Abstract
Gamma aminobutyric acid (GABA) type A receptors play a key role in brain inhibitory neurotransmission, and are ligand-activated chloride channels blocked by numerous convulsant ligands. Here we summarize data on binding of picrotoxin, tetrazoles, β-lactams, bicyclophosphates, butyrolactones and neurotoxic pesticides to GABA-A ionophore, and discuss functional and structural overlapping of their binding sites. The paper reviews data on convulsants’ binding sensitivity to different point mutations in ionophore-lining second transmembrane domains of GABA-A subunits, and maps possible location of convulsants’ sites within the chloride ionophore. We also discuss data on inhibition of glycine, glutamate, serotonin (5-HT3) and N-acetylcholine receptors by GABA-A channel blockers, and examine the applicability of this model to other homologous ionotropic receptors. Positioning various convulsant-binding sites within ionophore of GABA-A receptors, this model enables a better understanding of complex architectonics of ionotropic receptors, and may be used for developing new channel-modulating drugs.
Keywords: GABA-A receptors, ionophore, channel chemoconvulsants, binding sites, point mutagenesis
1. GABA and GABA-A receptor complex
Gamma-amino butyric acid (GABA) is the primary mediator of inhibitory transmission in the mammalian central nervous system (Akaike et al., 1987; Korpi et al., 2002; Leung and Xue, 2003). It has complex interactions with other neurotransmitter systems and acts through ionotropic A and metabotropic B type receptors (Martin and Dunn, 2002; Atack, 2003, 2005). Both receptors are a target for many endogenous and exogenous modulators that regulate normal and pathological brain mechanisms - sleep, memory, epilepsy and emotions (Kalueff and Nutt, 1997; Argyropoulos et al., 2000; Sandford et al., 2000; Nutt and Malizia, 2001; Vicini and Ortinski, 2004; Cryan and Kaupmann, 2005).
GABA-A receptors are crucial for controlling brain excitability, and represent ligand-gated ion channels composed of five subunits (belonging to eight families: α1–6, β1–3, γ1–3, δ, ε, π, θ and ρ1–3) around the ionophore (Baumann et al., 2001, 2002; Korpi et al., 2002; Rosahl, 2003; Vicini and Ortinski, 2004). Each subunit of GABA-A receptors consists of four transmembrane domains (TM1-4) (Perret et al., 1999; Engblom et al., 2002; Filippova et al., 2004) modulating the receptor activity. Binding of GABA opens up a Cl− channel, leading to neuronal inhibition (Wooltorton et al., 1997; Olsen et al., 2004). GABA-A receptors contain binding sites for GABA agonists and antagonists, as well as numerous positive and negative modulators (Olsen et al., 1990; Mathews et al., 1996; El-Etr et al., 1998; Argyropoulos et al., 2000; Nutt and Malizia, 2001; Leung and Xue, 2003). Positive modulators of GABA-A receptors barbiturates, benzodiazepines, steroids, ethanol and γ-butyrolactones (Holland et al., 1995; Canney et al., 1998; Olsen et al., 2004; Atack, 2003, 2006; Rudolph and Mohler, 2006). Neurosteroid antagonists, benzodiazepine inverse agonists and chloride channel blockers negatively modulate the receptor (Maksay, 1996; Akaike et al., 1987; Sousa and Ticku, 1997; Wooltorton et al., 1997; Leung and Xue, 2003), Tables 1–3.
Table 1.
Comparative analysis of pharmacological properties of traditional GABA-A ionophore blockers (+ present, − absent, ? unclear or conflicting effects)
| Properties | P | BPH | BL | PTZ | PC | LA |
|---|---|---|---|---|---|---|
| Character of channel blockage: | ||||||
| Competitive | −/+ | − | −/+ | +? | −? | + |
| Reversible | + | + | + | + | + | + |
| Voltage-dependent | +?* | + | + | + | − | + |
| Binding to closed channel | + | + | + | +? | + | |
|
| ||||||
| Effects of channel state: | ||||||
| open frequency | − | − | + | |||
| open duration | − | − | − | − | ||
| closed frequency | + | + | ||||
| closed duration | + | + | + | + | ||
|
| ||||||
| Molecular (structural) similarity: | ||||||
| Similarity to P | + | + | + | + | + | |
| Similarity to other ligands | BL,PTZ, BPH | PTZ | PC,BL | LA, PTZ | PC | |
| Ability to displace BPH | + | + | + | + | ||
| Hydrophobic molecule | + | + | − | − | ||
|
| ||||||
| Ability to inhibit GABA agonists binding | − | − | − | − | − | + |
|
| ||||||
| References | (I) | (II) | (III) | (IV) | (V) | (V,VI) |
P – picrotoxin, BPH – bicyclophosphates, BL – butyrolactones, PTZ – pentylenetetrazole, PC – penicillin, LA – other lactam antibiotics.
Several studies have shown that picrotoxin inhibition of the channel may be voltage-dependent (Newland and Cull-Candy, 1992; Yoon et al., 1993; Lynch et al., 1995; Yakushiji et al., 1987).
References: I (Newland and Cull-Candy, 1992), II (Hamann et al., 1990; Maksay et al., 1996; Sinkkonen et al., 2001), III (Holland et al., 1990, 1991, 1993; Maksay et al., 1994; Mathews et al., 1996), IV (Squires et al., 1984; Maksay et al., 1994; Huang et al., 2001), V (Akaike et al., 1987; Yakushiji et al., 1987; Twyman et al., 1992; Fujimoto et al., 1995; Behrends, 2000; Lindquist et al., 2004), VI (Fujimoto et al., 1995; Sugimoto et al., 2003).
Table 3.
Comparative analysis of the ability to inhibit different ionotropic receptors by GABA-active ionophore ligands (legend as in Fig. 1)
| Inhibition of ion channels
|
|||||||
|---|---|---|---|---|---|---|---|
| Receptors | P | BPH | BL | PTZ | PC | LA | NP |
| GABA-A | yes | yes | yes | yes | Yes | yes | yes |
| Invertebrate GABA | yes | yes | Yes | yes | |||
| Acetylcholine N type | yes | ||||||
| Glycine | yes | yes | |||||
| 5-HT3 serotonin | yes | no | yes | ||||
| Glutamate | yes | no | |||||
| References | (I) | (II) | (III) | (IV) | (I,V) | ||
References: I (Ffrench-Constant et al., 1993; Lynch et al., 1995; Zhang et al., 1995; Yoon et al., 1998; Etter et al., 1999; Shan et al., 2001, 2002; Le Corronc et al., 2002; Bloomquist, 2003; Das et al., 2003; Das and Dillon, 2003, 2005; Erkkila et al., 2004), II (Maksay et al., 1996; Yagle et al., 2003), III (Das et al., 2003), IV (Fujimoto et al., 1995; Hosie et al., 2006), V (Vale et al., 2003).
