/Sv mice were injected intratracheally with 100 μl of liposomes containing clodronate or saline as a control. Total numbers of cells recovered from bronchoalveolar lavage fluid were quantified at different time points after treatment (n = 3 per time point). Data are expressed as the percent of cells recovered relative to mice that did not receive either clodronate or saline liposomes (n = 3). Error bars represent SEM.
Some populations of dendritic cells may be depleted by clodronate liposomes (Leenen et al., 1998), although effects of this treatment on lung dendritic cells remain poorly defined. We quantified numbers of dendritic cells in lungs of mice treated with clodronate or control saline liposomes. Clodronate liposomes did not affect total numbers of CD11c(+), MHC class II(+) dendritic cells in the lung interstitium as compared with control liposomes. In both groups, dendritic cells comprised ≈ 0.8% of total cells in the lungs, which is consistent with our previous data from untreated mice. Because antigen presenting cells such as dendritic cells and macrophages migrate to regional lymph nodes to initiate adaptive immunity, we also quantified numbers of these cell types in the tracheobronchial lymph node of mice 2 days after injection of liposomes. There were no significant differences between clodronate and saline control mice in numbers or percentages of CD11c(+), MHC class II(+) dendritic cells, Mac-3(+) macrophages, or ERMP-20(+) late monocyte progenitors in these lymph nodes (data not shown). Furthermore, numbers or percentages of dendritic cells and macrophages in peripheral blood, as determined by these same cell surface markers, did not differ between mice 2 days following treatment with clodronate or saline liposomes (data not shown). Collectively, these data demonstrate that intratracheal administration of clodronate liposomes depleted only alveolar macrophages.
Alveolar macrophages limit vaccinia infection in vivo
We treated mice with intratracheal administration of clodronate liposomes to investigate effects of alveolar macrophages on host defense against respiratory infection with vaccinia virus. Mice were injected with clodronate liposomes and then infected with 4 × 105 pfu Vac-FL intranasally 2 days later at a time when alveolar macrophages are depleted maximally as shown above. Control mice received liposomes filled with saline 2 days prior to infection. Mice were inoculated with vaccinia intranasally to reproduce infection through the upper respiratory tract, the typical route of transmission for smallpox. In this and subsequent experiments, two mice each treated with clodronate or saline liposomes were euthanized at the time of infection with vaccinia to confirm effective depletion of alveolar macrophages relative to saline control (data not shown).
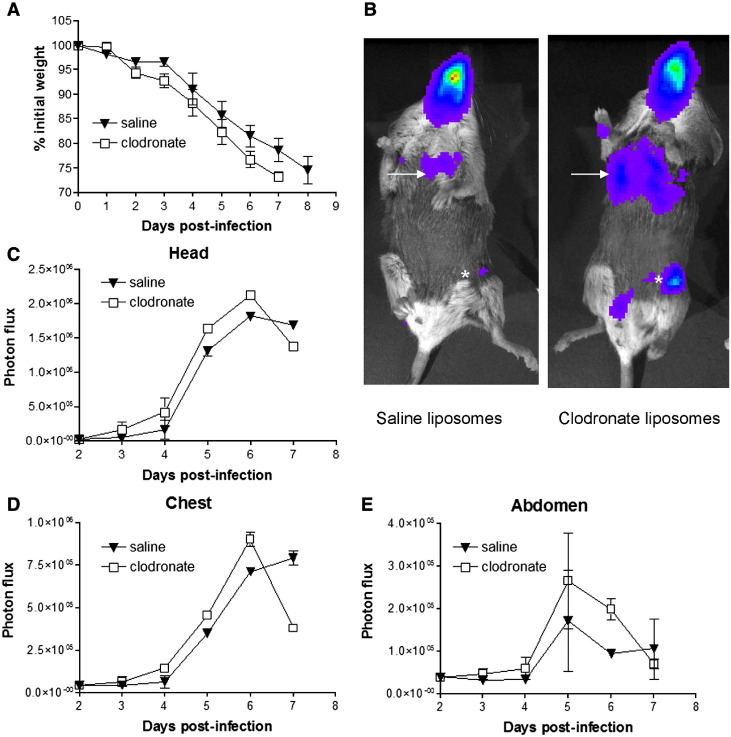
Depletion of alveolar macrophages increased the overall severity of vaccinia infection relative to saline controls, as evidenced by more pronounced weight loss over the course of infection (Fig. 2A). Differences in weight loss became apparent by day 3 after infection and persisted through day 7. Notably, mice treated with clodronate liposomes to deplete alveolar macrophages died on day 7 post-infection, while control animals survived through day 8 of acute infection.
Fig. 2.
Alveolar macrophages limit vaccinia virus infection. Mice were treated with intratracheal injection of clodronate or saline liposomes 2 days prior to infection with 4 × 105 pfu Vac-FL intranasally (n = 5 mice per group). (A) Mice were weighed daily to monitor overall progression of disease. Data are presented as mean ± SEM percent loss of initial weight. (B) Representative bioluminescent images on day 4 of Vac-FL infection in mice treated with clodronate or saline liposomes. Viral bioluminescence in the lungs (arrow) and spread to inguinal lymph node (asterisk) is greater in mice treated with clodronate liposomes to deplete alveolar macrophages. (C–E) Quantified photon flux data from the head (C), chest (D), and abdomen (E) regions-of-interest. Data are shown as mean values of background subtracted photon flux ± SEM.