Ionophore-lining TM2 are responsible for GABA-A channel activation and desensitization, ion selectivity and binding of various ionophore ligands (Buhr et al., 2001; Horenstein et al., 2001; Jensen et al., 2002; Scheller and Forman, 2002; Filippova et al., 2004). Similar structure is known for other ligand-gated ionotropic receptors – GABA receptors of invertebrates, N-acetylcholine, glycine, glutamate, and serotonin (5-HT3) receptors (Ffrench-Constant et al., 1993; Baumann et al., 2001; Jentsch et al., 2001; Bloomquist, 2003; Das and Dillon, 2003, 2005; Newell et al., 2004; Olsen et al., 2004).
Picrotoxin and picrotoxinin, pentylenetetrazole (PTZ) and other tetrazoles, penicillin and other β-lactam antibiotics, thio-butyrolactones, bicyclophosphates (such as t-butylbicyclophosphorothionate TBPS), U-93631 and neurotoxic pesticides (NP) are traditional ionophore-blocking convulsant ligands (Squires et al., 1984; Hamann et al., 1990; Holland et al., 1991; Lindane, 1991; Twyman et al., 1992; Dillon et al., 1993, 1995; Wang et al., 1995; Le Corronc et al., 2002; Omrani et al., 2003; Sugimoto et al., 2003; Vale et al., 2003; Hansen et al., 2004; Kaminski et al., 2004; Lindquist et al., 2004; Sinkkonen et al., 2005). All these agents have cyclic structures, allowing to consider them as a common group of “cage convulsants” (Olsen et al., 1980; Hamon et al., 1998; Maksay et al., 1998; Rossi et al., 2001; Chen et al., 2006). Moreover, many of them share a substantial similarity in chemical and conformational structures (Table 1; Maksay, 1996), and also block other non-GABAergic ionotropic receptors (Table 3; Bloomquist, 2003; Vale et al., 2003).
Although mechanisms of action of non-ionophore modulators of GABA-A receptors have been extensively studied, the effector part of the receptor – its ionophore – is much less understood. It has long been thought that various convulsants inhibit ion influx by physically plugging ionophore (rev.: Petter et al., 1999; Behrends, 2000) when bound in different positions to a common “convulsant” binding pocket. Described in the literature as picrotoxin(in) site or receptor (Holland et al., 1991; Ito and Ho, 1994; Nobrega et al., 1995; Bell-Horner et al., 2000; Olsen et al., 2004; Das and Dillon, 2005), PTZ/TBPS site (Holland et al., 1990, 1993; Kalueff, 2002), convulsant, ionophore or channel site (Olsen et al., 1980, 1990; Peris et al., 1991; Maksay et al., 1996; Yagle et al., 2003), this convulsant-binding pocket of GABA-A receptors is currently poorly understood.
Mounting data indicates that different convulsants bind to overlapping but not identical sites, also showing multiple mechanisms of binding with different kinetics of association and dissociation (Holland et al., 1991; Twyman et al., 1992; Hamon et al., 1998; Yoon et al., 1998; Dibas and Dillon, 2000; Le Corronc et al., 2002; Mortensen et al., 2003; Sinkkonen et al., 2005). Some convulsant ligands (picrotoxin, bicyclophosphates, butyrolactones, β-lactams) seem to reach their binding sites in closed state of the channel, further confirming that simple plugging of ionophore is not the actual single mechanism of their action (Table 1; also see: Dibas et al., 2002; Hawthorne and Lynch, 2005 for discussion).
While several groups have modeled different aspects of ionophore functioning (Maksay, 1996, 2005; Baumann et al., 2002; Maksay et al., 2003; Chou, 2004; Chen et al., 2006; Muroi et al., 2006) and ligand binding (Twyman et al., 1992; Canney et al., 1998; Zhorov and Bregestovski, 2000; Shan et al., 2002; Vale et al., 2003; Horenstein et al., 2005), further studies modeling ionophore organization and its sites are needed. If successful, such attempts may increase our understanding of pathogenetic mechanisms of channelopathies (Felix, 2000) and facilitate the development of novel selective ionophore-targeting drugs (Eldefrawi and Eldefrawi, 1987; Bloomquist, 2003).
Since channel ligands are thought to bind to heterogeneous binding sites within ionophore, the important question is the positioning of these binding sites within the ionophore and relative to each other. Point mutagenesis data may give an important information on which TM2 residues may be critical for binding of different ligands. For example, a common critical residue for two convulsants implies overlapping of their binding sites, whereas different critical residues for these ligands suggests their distinct binding sites. As recent extensive data provides important insights into functional properties of ionophore receptors (Ffrench-Constant et al., 1993; Gurley et al., 1995; Perret et al., 1999), we will systematically evaluate the available literature on pharmacology and mutagenesis of GABA-A and other homologous ionotropic receptors, in order to develop a model of ionophore binding sites for different classes of GABA-active ligands.
2. Ionophore sites and their ligands
Table 1 summarizes known physiological and pharmacological properties of traditional GABA-A ionophore blockers. Table 2 shows point mutagenesis data for major GABA-A chemoconvulsants, outlining critical residues for each class of ionophore ligands. Table 3 describes the ability of ionophore blockers to inhibit other ionotropic (glycine, glutamate, serotonin 5-HT3 and N-acetylcholine) receptors. Fig. 1 shows a model of GABA-A ionophore, developed based on data in Tables 1–3.
Table 2.
Mutagenesis-based data on critical residues for binding of selected ionophore ligands to TM2 domains of GABA-A receptor subunits (legend as in Fig. 1).
| Ligands | References | |||||||
|---|---|---|---|---|---|---|---|---|
| TM2 residue | P | PTZ | BPH | BL | PC | LA | NP * | |
| 2 | yes | no | yes | no | yes | (I) | ||
| 3 | yes | yes | (I) | |||||
| 6 | yes | yes | yes | yes ** | no | yes | (II) | |
| 9 | yes | no | no | no | no | (III) | ||
| 15 | yes | (IV) | ||||||
| 19 | yes | (IV) | ||||||
E.g., dieldrin.
partially sensitive (border of the binding pocket?).
References: I (Gurley et al., 1995; Edwards and Lees, 1997; Jursky et al., 2000; Buhr et al., 2001), II (Sugimoto et al., 2002), III (Tierney et al., 1996; Lindquist et al., 2004), IV (Findlay et al., 2001; Dibas et al., 2002)
Figure 1. Proposed model of convulsant-binding sites of GABA-A receptor ionophore.
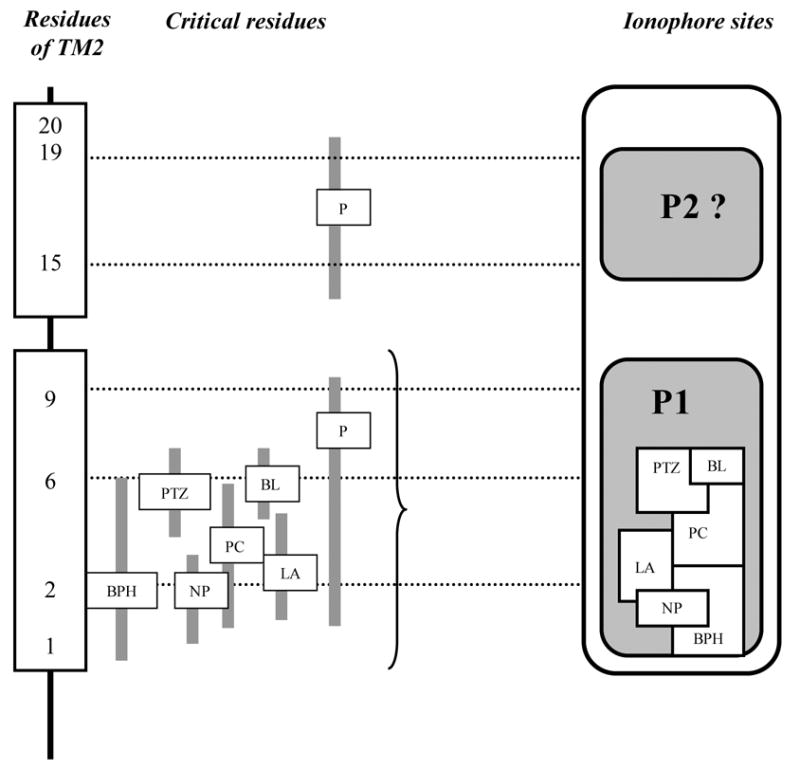
TM2 residues are conventionally numbered 1′–20′ from the N-terminal bottom to the C-terminal extracellular end of the helix (see Olsen, 2006 fore details). P – picrotoxin(in), BPH – bicyclophosphates, BL – butyrolactones, PTZ – pentylenetetrazole, PC – penicillin, LA – other lactam antibiotics, NP – neurotoxic pesticides, P1 – “main” picrotoxin binding site, P2 – hypothetical second “allosteric” picrotoxin site.
Picrotoxin site
As picrotoxin effectively inhibits chloride influx in GABA-A (Newland and Cull-Candy, 1992) and other ionotropic receptors (Table 3), it represents a universal “reference” channel blocker (Das et al., 2003; Olsen, 2006) with whom other ligands may be compared (Table 1). While the exact location of picrotoxin binding to ionophore is still unknown (Huang et al., 2001), its sensitivity to mutations in residues 2/3 and 6 of TM2 suggests that the site contains residues 2–6 (Table 2; Buhr et al., 2001). In line with this, Zhorov and Bregestovski (2000) suggested that picrotoxin penetrates deep inside the ionophore pore, binding with its hydrophobic moiety to residue 2 of TM2 (close to the pore) and forming hydrogen bounds with residue 6 in the middle of TM2. Importantly, while amino acid composition of residue 2 is variable in different ionotropic receptors, the composition of residue 6 is highly conservative, implying that it is crucial for picrotoxin binding to ionophore, and most likely representing the epicenter of its binding pocket (Fig. 1).
While residue 15 is critical for picrotoxin binding to glycine receptors, its mutation in GABA-A receptors inhibited (but not abolished) use-dependent ionophore block by picrotoxin, suggesting that this residue may be important for interplay between GABA and picrotoxin binding sites (Dibas et al., 2002). Other studies implicate residues 9 and 15 in the regulation of channel properties, such as desensitization, stabilization of open states and gating (Findlay et al., 2001; Scheller and Forman, 2002). Taken together, this indirectly supports the possibility of a second “modulatory” (allosteric) binding site of picrotoxin including residues 15–19. In line with this, residue 17 is important for picrotoxin modulation of GABA-A receptors (Horenstein et al., 2001). The hypothesis of an additional allosteric picrotoxin site (Fig. 1) is also indirectly supported by recent data (Mortensen et al., 2003) showing that potency of picrotoxin binding is highly dependent on the level of spontaneous activity of GABA-A receptors.
PTZ (tetrazole) site
Although early studies hypothesized PTZ binding to benzodiazepine site of GABA-A receptors, similarity to picrotoxin (in terms or molecular structure, use-dependent voltage-independent action and displacement of TBPS) prompted its activity at ionophore “convulsant” site (Table 1; Dibas and Dillon, 2000). Some butyrolactones and PTZ share stereo-structural similarity and synergetically affected TBPS binding (Maksay et al., 1994). This, and the ability of selected butyrolactones to inhibit binding of other cage convulsants – picrotoxin, PTZ or bicyclophosphates (rev.: Dibas and Dillon, 2000; Huang et al., 2001), supports the idea that all these binding sites overlap. Finally, similar sensitivity of picrotoxin and PTZ binding to some mutations in TM2 further confirms this notion (Dibas and Dillon, 2000).
However, there are several distinctions between picrotoxin and PTZ actions, including different affinity and dynamics of association with ionophore and Cl- current inhibition (Huang et al., 2001). Another dissimilarity is the lack of complex (competitive + non-competitive) effects in PTZ (Table 1), and insensitivity of PTZ binding to ablation of α-subunits (abolishing binding of picrotoxin and NP) (Huang et al., 2001). Finally, PTZ binding is insensitive to point mutations in residue 2, suggesting the location of this residue outside of the PTZ site (Huang et al., 2001), as indicated in Fig. 1.
TBPS (bicyclophosphate) site
TBPS is a non-competitive ionophore blocker, traditionally thought to act via classical picrotoxin/convulsant site, although with different kinetics (Peris et al., 1991; Ito and Ho, 1994; Nobrega et al., 1995; Maksay et al., 1996; Luddens et al., 1998; Jursky et al., 2000; Sinkkonen et al., 2001, 2005). Since mutations in residue 2 are critical for TBPS binding to ionophore, it is possible to assume that its binding site includes this residue (Jursky et al., 2000). TM2 residues 1, 2 and 3 of β-subunits of GABA-A receptors were important to form TBPS binding site in chimeric receptors, implying that these residues form the TBPS binding pocket (Jursky et al., 2000) (Fig. 1).
Butyrolactone site
Earlier reports on competitive inhibition of TBPS binding by convulsant butyrolactones suggested that they bind to a common TBPS/picrotoxin “convulsant” ionophore site (Holland et al., 1991, 1995; Mathews et al., 1996; Canney et al., 1998; Gonzales et al., 2003). This is also in line with similar chemical structures of these ligands (e.g., picrotoxin molecule contains a butyrolactone ring) (Williams et al., 1997). Likewise, butyrolactones share similar physiological and pharmacological mechanisms of action with PTZ (Maksay et al., 1994), and are able to allosterically modulate TBPS binding (Holland et al., 1990, 1991, 1993). Collectively, this implies that picrotoxin, PTZ, TBPS and butyrolactones may bind to overlapping ionophore binding sites (Fig. 1).
Although butyrolactone binding site is not yet identified (Gonzales et al., 2003), the sensitivity of butyrolactone binding to point mutations in residue 6 (Huang et al., 2001) indicates its location within a common binding area for these convulsants. Since mutations affecting picrotoxin binding also affect that of butyrolactones (Huang et al., 2001), it is indeed likely that binding sites for picrotoxin and butyrolactones significantly overlap (Fig. 1). In contrast, other studies have demonstrated anticonvulsant effects of some butyrolactones, suggesting either antagonism of the picrotoxin receptor, or a second positive (modulatory) “lactone” site (Holland et al., 1991, 1993, 1995; Williams et al., 1997; Gonzales et al., 2003).