We used bioluminescence imaging to quantify viral replication and dissemination over the course of infection. Mice treated with clodronate liposomes had higher levels of luciferase activity in the head, chest, and abdomen on days 4–6 post-infection than control mice (Figs. 2B–E). By area-under-curve (AUC) analysis of these days, total bioluminescence for each region of interest was significantly greater in mice treated with clodronate liposomes (Table 1 ) (p < 0.05). On day 7 after infection, amounts of luciferase activity at these various sites were lower in mice treated with clodronate liposomes compared with control animals. However, this decrease in bioluminescence may be due to poor perfusion of luciferin to sites of Vac-FL in these mice because of severe systemic illness in mice treated with clodronate liposomes rather than an actual decrease in vaccinia virus. Collectively, these data show that alveolar macrophages decrease the overall extent of vaccinia viral infection after respiratory inoculation.
Table 1.
Area-under-the-curve (AUC) photon flux days 4–6 from 4 × 105 pfu infection
| Liposomes | Head | Chest | Abdomen |
|---|---|---|---|
| Saline | 2.3 × 106 | 7.36 × 105 | 2.94 × 105 |
| Clodronate | 2.9 × 106 | 9.78 × 105 | 3.95 × 105 |
AUC values for mice treated with clodronate liposomes are significantly greater than those for control saline liposomes (p < 0.05) (n = 5 mice per group).
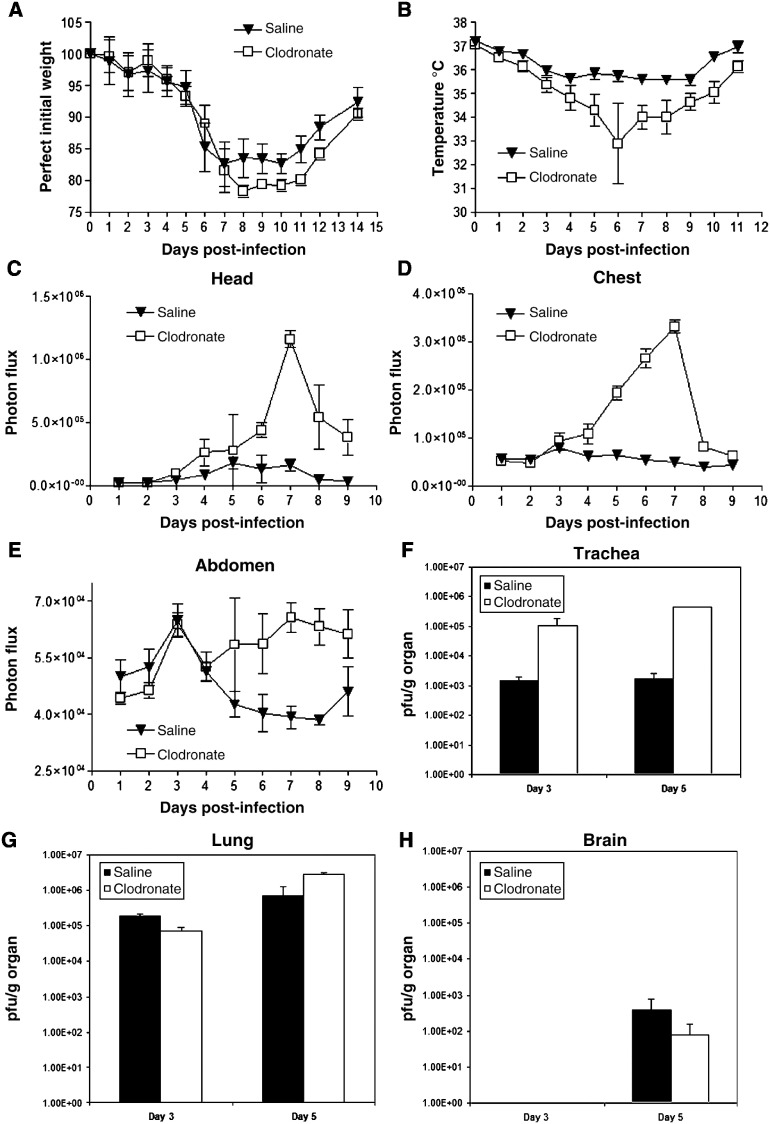
To build upon the data presented above with a lethal inoculum of vaccinia virus, we then investigated functions of alveolar macrophages in the context of a non-lethal infection. Mice were treated with clodronate or control saline liposomes and infected with 4 × 104 pfu Vac-FL 2 days later at a time coinciding with maximum depletion of alveolar macrophages by clodronate. Under these conditions, mice lost comparable amounts of weight until day 8 post-infection, when weight loss was greater in animals treated with clodronate liposomes. These differences in weight loss persisted through day 12 (Fig. 3A).
Fig. 3.
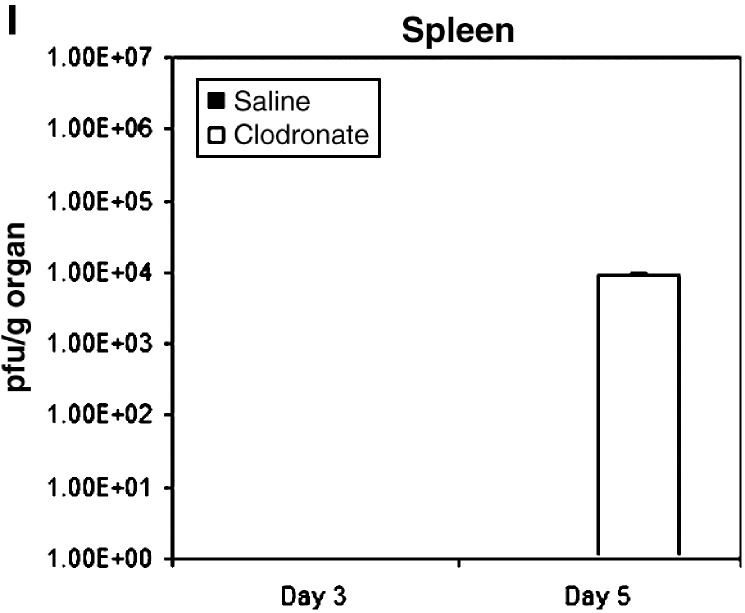
Protective effects of alveolar macrophages in sublethal vaccinia infection. Mice (n = 10) were injected intratracheally with clodronate or saline liposomes and then infected 2 days later with 4 × 104 pfu of Vac-FL i.n. (A) Weight and (B) rectal temperature were measured daily to monitor overall progression of disease. Data are mean values ± SEM for percent weight loss or temperature, respectively. (C–E) Photon flux from regions-of-interest defined for the head (C), chest (D), and abdomen (E) on bioluminescent images over the course of infection. Data are mean values of background subtracted photon flux ± SEM. (F–I) Viral titers were quantified on days 3 and 5 post-infection (n = 3 per group) in the trachea (F), lung (G), brain (H), and spleen (I). Data are presented as mean values for viral titers ± SEM. 30 pfu/g tissue is the lower limit of detection for viral titers.
In addition to weight, we measured rectal temperatures as another determinant of overall severity of disease produced by Vac-FL. Mice depleted of alveolar macrophages had a greater loss of body temperature over the course of infection, and differences between groups occurred earlier than we observed with body weight (Fig. 3B). By day 6, mean body temperatures in these mice had dropped by > 4 °C, while control animals lost < 2 °C. Collectively, these data demonstrate that alveolar macrophages reduce the overall severity of system disease produced by non-lethal infection with vaccinia virus.
Differences in viral replication as quantified by bioluminescence imaging and plaque assay corresponded with protective effects of alveolar macrophages in vaccinia infection. Luciferase activity produced by Vac-FL in the head, chest, and abdomen regions-of-interest was higher in mice treated with clodronate liposomes, with differences becoming apparent by days 4–5 post-infection (Figs. 3C–E). In the abdomen, images showed that Vac-FL localized predominantly to the spleen (data not shown). By AUC analysis of photon flux over days 4–9 post-infection, bioluminescence from Vac-FL was significantly greater in these three regions of interest for infection (Table 2 ) (p < 0.01).
Table 2.
AUC photon flux days 4–9 from 4 × 104 pfu infection
| Liposomes | Head | Chest | Abdomen |
|---|---|---|---|
| Saline | 5.82 × 105 | 2.61 × 105 | 2.09 × 105 |
| Clodronate | 2.74 × 106 | 9.59 × 105 | 3.03 × 105 |
AUC values for mice treated with clodronate liposomes are significantly greater than those for control saline liposomes (p < 0.01) (n = 7 mice per group through day 5, then n = 4 per group on days 6–9).
To confirm imaging data, we euthanized three mice from each cohort on days 3 and 5 post-infection to quantify amounts of virus in selected organs by plaque assay. Titers of Vac-FL in tracheas were approximately 2 logs greater in mice treated with clodronate liposomes compared with control animals on days 3 and 5 post-infection (Figs. 3F–I). Interestingly, viral titers in the lungs were comparable between both groups, implying that differences in bioluminescence in the chest region-of-interest may be the result of greater amounts of Vac-FL in tracheas. Systemic spread of Vac-FL to the spleen was detected in mice treated with clodronate liposomes on day 5, while animals treated with saline liposomes had no detectable virus in the spleen on either day. Neither group had detectable amounts of Vac-FL in liver. Amounts of Vac-FL in brain were comparable on day 5, which is consistent with our previous data suggesting that vaccinia spreads to the brain by local extension from the nasopharynx rather than systemic dissemination (Luker et al., 2005). We also analyzed bronchoalveolar lavage fluids from these mice for levels of interferon β, TNF-α, IL-6, and MCP-1, but we detected no consistent differences in levels of these cytokines produced by mice treated with clodronate versus saline liposomes (data not shown). Overall, these data further demonstrate that alveolar macrophages limit local infection with vaccinia virus and emphasize that protective effects of these cells extend beyond the lungs and impact the overall severity of infection.
Depletion of alveolar macrophages enhances recruitment of leukocytes to the lungs
Alveolar macrophages have been shown to regulate recruitment of inflammatory cells to the lungs during infection with bacterial pathogens including Klebsiella pneumoniae and Streptococcus pneumoniae (Broug-Holub et al., 1997, Knapp et al., 2003). To determine up to what extent alveolar macrophages regulate leukocytes in the lungs during vaccinia infection, we depleted alveolar macrophages with liposomal clodronate and then analyzed total numbers and types of immune cells in the lung interstitium on days 3 and 5 after infection with 4 × 105 pfu Vac-FL. Numbers of cells in uninfected mice also were determined and subtracted from values obtained in the infected animals.