Penicillin and lactam site(s)
Despite early studies (implying action via benzodiazepine site; Shiraishi et al., 1993), later data demonstrated binding of penicillin and other β-lactams to an ionophore site, with a physical blockage of GABA-A channel (Twyman et al., 1992; Fujimoto et al., 1995; Lindquist et al., 2004) and complex multiphasic kinetics (Katayama et al., 2002). Structural similarity and interference with psychopharmacological effects of picrotoxin and PTZ (Kalueff, 2002) confirms that penicillin, like other ionophore ligands, acts via a common convulsant binding site. Sensitivity of penicillin binding to mutations in TM2 residue 6 (Table 2) indicates that it may be a common site for picrotoxin, PTZ and penicillin (Fig. 1).
However, the fact that such mutation completely abolished picrotoxin binding, but only reduced penicillin binding (Sugimoto et al., 2002), suggests that residue 6 may be on a border of penicillin binding pocket, as suggested in Fig. 1. Insensitivity of penicillin (but not picrotoxin) binding to a point mutation in residue 9 indicates that this residue is not a critical element of penicillin binding site, and is most likely located outside the penicillin site (Tierney et al., 1996; Lindquist et al., 2004). Given partial effects of residue 6 mutations on penicillin binding, this indirectly suggests that penicillin binding site may be located below residue 6 of TM2 (Fig. 1).
Notably, other β-lactams, such as cephalosporines and penems, are known as competitive voltage-independent inhibitors of GABA-A ionophore, strikingly dissimilar to non-competitive voltage-dependent action of penicillin (Fujimoto et al., 1995; Sugimoto et al., 2002, 2003). Collectively, this implies different mechanisms of action (and binding sites) of penicillin and other lactams (Sugimoto et al., 2003). Since mounting data shows heterogeneity of penicillin and other β-lactam binding sites, it is possible to assume distinct binding sites for penicillin and other β-lactams. Insensitivity of β-lactam binding to mutated residue 6 (Sugimoto et al., 2002) suggests that this additional “lactam” binding site is not within penicillin binding pocket (see model in Fig. 1).
Neurotoxic pesticides
NP, such as lindane, α-endosulphan and dieldrin, share structural similarity (and compete for the binding site) with picrotoxin, inhibit TBPS binding, induce seizures and block Cl-currents through ionophore (Lindane, 1991; Ffrench-Constant et al., 1993; Edwards and Lees, 1997; Le Corronc et al., 2002; Kaminski et al., 2004). Together, this implies similar mechanisms of their action, also see (Chen et al., 2006) for discussion. However, some differences of insect GABA receptors in sensitivity to picrotoxin and NP blockage (Le Corronc et al., 2002), and of rat and fish GABA-A receptors to TBPS and lindane (Thompson et al., 1990), suggest that binding sites of these ligands are overlapping but not identical. Sensitivity of some NP-mediated effects to point mutation in residue 2 (Edwards and Lees, 1997) (Table 2) suggests the location of NP site(s) close to this residue (Fig. 1), rather than to residue 6. Positioning bicyclophosphate site close to NP binding site in this model is also in line with numerous above-mentioned data on overlapping pharmacological mechanisms of their action.
3. Non-GABA-A receptors
A substantial homology in molecular structures of different ionophore receptors (Eldefrawi and Eldefrawi, 1987; Vassilatis et al., 1997; Yoon et al., 1998; Bloomquist, 2003; Erkkila et al., 2004) implies similar actions of their ionophore ligands (Table 3); also see (Thompson et al., 1999; Horenstein et al., 2001; Jensen et al., 2002; Chen et al., 2006) for discussion. Can the model suggested for GABA-A receptor ionophore (Fig. 1) be generally applied to other ionophore channels? While mounting neurogenetic data generally supports this notion, it also reveals some interesting receptor-specific differences.
For example, residues 2, 6, 15 and 19 of TM2-domain of glycine receptor β-subunits are critical determinants for picrotoxin binding, whereas residue 6 is important for picrotoxin binding to glutamate and serotonin 5-HT3 receptors, although reducing but not abolishing picrotoxin sensitivity in the latter (Lynch et al., 1995; Shan et al., 2001; Dibas et al., 2002; Das and Dillon, 2005). Residue 6 of TM2 is critical for picrotoxin and NP binding by insect GABA receptors (Ffrench-Constant et al., 1993; Jursky et al., 2000). Unlike GABA-A and glycine receptors, residue 2 is not required for picrotoxin binding to 5-HT3 receptors, reflecting different functions of this residue in different receptors (see, however, an additional modulatory role of residue 7 in Das and Dillon, 2005). Interestingly, while picrotoxin binding to glycine receptors is use- and voltage-independent (Lynch et al., 1995), it was use-dependent for N-acetylcholine (Erkkila et al., 2004) and glutamate (Etter et al., 1999) receptors, suggesting that amino acids in position 15 (different in these receptors) may modulate use-dependent character of picrotoxin binding (Dibas et al., 2002).
There are other examples of receptor-specific differences in ionophore binding of convulsant ligands. For instance, in addition to GABA-A receptors, NP also inhibit glycine receptors (Table 3), supporting common mechanism of their action at ion channels (Vale et al., 2003). While NP lindane was equally effective in blocking both receptors, endosulphan and dieldrin were more active at GABA-A channels (Vale et al., 2003); also see GABA-A-selectivity for another related compound BIDN (Hamon et al., 1998). Likewise, TBPS binds to GABA-A receptors but shows much weaker binding to invertebrate GABA receptors (Yagle et al., 2003). Collectively, these findings further confirm the notion that different ionophore ligands may have complex interactions with ionophores at different receptors (Das and Dillon, 2003; Hosie et al., 2006).
4. Concluding remarks
Mounting data evidences that ionophore binding sites of GABA-A and other ionotropic receptors demonstrate sufficient homology and show heterogeneous overlapping binding sites for different convulsant ligands (Fig. 1). Ligand-binding area of ionophore can be considered as a “big picrotoxin binding pocket”, representing a conservative basis for clustering sites of other channel ligands. The ability of some mutant channels to be picrotoxin-resistant and yet sensitive to other similar ligands (e.g., data for penicillin in Tierney et al., 1996) suggests a relative autonomy of ionophore blockage by different ligands. Moreover, many classes of convulsant drugs discussed here have been suggested to have dual mechanisms of ionophore action, including both inhibitory and stimulatory effects (e.g., Williams et al., 1997 (butyrolactones); Dibas and Dillon, 2000 (PTZ); Kalueff, 2002 (penicillin)). In line with this, Lynch et al. (1995) have shown that a single mutation in glycine receptor may convert picrotoxin from antagonist into allosteric potentiator. Given similarity of various ionophore receptors, it is possible to expect that similar phenomenon may exist for other receptors, including GABA-A.
Finally, it is possible to assume that GABA-A ionophore binding sites may have complex 3D architectonics, determining the accessibility for, and interactions with, various channel convulsants. Clearly, an in-depth analysis of 3D structures of GABA-A receptor channels may improve the present ionophore model (Fig. 1) and help clarify the impact of individual TM2 point mutations on binding of different channel ligands. Moreover, in addition to ligand binding, the effects on channel functions may be related transduction mechanisms (Miyazawa et al., 2003; Unwin, 2005). Recent homology 3D models of receptor channels (Miyazawa et al., 2003; Maksay, 2005; Reeves et al., 2005), based on the electron microscopic structures of the nicotinic receptor channel (Unwin, 1995, 2003, 2005), may be a useful approach to further modeling of GABA-A receptor ionophore.
In conclusion, although the model of ionophore suggested here (Fig. 1) needs further sophistication and elaboration, it paves the way to reconstructing ionophore binding pockets in GABA-A and other receptors, based on data from neurogenetics and neurochemistry. Understanding how different binding sites may be located relative to each other would help to design new selective ligands that will target several overlapping sites or bind simultaneously to several distinct neighboring sites. Based on modulation of “ionophore” binding sites, this may lead to creation of novel classes of selective GABA-ergic channel-active neurotropic drugs (also see Lynch et al., 1995; Dawson et al., 2000).
Acknowledgments
This research was supported by the NIH/NIMH Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akaike N, Yakushiji T, Tokutomi N, Carpenter DO. Multiple mechanisms of antagonism of gamma-aminobutyric acid (GABA) responses. Cell Mol Neurobiol. 1987;7:97–103. doi: 10.1007/BF00734993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argyropoulos SV, Sandford JJ, Nutt DJ. The psychobiology of anxiolytic drug. Part 2: Pharmacological treatments of anxiety. Pharmacol Ther. 2000;88:213–227. doi: 10.1016/s0163-7258(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 3.Atack JR. Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site. Curr Drug Targets CNS Neurol Disord. 2003;2:213–232. doi: 10.2174/1568007033482841. [DOI] [PubMed] [Google Scholar]
- 4.Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- 5.Bloomquist JR. Chloride channels as tools for developing selective insecticides. Arch Insect Biochem Physiol. 2003;54:145–156. doi: 10.1002/arch.10112. [DOI] [PubMed] [Google Scholar]
- 6.Baumann SW, Baur R, Sigel E. Subunit arrangement of y-aminobuturic acid type A receptor. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 7.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 8.Behrends JC. Modulation by bicuculline and penicillin of the block by t-butyl-bicyclo-phosphorothionate (TBPS) of GABA(A)-receptor mediated Cl(−)-current responses in rat striatal neurones. Br J Pharmacol. 2000;129:402–408. doi: 10.1038/sj.bjp.0703063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell-Horner CL, Dibas M, Huang RQ, Drewe JA, Dillon GH. Influence of subunit configuration on the interaction of picrotoxin-site ligands with recombinant GABA(A) receptors. Mol Brain Res. 2000;76:47–55. doi: 10.1016/s0169-328x(99)00330-7. [DOI] [PubMed] [Google Scholar]
- 10.Buhr A, Wagner C, Fuchs K, Sieghart W, Sigel E. Two novel residues in M2 of the gamma-aminobutyric acid type A receptor affecting gating by GABA and picrotoxin affinity. J Biol Chem. 2001;276:7775–7781. doi: 10.1074/jbc.M008907200. [DOI] [PubMed] [Google Scholar]
- 11.Canney DJ, Lu HF, McKeon AC, Yoon KW, et al. Structure-activity studies of fluoroalkyl-substituted gamma-butyrolactone and gamma-thiobutyrolactone modulators of GABA(A) receptor function. Bioorg Med Chem. 1998;6:43–55. doi: 10.1016/s0968-0896(97)10006-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Durkin KA, Casida JE. Structural model for gamma-aminobutyric acid receptor noncompetitive antagonist binding: widely diverse structures fit the same site. Proc Natl Acad Sci USA. 2006;103:5185–5190. doi: 10.1073/pnas.0600370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou K-C. Modelling extracellular domains of GABA-A receptors: subtypes 1, 2, 3, and 5. Biochem Biopsych Res Commun. 2004;316:636–642. doi: 10.1016/j.bbrc.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 14.Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2004;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Das P, Bell-Horner CL, Machu TK, Dillon GH. The GABA(A) receptor antagonist picrotoxin inhibits 5-hydroxytryptamine type 3A receptors. Neuropharmacol. 2003;44:431–438. doi: 10.1016/s0028-3908(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 16.Das P, Dillon GH. The 5-HT3B subunit confers reduced sensitivity to picrotoxin when co-expressed with the 5-HT3A receptor. Mol Brain Res. 2003;119:207–212. doi: 10.1016/j.molbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Das P, Dillon GH. Molecular determinants of picrotoxin inhibition of 5-hydroxytryptamine type 3 receptors. J Pharmacol Exp Ther. 2005;314:320–328. doi: 10.1124/jpet.104.080325. [DOI] [PubMed] [Google Scholar]
- 18.Dawson GR, Wafford KA, Smith A, Marshall GR, et al. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 2000;295:1051–1060. [PubMed] [Google Scholar]
- 19.Dibas MI, Dillon GH. The central nervous system convulsant pentylenetetrazole stimulates gamma-aminobutyric acid (GABA)-activated current in picrotoxin-resistant GABA(A) receptors in HEK293 cells. Neurosci Lett. 2000;285:193–196. doi: 10.1016/s0304-3940(00)01064-8. [DOI] [PubMed] [Google Scholar]
- 20.Dibas MI, Gonzales EB, Das P, Bell-Horner CL, Dillon GH. Identification of a novel residue within the second transmembrane domain that confers use-facilitated block by picrotoxin in glycine alpha 1 receptors. J Biol Chem. 2002;277:9112–9117. doi: 10.1074/jbc.M111356200. [DOI] [PubMed] [Google Scholar]
- 21.Dillon GH, Im HK, Hamilton BJ, Carter DB, Gammill RB, Judge TM, Im WB. U-93631 causes rapid decay of gamma-aminobutyric acid-induced chloride currents in recombinant rat gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993;44:860–865. [PubMed] [Google Scholar]
- 22.Dillon GH, Im WB, Pregenzer JF, Carter DB, Hamilton BJ. [4-Dimethyl-3-t-butylcarboxyl-4,5-dihydro (1,5-a) quinoxaline] is a novel ligand to the picrotoxin site on GABAA receptors, and decreases single-channel open probability. J Pharmacol Exp Ther. 1995;272:597–603. [PubMed] [Google Scholar]
- 23.Edwards MD, Lees G. Modulation of a recombinant invertebrate gamma-aminobutyric acid receptor-chloride channel complex by isoflurane: effects of a point mutation in the M2 domain. Br J Pharmacol. 1997;122:726–732. doi: 10.1038/sj.bjp.0701417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldefrawi AT, Eldefrawi ME. Receptors for gamma-aminobutyric acid and voltage-dependent chloride channels as targets for drugs and toxicants. FASEB J. 1987;1:262–271. doi: 10.1096/fasebj.1.4.2443413. [DOI] [PubMed] [Google Scholar]
- 25.El-Etr M, Akwa Y, Robel P, Baulieu EE. Opposing effects of different steroid sulfates on GABAa receptor-mediated chloride uptake. Brain Res. 1998;790:334–338. doi: 10.1016/s0006-8993(98)00145-0. [DOI] [PubMed] [Google Scholar]