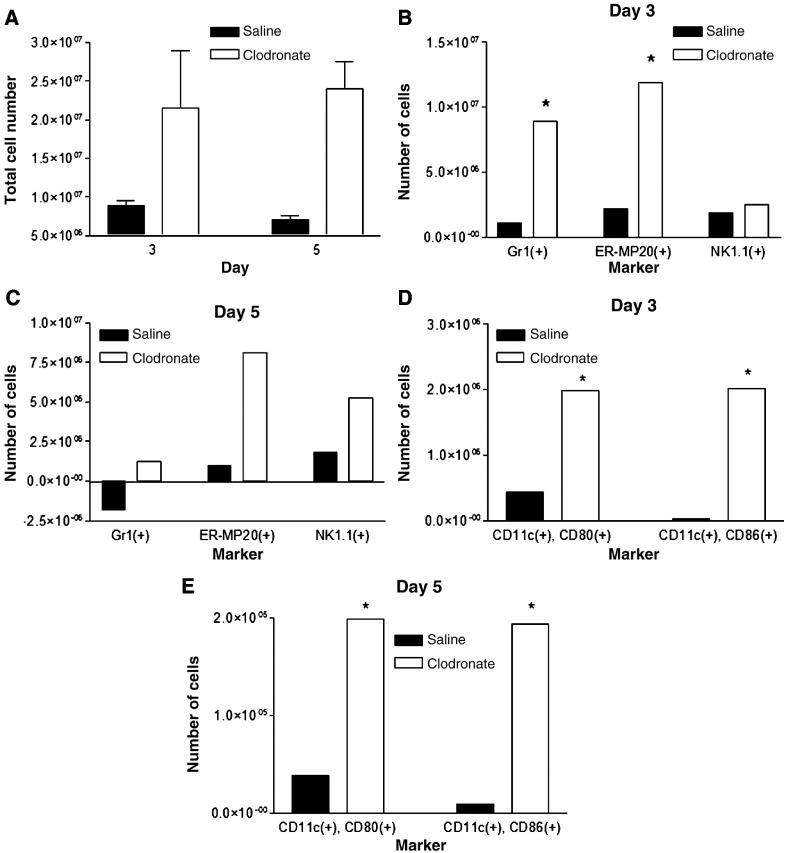
Depletion of alveolar macrophages with liposomal clodronate significantly increased total numbers of CD45+ leukocytes recruited to the lungs of infected mice (Fig. 4A). On both days 3 and 5 post-infection, there were approximately 3-fold more total leukocytes present in mice treated with liposomal clodronate and then infected with Vac-FL. By comparison, total numbers of leukocytes in uninfected lungs did not differ between mice injected with clodronate or saline liposomes (data not shown).
Fig. 4.
Alveolar macrophages reduce numbers of leukocytes in the lung during vaccinia infection. (A) Mice were injected intratracheally with clodronate or saline control liposomes on then infected with 5 × 105 pfu Vac-FL i.n. 2 days later (day 0). Total numbers of CD45(+) leukocytes in the lungs of mice were quantified on days 3 and 5 post-infection (n = 3 mice per group at each time point). Numbers of cells in mice treated with clodronate or saline liposomes and then mock infected were subtracted from the presented data. *Denotes p < 0.05. (B, C) Subpopulations of CD45(+) leukocytes present in the lung minces were analyzed by flow cytometry on days 3 (B) and 5 (C) post-infection. Percentages of various cell types were multiplied by the total number of CD45(+) leukocytes to determine numbers of cells expressing each cell surface marker. Flow cytometry data are pooled samples from 4–5 mice per treatment normalized to numbers of cells present in mock-infected mice injected with clodronate or saline liposomes, respectively. (D, E) Numbers of CD11c(+) cells expressing co-stimulatory molecules CD80 or CD86 on days 3 (D) and 5 (E) post-infection. *Denotes p < 0.05, #Denotes p < 0.01.
We used flow cytometry to identify specific types of leukocytes present in the lungs and effects of alveolar macrophages on numbers of various cells present during vaccinia infraction with 4 × 105 pfu. On day 3 post-infection, mice treated with clodronate liposomes had significantly greater numbers of Gr1(+) granulocytes relative to saline control (Fig. 4B). Depletion of alveolar macrophages also increased numbers of cells expressing ER-MP20, a marker of precursor cells for granulocytes/monocytes, on day 3 after infection with Vac-FL. Numbers of Gr1(+) and ER-MP20(+) cells on day 5 of infection remained higher in mice treated with clodronate liposomes as compared with control, although total numbers of cells expressing either marker decreased from day 3 in both experimental groups (Fig. 4C). In addition, there were significantly greater numbers of NK1.1(+) natural killer cells present in lungs of mice treated with clodronate liposomes on day 5. We did not detect differences in numbers of cells with markers of T or B lymphocytes on either day (data not shown). Although the alveolar space ultimately is repopulated by cells recruited from the circulation, this process does not make a significant impact on leukocytes in the lungs at these early times. Overall, these data show that alveolar macrophages directly or indirectly limit influx of selected populations of leukocytes into the lungs during the course of infection with vaccinia virus.
Because alveolar macrophages have been proposed to limit antigen presentation by resident dendritic cells in the pulmonary interstitium, we also used flow cytometry to analyze expression of co-stimulatory molecules on CD11c(+) cells in lung tissue. Using CD11c as a marker of pulmonary dendritic cells, there were no differences in total numbers of these cells in the lungs between mice treated with clodronate or saline liposomes, respectively (data not shown). However, depletion of alveolar macrophages greatly enhanced the number of activated CD11c(+) cells on both days 3 and 5 post-infection, as determined by increased expression of co-stimulatory molecules CD80 or CD86 (Figs. 4D, E). These data for CD11c(+) dendritic cells suggest that alveolar macrophages limit activation of dendritic cells during vaccinia infection, potentially through direct suppression or indirectly by reducing amounts of antigen available to dendritic cells.
Co-culture of alveolar macrophages with respiratory epithelium reduces vaccinia infection and replication in vitro
Because alveolar macrophages limited replication of vaccinia virus in vivo, we further analyzed effects of these cells on viral infection in respiratory epithelium in cultured cell lines derived from alveolar macrophages and lung epithelium. MH-S alveolar macrophages and LA-4 lung epithelial cells, which are murine cell lines that have been used previously to analyze host responses to infection (Farbermann et al., 2004) (Tuthill et al., 2003), were co-cultured in direct contact to model the dynamic interactions between these cell types during infection in vivo. For these experiments, we focused on co-cultures with 1% and 5% MH-S cells added to LA-4 cells for 48 h prior to infection. Although it is difficult to determine the actual ratio of alveolar macrophages to respiratory epithelium at a site of infection, these percentages are lower than those published previously for co-culture models and may more accurately reproduce conditions in vivo (Hjort et al., 2003) (Ishii et al., 2005).