- 26.Engblom AC, Carlson BX, Olsen RW, Schousboe A, Kristiansen U. Point mutation in the first transmembrane region of the beta 2 subunit of the gamma-aminobutyric acid type A receptor alters desensitization kinetics of gamma-aminobutyric acid- and anesthetic-induced channel gating. J Biol Chem. 2002;277:17438–17447. doi: 10.1074/jbc.M111215200. [DOI] [PubMed] [Google Scholar]
- 27.Erkkila BE, Weiss DS, Wotring VE. Picrotoxin-mediated antagonism of alpha3beta4 and alpha7 acetylcholine receptors. Neuroreport. 2004;15:1969–1973. doi: 10.1097/00001756-200408260-00027. [DOI] [PubMed] [Google Scholar]
- 28.Etter A, Cully DF, Liu KK, Reiss B, et al. Picrotoxin blockade of invertebrate glutamate-gated chloride channels: subunit dependence and evidence for binding within the pore. J Neurochem. 1999;72:318–326. [PubMed] [Google Scholar]
- 29.Felix R. Channelopathies: ion channel defects linked to heritable clinical disorders. J Med Genet. 2000;37:729–740. doi: 10.1136/jmg.37.10.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ffrench-Constant RH, Rocheleau TA, Steichen JC, Chalmers AE. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- 31.Filippova N, Wotring VE, Weiss DS. Evidence that the TM1-TM2 loop contributes to the rho1 GABA receptor pore. J Biol Chem. 2004;279:20906–20914. doi: 10.1074/jbc.M401012200. [DOI] [PubMed] [Google Scholar]
- 32.Findlay GS, Ueno S, Harrison NL, Harris RA. Allosteric modulation in spontaneously active mutant gamma-aminobutyric acidA receptors. Neurosci Lett. 2001;305:77–80. doi: 10.1016/s0304-3940(01)01646-9. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto M, Munakata M, Akaike N. Dual mechanisms of GABAA response inhibition by beta-lactam antibiotics in the pyramidal neurones of the rat cerebral cortex. Br J Pharmacol. 1995;116:3014–3020. doi: 10.1111/j.1476-5381.1995.tb15957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzales EB, Bell-Horner CL, de la Cruz MA, Ferrendelli JA, Covey DF, Dillon GH. Enantioselectivity of alpha-benzyl-alpha-methyl-gamma-butyrolactone-mediated modulation of anticonvulsant activity and GABA(A) receptor function. J Pharmacol Exp Ther. 2004;309:677–683. doi: 10.1124/jpet.103.063008. [DOI] [PubMed] [Google Scholar]
- 35.Gurley D, Amin J, Ross PC, Weiss DS, White G. Point mutations in the M2 region of the alpha, beta, or gamma subunit of the GABAA channel that abolish block by picrotoxin. Recept Channels. 1995;3:13–20. [PubMed] [Google Scholar]
- 36.Hamon A, Le Corronc H, Hue B, Rauh JJ, Sattelle DB. BIDN, a bicyclic dinitrile convulsant, selectively blocks GABA-gated Cl- channels. Brain Res. 1998;780:20–26. doi: 10.1016/s0006-8993(97)00895-0. [DOI] [PubMed] [Google Scholar]
- 37.Hamann M, Desarmenien M, Vanderheyden P, et al. Electrophysiological study of tert-butylbicyclo-phosphorothionate induced block of spontaneous chloride channels. Mol Pharmacol. 1990;37:578–582. [PubMed] [Google Scholar]
- 38.Hansen SL, Sperling BB, Sanchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:105–113. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 39.Hawthorne R, Lynch JW. A picrotoxin-specific conformational change in the glycine receptor M2-M3 loop. J Biol Chem. 2005;280:35836–35843. doi: 10.1074/jbc.M506645200. [DOI] [PubMed] [Google Scholar]
- 40.Holland KD, McKeon AC, Covey DF, Ferrendelli JA. Binding interactions of convulsant and anticonvulsant gamma-butyrolactones and gamma-thiobutyrolactones with the picrotoxin receptor. J Pharmacol Exp Ther. 1990;254:578–583. [PubMed] [Google Scholar]
- 41.Holland KD, Yoon KW, Ferrendelli JA, Covey DF, Rothman SM. Gamma-butyrolactone antagonism of the picrotoxin receptor: comparison of a pure antagonist and a mixed antagonist/inverse agonist. Mol Pharmacol. 1991;39:79–84. [PubMed] [Google Scholar]
- 42.Holland KD, Bouley MG, Covey DF, Ferrendelli JA. Alkyl-substituted gamma-butyrolactones act at a distinct site allosterically linked to the TBPS/picrotoxinin site on the GABAA receptor complex. Brain Res. 1993;615:170–174. doi: 10.1016/0006-8993(93)91128-f. [DOI] [PubMed] [Google Scholar]
- 43.Holland KD, Mathews GC, Bolos-Sy AM, et al. Dual modulation of the gamma-aminobutyric acid type A receptor/ionophore by alkyl-substituted gamma-butyrolactones. Mol Pharmacol. 1995;47:1217–1223. [PubMed] [Google Scholar]
- 44.Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABA(A) receptor pore-lining M2 segment. Nat Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- 45.Horenstein J, Riegelhaupt P, Akabas MH. Differential protein mobility of the gamma-aminobutyric acid, type A, receptor alpha and beta subunit channel-lining segments. J Biol Chem. 2005;280:1573–1581. doi: 10.1074/jbc.M410881200. [DOI] [PubMed] [Google Scholar]
- 46.Hosie AM, Buckingham SD, Hamon A, Sattelle DB. Replacement of asparagine with arginine at the extracellular end of the second transmembrane (M2) region of insect GABA receptors increases sensitivity to penicillin G. Invert Neurosci. 2006;6:75–79. doi: 10.1007/s10158-006-0020-4. [DOI] [PubMed] [Google Scholar]
- 47.Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–995. [PubMed] [Google Scholar]
- 48.Ito Y, Ho IK. Studies on picrotoxin binding sites of GABAA receptors in rat cortical synaptoneurosomes. Brain Res Bull. 1994;33:373–378. doi: 10.1016/0361-9230(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 49.Jensen ML, Timmermann DB, Johansen TH, Schousboe A, Varming T, Ahring K. The beta subunit determines the ion selectivity of the GABAA receptor. J Biol Chem. 2002;277:41438–41447. doi: 10.1074/jbc.M205645200. [DOI] [PubMed] [Google Scholar]
- 50.Jentsch TJ, Stein V, Weinreich F, Zdebnik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2001;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 51.Jursky F, Fuchs K, Buhr A, Tretter V, Sigel E, Sieghart W. Identification of amino acid residues of GABA(A) receptor subunits contributing to the formation and affinity of the tert-butylbicyclophosphorothionate binding site. J Neurochem. 2000;74:1310–1316. doi: 10.1046/j.1471-4159.2000.741310.x. [DOI] [PubMed] [Google Scholar]
- 52.Kalueff AV, Nutt DJ. Role of GABA in memory and anxiety. Anxiety Depress. 1997;4:100–110. doi: 10.1002/(SICI)1520-6394(1996)4:3<100::AID-DA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 53.Kalueff AV. Neurotropic effects of benzylpenicillin and related compounds in rats. Academic Dissertation; RUDN: 2002. p. 150. [Google Scholar]
- 54.Kaminski RM, Tochman AM, Dekundy A, Turski WA, Czuczwar SJ. Ethosuximide and valproate display high efficacy against lindane-induced seizures in mice. Toxicol Lett. 2004;154:55–60. doi: 10.1016/j.toxlet.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Katayama N, Tokutomi N, Nabekura J, Akaike N. Penicillin-induced triphasic modulation of GABAA receptor-operated chloride current in frog sensory neuron. Brain Res. 1992;595:249–255. doi: 10.1016/0006-8993(92)91057-l. [DOI] [PubMed] [Google Scholar]
- 56.Korpi ER, Grunder G, Luddens H. Drug interactions at GABAa receptors. Progr Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 57.Le Corronc H, Alix P, Hue B. Differential sensitivity of two insect GABA-gated chloride channels to dieldrin, fipronil and picrotoxinin. J Insect Physiol. 2002;48:419–431. doi: 10.1016/s0022-1910(02)00061-6. [DOI] [PubMed] [Google Scholar]
- 58.Leung JW, Xue H. GABAergic functions and depression: from classical therapies to herbal medicine. Curr Drug Targets CNS Neurol Disord. 2003;2:363–374. doi: 10.2174/1568007033482715. [DOI] [PubMed] [Google Scholar]
- 59.Lindane . WHO Report. WHO; Geneva: 1991. Environmental health criteria 124; p. 208. [Google Scholar]
- 60.Lindquist CE, Dalziel JE, Cromer BA, Birnir B. Penicillin blocks human alpha 1 beta 1 and alpha 1 beta 1 gamma 2S GABAA channels that open spontaneously. Eur J Pharmacol. 2004;496:23–32. doi: 10.1016/j.ejphar.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Luddens H, Lang HJ, Korpi ER. Structure-activity relationship of furosemide-derived compounds as antagonists of cerebellum-specific GABA(A) receptors. Eur J Pharmacol. 1998;344:269–277. doi: 10.1016/s0014-2999(97)01577-x. [DOI] [PubMed] [Google Scholar]
- 62.Lynch JW, Rajendra S, Barry PH, Schofield PR. Mutations affecting the glycine receptor agonist transduction mechanism convert the competitive antagonist, picrotoxin, into an allosteric potentiator. J Biol Chem. 1995;270:13799–13806. doi: 10.1074/jbc.270.23.13799. [DOI] [PubMed] [Google Scholar]
- 63.Maksay G. From kinetics and thermodynamics of GABAa receptor binding to ionophore function. Neurochem Int. 1996;29:361–370. doi: 10.1016/0197-0186(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 64.Maksay G. Activation of ionotropic receptors and thermodynamics of binding. Neurochem Int. 2005;46:281–291. doi: 10.1016/j.neuint.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Maksay G, Molnar P, Gruber L. Common modes of action of gamma-butyrolactones and pentylenetetrazole on the GABAA receptor-ionophore complex. Eur J Pharmacol. 1994;288:61–68. doi: 10.1016/0922-4106(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 66.Maksay G, Molnar P, Simonyi M. Thermodynamics and kinetics of t-butylbicyclophosphorothionate binding differentiate convulsant and depressant barbiturate stereoisomers acting via GABAA ionophores. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:306–313. doi: 10.1007/BF00168633. [DOI] [PubMed] [Google Scholar]
- 67.Maksay G, Korpi ER, Uusi-Oukari M. Bimodal action of furosemide on convulsant (3H)EBOB binding to cerebellar and cortical GABA(A) receptors. Neurochem Int. 1998;33:353–358. doi: 10.1016/s0197-0186(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 68.Maksay G, Thompson SA, Wafford KA. The pharmacology of spontaneously open alpha 1 beta 3 epsilon GABA A receptor-ionophores. Neuropharmacol. 2003;44:994–1002. doi: 10.1016/s0028-3908(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 69.Martin IL, Dunn SM. GABA receptors. Tocris Revs. 2002;20:1–8. [Google Scholar]
- 70.Matthews GG, Bolos-Sy AM, Covey DF, et al. Physiological comparison of a-ethyl-a-methyl-y-thiobutyrolactone with benzodiazepine and barbiturate modulators of GABAa receptors. Neuropharmacol. 1996;35:123–136. doi: 10.1016/0028-3908(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 71.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 72.Mortensen M, Wafford KA, Wingrove P, Ebert B. Pharmacology of GABA(A) receptors exhibiting different levels of spontaneous activity. Eur J Pharmacol. 2003;476:17–24. doi: 10.1016/s0014-2999(03)02125-3. [DOI] [PubMed] [Google Scholar]
- 73.Muroi Y, Czajkowski C, Jackson MB. Local and global ligand-induced changes in the structure of the GABA(A) receptor. Biochemistry. 2006;45:7013–7022. doi: 10.1021/bi060222v. [DOI] [PubMed] [Google Scholar]
- 74.Newell JG, McDevitt RA, Czajkowski C. Mutation of glutamate 155 of the GABAA receptor beta2 subunit produces a spontaneously open channel: a trigger for channel activation. J Neurosci. 2004;24:11226–11235. doi: 10.1523/JNEUROSCI.3746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newland CF, Cull-Candy SG. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurons of the rat. J Physiol. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nobrega JN, Richter A, Burnham WM, Loscher W. Alterations in the brain GABAA/benzodiazepine receptor-chloride ionophore complex in a genetic model of paroxysmal dystonia: a quantitative autoradiographic analysis. Neuroscience. 1995;64:229–239. doi: 10.1016/0306-4522(94)00334-2. [DOI] [PubMed] [Google Scholar]
- 77.Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- 78.Olsen RW, Leeb-Lundberg F, Napioas C. Picrotoxin and convulsant binding sites in mammalian brain. Brain Res Bull. 1980;5:217–221. doi: 10.1016/0006-8993(81)90141-4. [DOI] [PubMed] [Google Scholar]
- 79.Olsen RW, McCabe RT, Wamsley JK. GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- 80.Olsen RW, Chang C-SS, Li G, Hanchar HJ, Wallner M. Fishing for allosteric sites on GABAa receptors. Biochem Pharmacol. 2004;68:1675–1684. doi: 10.1016/j.bcp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 81.Olsen RW. Picrotoxin-like channel blockers of GABAA receptors. Proc Natl Acad Sci USA. 2006;103:6081–6082. doi: 10.1073/pnas.0601121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omrani A, Fathollahi Y, Almasi M, Semnanian S, et al. Contribution of ionotropic glutamate receptors and voltage-dependent calcium channels to the potentiation phenomenon induced by transient pentylenetetrazol in the CA1 region of rat hippocampal slices. Brain Res. 2003;959:173–181. doi: 10.1016/s0006-8993(02)03830-1. [DOI] [PubMed] [Google Scholar]
- 83.Peris J, Shawley A, Dawson R, Abendschein KH. Regulation of 35S-TBPS binding by bicuculline is region specific in rat brain. Life Sci. 1991;49:PL49–PL54. doi: 10.1016/0024-3205(91)90250-f. [DOI] [PubMed] [Google Scholar]