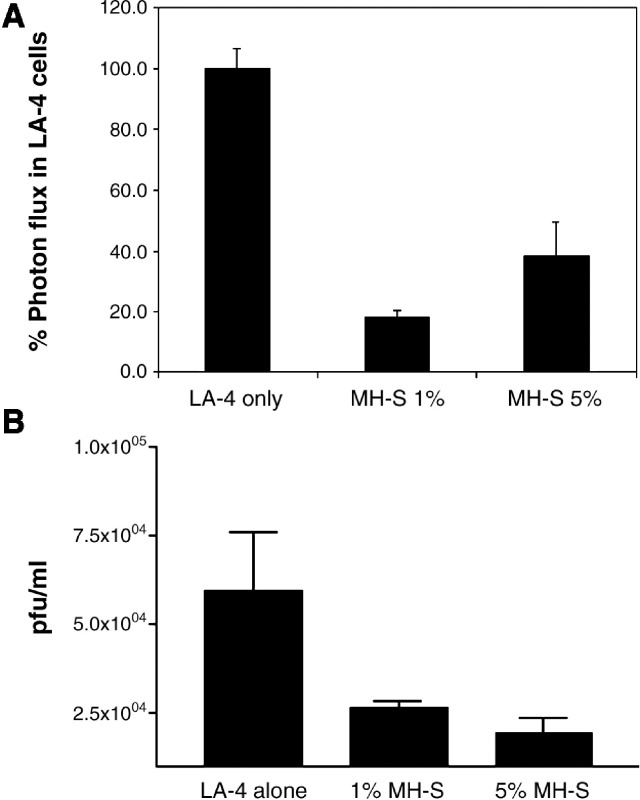
Cells were infected with Vac-FL, adjusting the total amount of virus to account for differing total numbers of LA-4 and MH-S cells in various wells, and we then quantified bioluminescence 24 h after infection. Wells with co-cultured MH-S and LA-4 cells had significantly less bioluminescence from Vac-FL as compared with LA-4 cells alone (Fig. 5A). Addition of as few as 1% MH-S cells significantly limited expression of the reporter gene, and protective effects were evident under both co-culture conditions (p < 0.05). To establish that co-cultures of respiratory epithelium and alveolar macrophages also limited production of new viruses, we quantified viral replication by plaque assay 24 h after infection with Vac-FL at an MOI of 0.1. Addition of either 1% or 5% MH-S macrophages to LA-4 epithelial cells significantly decreased replication of Vac-FL relative to LA-4 cells alone (p < 0.01) (Fig. 5B). Collectively, these data demonstrate that alveolar macrophages limit vaccinia infection in respiratory epithelium, which may contribute in part to protective effects of alveolar macrophages during vaccinia infection in mice.
Fig. 5.
Co-culture of lung epithelial cells with alveolar macrophages limits vaccinia infection. LA-4 lung epithelial cells were cultured alone or with 1% or 5% added MH-S alveolar macrophages for 2 days prior to infection with Vac-FL at MOI of 0.1. Numbers of viral pfu were adjusted to account for differing total numbers of cells in various wells. (A) Photon flux data are shown for Vac-FL 24 h after infection at MOI of 0.1. Data are expressed as mean values ± SEM for background subtracted photon flux normalized to bioluminescence produced from infection of LA-4 cells alone. (B) Titers of Vac-FL produced 24 h after infection of LA-4 cells or LA-4 cells cultured with 1% or 5% MH-S cells. Data are presented as mean values for viral pfu/ml ± SEM (n = 4 samples per condition). The lower limits of detection for the assay are 30 pfu/ml. Results are representative of two independent experiments.
Type I interferons are key components of host defense against vaccinia, which suggests that production of these cytokines or genes activated by type I interferons could account for protective effects of MH-S cells on infection of respiratory epithelium by vaccinia. We measured levels of interferon β, a key type I interferon regulated by transcription factors including IRF-3 and IRF-7 and produced early in infection (Wathelet et al., 1998). By quantitative RT-PCR, MH-S cells increased expression of interferon β by 2- to 3-fold in response to vaccinia infection at MOI 0.1. However, there was no change in the expression of ISG15, RANTES, or IP10, each of which can be induced primarily by IRF-3 or induced secondarily by type I interferon signaling through its cognate receptor (Nakaya et al., 2001). These data suggest that activation of a type I interferon response to vaccinia infection is incomplete in MH-S cells, which is consistent with previous work showing that primary alveolar macrophages from mice can respond to but not produce interferon β (Punturieri et al., 2004). Therefore, other anti-viral signaling molecules and/or mechanisms of host defense, such as phagocytosis, likely enable MH-S cells to reduce vaccinia infection in co-culture with respiratory epithelium.
Discussion
We demonstrate that alveolar macrophages confer protection against vaccinia virus infection, functioning to limit local and systemic replication of virus as quantified by bioluminescence imaging and plaque assays on excised tissues. Depletion of alveolar macrophages increases weight loss and changes in body temperature, showing that these cells limit the overall severity of disease produced by vaccinia. In the setting of lethal infection, alveolar macrophages also had a modest effect to prolong survival of mice. Although images from selected mice show that alveolar macrophages limit vaccinia infection in the lungs, data from plaque assays for the entire cohort of mice demonstrate that alveolar macrophages consistently reduce viral replication in the trachea and not the lungs. Alveolar macrophages limited systemic dissemination of virus to spleen and lymph nodes in the abdomen. Viral titers in the brain were not affected by treatment with clodronate versus saline liposomes, although our previous work suggests that brain infection with vaccinia is due to direct spread through the olfactory tract rather than systemic dissemination (Luker et al., 2005). Collectively, these data emphasize the critical function of alveolar macrophages to regulate effective host immunity against vaccinia.
Alveolar macrophages function in part by limiting the total number of inflammatory cells recruited to the lungs in response to vaccinia infection. In particular, depletion of alveolar macrophages increases Gr1(+) granulocytes and ER-MP20(+) cells, the latter of which are progenitors of granulocytes and monocytes. Prior studies performed predominantly in vitro show that monocytes/macrophages and potentially neutrophils have direct roles in controlling replication of vaccinia virus (Jones, 1982, Karupiah and Harris, 1995, West et al., 1987). However, our data show that the enhanced inflammatory response is associated with more severe disease, indicating that enhanced recruitment of these cells is detrimental to the host. Our results are consistent with previous studies showing that the inflammatory response to particulate materials and pathogens in the lungs disrupts integrity of respiratory epithelium and capillaries, thereby impairing lung exchange and increasing severity of disease (Moraes et al., 2006). In addition, depletion of alveolar macrophages increases numbers of CD11c(+) dendritic cells in the lungs that express co-stimulatory molecules CD80 and CD86 as markers of activation. It has been demonstrated previously that depletion of pulmonary macrophages from rats enhances the ability of dendritic cells to present a model antigen to T lymphocytes (Holt et al., 1993). Overall, our results establish that one key function of alveolar macrophages is to limit the inflammatory response to vaccinia in the lungs, which reduces viral dissemination of systemic effects of infection.
In addition to reducing numbers of leukocytes in infected lungs, alveolar macrophages also interact with lung epithelium to limit infection with vaccinia. Using an in vitro co-culture model of alveolar macrophages and lung epithelium, we demonstrated that addition of as few as 1% alveolar macrophages to lung epithelium significantly reduced viral gene expression and replication after infection at low MOI. Although it is difficult to estimate relative proportions of alveolar macrophages to respiratory epithelium at sites of infection, alveolar macrophages are known to be recruited actively to areas of infection and inflammation in the lungs. Therefore, the ability of alveolar macrophages to reduce vaccinia infection of lung epithelium in a cell culture model likely represents another critical function of alveolar macrophages in host defense against poxviruses in vivo.
Secretion of cytokines and/or phagocytosis are two possible mechanisms through which alveolar macrophages limit infection with vaccinia virus. We analyzed the expression of interferon β and selected interferon stimulated genes in alveolar macrophages as candidate molecules to reduce viral replication. Although MH-S alveolar macrophages infected with vaccinia virus upregulated expression of interferon β, we did not detect changes in the expression of other interferon-regulated genes. These data are consistent with previous research showing that alveolar macrophages lack autocrine stimulation and feed-forward amplification of interferon signaling in response to stimulation with poly I:C, although these cells are able to respond to exogenous type I interferon (Punturieri et al., 2004). Because we were unable to reliably separate small numbers of alveolar macrophages from lung epithelial cells, we could not analyze the expression of interferon-responsive genes in alveolar macrophages in co-culture conditions. While lung epithelial cells may secrete type I interferon to activate interferon signaling in alveolar macrophages, these data suggest that protective effects of alveolar macrophages may be mediated by other cytokines or molecules such as nitric oxide (Karupiah and Harris, 1995). Protective effects of alveolar macrophages also may be mediated through phagocytosis of virus particles, thereby sequestering vaccinia from lung epithelium and limiting infection. This mechanism of host defense is supported by previous research showing that both immune and non-immune macrophages ingest vaccinia virus (Greer et al., 1974). Further studies are needed to establish up to what extent secretion of cytokines, phagocytosis, and/or other mechanisms such as cell-to-cell contact promote protective effects of alveolar macrophages in vitro and in vivo.
In conclusion, the current study establishes that alveolar macrophages have an essential protective function against infection with orthopoxviruses. These cells limit the inflammatory response to vaccinia in the lungs, which reduces viral replication, dissemination, and overall severity of disease. Modulating the recruitment of neutrophils and other inflammatory cells into the lungs potentially may be effective in reducing disease severity produced by a natural or intentional infection with poxviruses.
Materials and methods
Cells
MH-S cells (ATCC) are an SV40 transformed murine alveolar macrophage cell line derived from Balb/c mice. These cells retain many morphologic and functional features of alveolar macrophages and express surface antigens MHC class I H2d, class II Ia, and CD11B (Mbawuike and Herscowitz, 1989). LA-4 cells are a murine lung epithelial cell line with characteristics of type II pneumocytes (Stoner et al., 1975). LA-4 cells were derived from an A/He mouse and have MHC class I haplotype H2 k. MH-S and Vero cells were cultured in DMEM medium (Invitrogen) with 10% heat-inactivated fetal bovine serum, 1% l-glutamine, and 0.1% penicillin–streptomycin in a 5% CO2 incubator at 37 °C. LA-4 cells were cultured in DMEM medium containing 15% heat-inactivated fetal bovine serum.
Vaccinia virus
Stocks of Vac-FL, a recombinant Western Reserve vaccinia virus that expresses firefly luciferase, were prepared and titered as described previously (Earl et al., 1998, Luker et al., 2005).
Monoclonal antibodies
The following mAbs were used for flow cytometry (BD Pharmingen): RM4-4 (anti-murine CD4, rat IgG2b), 53-6.72 (anti-murine CD8, rat IgG2b), M1/70 (anti-murine CD11b, rat IgG2b), HL3 (anti-murine CD11c, hamster IgG1), 2.4G2 (anti-murine CD16/CD32 Fc block, rat IgG2b), 3/23 (anti-murine CD40, rat IgG2a), RA-36B2 (anti-murine CD45R/B220, rat IgG2a), 30-F11 (anti-murine CD45, rat IgG2b), 16-10A1 (anti-murine CD80, hamster IgG2), GL1 (anti-murine CD86, rat IgG2a), AF6-120.1 (anti-murine I-Ab MHC class II, mouse IgG2a), and RB6-8C5 (anti-murine Ly6G Gr-1, rat IgG2b). mAbs were primarily conjugated with FITC, biotin, or PE; biotinylated Abs were visualized using streptavidin-PerCP (BD Pharmingen). Isotype-matched irrelevant control mAbs (BD Pharmingen) were tested simultaneously in all experiments.
In vitro co-culture experiments
1 × 104 LA-4 cells were plated in 96-well plates, allowed to adhere for ≈ 4 h, and then 0–5 × 102 MH-S cells were added to wells for 48 h prior to infection. Cells were infected with Vac-FL at MOI 0.1, adjusting the inoculum for the total number of cells in wells. Bioluminescence from Vac-FL was quantified using the IVIS (Xenogen) 24 h after infection (Luker et al., 2005). Viral titers were determined by plaque assay at these same time points.
Mouse procedures
All animal procedures were approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan. Seven- to ten-week-old male 129 Ev/Sv mice (Taconic) were used for all experiments. To deplete alveolar macrophages, mice were injected intratracheally with 100 μl of liposomes containing clodronate (clodronate liposomes). Control mice received liposomes containing saline. Mice were infected intranasally with various amounts of Vac-FL in 20 μl sterile DMEM as described in the figure legends. Anesthetized mice were shaved with clippers to decrease absorption and scattering of light for bioluminescence imaging. Animal weights were recorded daily following infection, and rectal temperatures were measured every 24 h in selected experiments. Bronchoalveolar lavage and removal of the lungs for analysis of cells in the pulmonary interstitium were performed as described previously (Curtis and Kaltreider, 1989) (Osterholzer et al., 2005).
Analysis of BAL samples
Total cells recovered from BAL were counted manually with a hemocytometer and then stained with Wright–Giemsa for differential cell counts. The first 1 ml of BAL fluid recovered from the lungs was used for ELISA studies of cytokines.
Antibody staining and flow-cytometric analysis
Staining of cells in the pulmonary interstitium, including blockade of FcRs, and analysis by flow cytometry were performed as described previously (Osterholzer et al., 2005). Data were collected on a FACScan flow cytometer using CellQuest software (both from BD Immunocytometry Systems) and analyzed using FlowJo software (Tree Star). A minimum of 10,000 cells were analyzed per sample. For all analyses, percentages for matched isotype control antibodies were subtracted from values obtained for staining with specific antibodies for individual markers.
Quantitative RT-PCR
RNA was isolated from cultured cells using Trizol (Invitrogen) and RNeasy columns (Qiagen) according to the manufacturers' protocols. Quantitative RT-PCR using Syber green detection was performed with the iScript One-Step Kit (Bio-Rad) on a MX3000P instrument (Stratagene). Primer sequences for amplified mouse genes are listed below:
| Interferon β | Forward 5′ AGCTCCAAGAAAGGACGAACAT 3′ |
| Reverse 5′ GCCCTGTAGGTGAGGTTGATCT 3′ | |
| ISG15 | Forward 5′ CAGGACGGTCTTACCCTTTCC 3′ |
| Reverse 5′ AGGCTCGCTGCAGTTCTGTAC 3′ | |
| IP10 | Forward 5′ CCTGCCCACGTGTTGAGAT 3′ |
| Reverse 5′ TGATGGTCTTAGATTCCGGATTC 3′ | |
| RANTES | Forward 5′ GCCCACGTCAAGGAGTATTTCTA 3′ |
| Reverse 5′ ACACACTTGGCGGTTCCTTC 3′ | |
| GAPDH | Forward 5′ TATGTCGTGGAGTCTACTGGT 3′ |
| Reverse 5′ GAGTTGTCATATTTCTCGTGG 3′ |
Data for target gene expression were normalized to GAPDH as a control.
Virus titration
Viral titers in cells and organs were analyzed by serial dilution on Vero cells as described previously (Luker et al., 2005).
Bioluminescence imaging
Bioluminescence imaging was performed with a cryogenically cooled CCD camera (IVIS) (Xenogen) as described previously (Luker et al., 2002). ROIs corresponding to the head, chest, and abdomen of infected mice were used to quantify bioluminescence as photon flux using software provided with the IVIS.
Statistics
Pairs of data points were analyzed by t test for statistically significant differences (p < 0.05). Statistics for correlation coefficients and area-under-the-curve analyses were performed with commercially available software (Graphpad, Prism).
Acknowledgments
Research was supported by R21AI066192 and RO1 HL082480 from the UHPHS, and Merit Review funds from the Department of Veterans Affairs. Support for imaging experiments was provided by NIH R24CA083099 for the University of Michigan Small Animal Imaging Resource.
References
- Broug-Holub E., Toews G., van Iwaarden J., Strieter R., Kunkel S., Paine R.R., Standiford T. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumoniae: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 1997;65(4):1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Kaltreider H. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am. Rev. Respir. Dis. 1989;139(2):393–400. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- Earl P., Cooper N., Wyatt L., Moss B. In: Current Protocols in Molecular Biology. Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K., editors. John Wiley and Sons, Inc; 1998. Preparation of cell cultures and vaccinia virus stocks; pp. 16.16.1–16.16.11. [Google Scholar]
- Farbermann M., Hoffmann J., Ryerse J., Demello D. fusible signal to murine alveolar macrophages from lipopolysaccharide- and Escherichia coli-stimulated lung Type II epithelial cells. Inflammation Res. 2004;53(9):475–483. doi: 10.1007/s00011-004-1289-6. [DOI] [PubMed] [Google Scholar]
- Fenner F., Henderson D., Arita I., Jezek Z., Ladnyi I. Smallpox and its eradication. World Health Organization, Geneva. 1988 [Google Scholar]
- Greer B., Delfs D., McElree H. Electron microscope study of the interaction of vaccinia virus with macrophages from immunized and nonimmunized rabbits. Infect. Immun. 1974;9(2):452–459. doi: 10.1128/iai.9.2.452-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort M., Brenyo A., Finkelstein J., Frampton M., LoMonaco M., Stewart J., Johnston C., D'Angio C. Alveolar epithelial cell–macrophage interactions affect oxygen-stimulated interleukin-8 release. Inflammation. 2003;27(3):137–145. doi: 10.1023/a:1023817811850. [DOI] [PubMed] [Google Scholar]
- Holt P., Oliver J., Bilyk N., McMenamin C., McMenamin P., Kraal G., Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 1993;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Hayashi S., Hogg J., Fujii T., Goto Y., Sakamoto N., Mukae H., Vincent R., van Eeden S. Alveolar macrophage–epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Respir. Res. 2005;6:87. doi: 10.1186/1465-9921-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. Interactions between human neutrophils and vaccinia virus: induction of oxidative metabolism and virus inactivation. Pediatr. Res. 1982;16(7):525–529. doi: 10.1203/00006450-198207000-00005. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Harris N. Inhibition of viral replication by nitric oxide and its reversal by ferrous sulfate and tricarboxylic acid cycle metabolites. J. Exp. Med. 1995;181(6):2171–2179. doi: 10.1084/jem.181.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Leemans J., Florquin S., Branger J., Maris N., Pater J., van Rooijen N., van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- Leenen P., Radosevic K., Voerman J., Salomon B., van Rooijen N., Klatzmann D., van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J. Immunol. 1998;160(5):2166–2173. [PubMed] [Google Scholar]
- Luker G., Bardill J., Prior J., Pica C., Piwnica-Worms D., Leib D. Noninvasive bioluminescence imaging of herpes simplex virus type 1 infection and therapy in living mice. J. Virol. 2002;76(23):12149–12161. doi: 10.1128/JVI.76.23.12149-12161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker K., Hutchens M., Schultz T., Pekosz A., Luker G. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005;341(2):284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- MacLean J., Zia W., Pinto C., Zhao L., Liu H., Kradin R. Sequestration of inhaled particulate antigens by lung phagocytes. A mechanism for the effective inhibition of pulmonary cell-mediated immunity. Am. J. Pathol. 1996;148:657–666. [PMC free article] [PubMed] [Google Scholar]
- Mbawuike I., Herscowitz H. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J. Leukoc. Biol. 1989;46(2):119–127. doi: 10.1002/jlb.46.2.119. [DOI] [PubMed] [Google Scholar]
- Moraes T., Zurawska J., Downey G. Neutrophil granule contents in the pathogenesis of lung injury. Curr. Opin. Hematol. 2006;13(1):21–27. doi: 10.1097/01.moh.0000190113.31027.d5. [DOI] [PubMed] [Google Scholar]
- Nakaya T., Sato M., Hata N., Asagiri M., Suemori H., Noguchi S., Tanaka N., Taniguchi T. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 2001;283(5):1150–1156. doi: 10.1006/bbrc.2001.4913. [DOI] [PubMed] [Google Scholar]
- Osterholzer J., Ames T., Polak T., Sonstein J., Moore B., Chensue S., Toews G., Curtis J. CCR2 and CCR6, but not endothelial selectins, mediate the accumulation of immature dendritic cells within the lungs of mice in response to particulate antigen. J. Immunol. 2005;175(2):874–883. doi: 10.4049/jimmunol.175.2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punturieri A., Alviani R., Polak T., Copper P., Sonstein J., Curtis J. Specific engagement of TLR4 or TLR3 does not lead to IFN-beta-mediated innate signal amplification and STAT1 phosphorylation in resident murine alveolar macrophages. J. Immunol. 2004;173(2):1033–1042. doi: 10.4049/jimmunol.173.2.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q., Jutila M., van Rooijen N., Cutler J. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 1994;152(10):5000–5008. [PubMed] [Google Scholar]
- Stoner G., Kikkawa Y., Kniazeff A., Miyai K., Wagner R. Clonal isolation of epithelial cells from mouse lung adenoma. Cancer Res. 1975;35(8):2177–2185. [PubMed] [Google Scholar]
- Thepen T., Kraal G., Holt P. The role of alveolar macrophages in regulation of lung inflammation. Ann. N. Y. Acad. Sci. 1994;725:200–206. doi: 10.1111/j.1749-6632.1994.tb39802.x. [DOI] [PubMed] [Google Scholar]
- Tuthill T., Papadopoulos N., Jourdan P., Challinor L., Sharp N., Plumpton C., Shah K., Barnard S., Dash L., Burnet J., Killington R., Rowlands D., Clarke N., Blair E., Johnston S. Mouse respiratory epithelial cells support efficient replication of human rhinovirus. J. Gen. Virol. 2003;84:2829–2836. doi: 10.1099/vir.0.19109-0. [DOI] [PubMed] [Google Scholar]
- van den Broek M., Muller U., Huang S., Aguet M., Zinkernagel R. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69(8):4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanRooijen N. The liposome-mediated macrophage ‘suicide’ technique. J. Immunol. Methods. 1989;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- Wathelet M., Lin C., Parekh B., Ronco L., Howley P., Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell. 1998;1(4):507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- West B., Eschete M., Cox M., King J. Neutrophil uptake of vaccinia virus in vitro. J. Infect. Dis. 1987;156(4):597–606. doi: 10.1093/infdis/156.4.597. [DOI] [PubMed] [Google Scholar]