- 84.Perret P, Sarda X, Wolff M, Wu TT, Bushey D, Goeldner M. Interaction of non-competitive blockers within the gamma-aminobutyric acid type A chloride channel using chemically reactive probes as chemical sensors for cysteine mutants. J Biol Chem. 1999;274:25350–25354. doi: 10.1074/jbc.274.36.25350. [DOI] [PubMed] [Google Scholar]
- 85.Reeves DC, Jansen M, Bali M, Lemster T, Akabas MH. A role for the beta 1-beta 2 loop in the gating of 5-HT3 receptors. J Neurosci. 2005;25:9358–9366. doi: 10.1523/JNEUROSCI.1045-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosahl TW. Validation of GABA(A) receptor subtypes as potential drug targets by using genetically modified mice. Curr Drug Targets CNS Neurol Disord. 2003;2:207–212. doi: 10.2174/1568007033482823. [DOI] [PubMed] [Google Scholar]
- 87.Rossi J, Ritche GD, McInturf S, Nordholm AF. Reduction of motor seizures in rats induced by the ethyl bicyclophosphate trimethylolpropane phosphate (TMPP) Prog Neuro-Psychopharmacol Biol Psychiatry. 2001;25:1323–1340. doi: 10.1016/s0278-5846(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 88.Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 89.Sandford JJ, Argyropoulos SV, Nutt DJ. The psychobiology of anxiolytic drugs. Part 1: Basic neurobiology. Pharmacol Ther. 2000;88:197–212. doi: 10.1016/s0163-7258(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 90.Scheller M, Forman SA. Coupled and uncoupled gating and desensitization effects by pore domain mutations in GABA(A) receptors. J Neurosci. 2002;22:8411–8421. doi: 10.1523/JNEUROSCI.22-19-08411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shan Q, Haddrill JL, Lynch JW. A single beta subunit M2 domain residue controls the picrotoxin sensitivity of alpha-beta glycine receptor chloride channels. J Neurochem. 2001;76:1109–1120. doi: 10.1046/j.1471-4159.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 92.Shan Q, Haddrill JL, Lynch JW. Comparative surface accessibility of a pore-lining threonine residue (T6′) in the glycine and GABA(A) receptors. J Biol Chem. 2002;277:44845–44853. doi: 10.1074/jbc.M208647200. [DOI] [PubMed] [Google Scholar]
- 93.Shiraishi H, Ito M, Go T, Mikawa H. High doses of penicillin decreases (3H)flunitrazepam binding sites in rat neuron primary culture. Brain Dev. 1993;15:356–361. doi: 10.1016/0387-7604(93)90121-n. [DOI] [PubMed] [Google Scholar]
- 94.Sinkkonen ST, Uusi-Oukari M, Tupala E. Characterization of gamma-aminobutyrate type A receptors with atypical coupling between agonist and convulsant binding sites in discrete brain regions. Mol Brain Res. 2001;86:168–178. doi: 10.1016/s0169-328x(00)00275-8. [DOI] [PubMed] [Google Scholar]
- 95.Sinkkonen ST, Rabe H, Luddens H, Korpi ER. Evidence for a reduction of coupling between GABAA receptor agonist and ionophore binding sites by inorganic phosphate. Neurochem Res. 2005;30:1471–1482. doi: 10.1007/s11064-005-8824-x. [DOI] [PubMed] [Google Scholar]
- 96.Sousa A, Ticku MK. Interactions of the neurosteroid dehydroepiandrosterone sulfate with the GABA(A) receptor complex reveals that it may act via the picrotoxin site. J Pharmacol Exp Ther. 1997;282:827–833. [PubMed] [Google Scholar]
- 97.Squires RF, Saederup E, Crawley JN, Skolnick P, Paul SM. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984;35:1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- 98.Sugimoto M, Fukami S, Kayakiri H, Yamazaki S, et al. The beta-lactam antibiotics, penicillin-G and cefoselis have different mechanisms and sites of action at GABA(A) receptors. Br J Pharmacol. 2002;135:427–432. doi: 10.1038/sj.bjp.0704496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugimoto M, Uchida I, Mashimo T, Yamazaki S, et al. Evidence for the involvement of GABAa receptor blockade in convulsions induced by cephalosporins. Neuropharmacol. 2003;45:304–314. doi: 10.1016/s0028-3908(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 100.Thompson RG, Menking DE, Valdes JJ. Comparison of lindane, bicyclophosphate and picrotoxin binding to the putative chloride channel sites in rat brain and Torpedo electric organ. Neurotoxicol Teratol. 1990;12:57–63. doi: 10.1016/0892-0362(90)90113-q. [DOI] [PubMed] [Google Scholar]
- 101.Thompson SA, Smith MZ, Wingrove B, Whiting PJ, Wafford KA. Mutation at the putative GABA(A) ion-channel gate reveals changes in allosteric modulation. Br J Pharmacol. 1999;127:1349–1358. doi: 10.1038/sj.bjp.0702687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tierney ML, Birnir B, Pillai NP, Clements JD, et al. Effects of mutating leucine to threonine in the M2 segment of alpha1 and beta1 subunits of GABAA alpha1beta1 receptors. J Membr Biol. 1996;154:11–21. doi: 10.1007/s002329900128. [DOI] [PubMed] [Google Scholar]
- 103.Twyman RE, Green RM, MacDonald RL. Kinetics of open channel block by penicillin of single GABAA receptor channels from mouse spinal cord neurones in culture. J Physiol. 1992;445:97–127. doi: 10.1113/jphysiol.1992.sp018914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 105.Unwin N. Structure and action of the nicotinic acetylcholine receptor explored by electron microscopy. FEBS Lett. 2003;555:91–95. doi: 10.1016/s0014-5793(03)01084-6. [DOI] [PubMed] [Google Scholar]
- 106.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 107.Vale C, Fonfria E, Bujons J, Messeguer A, Rodriguez-Farre E, Sunol C. The organochlorine pesticides gamma-hexachlorocyclohexane (lindane), alpha-endosulfan and dieldrin differentially interact with GABA(A) and glycine-gated chloride channels in primary cultures of cerebellar granule cells. Neuroscience. 2003;37:397–403. doi: 10.1016/s0306-4522(02)00875-8. [DOI] [PubMed] [Google Scholar]
- 108.Vassilatis DK, Elliston KO, Paress PS, Hamelin M, et al. Evolutionary relationship of the ligand-gated ion channels and the avermectin-sensitive, glutamate-gated chloride channels. J Mol Evol. 1997;44:501–508. doi: 10.1007/pl00006174. [DOI] [PubMed] [Google Scholar]
- 109.Vicini S, Ortinski P. Genetic manipulations of GABAA receptor in mice make inhibition exciting. Pharmacol Ther. 2004;103:109–120. doi: 10.1016/j.pharmthera.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Wang TL, Hackam AS, Guggino WB, Cutting GR. A single amino acid in gamma-aminobutyric acid rho 1 receptors affects competitive and noncompetitive components of picrotoxin inhibition. Proc Natl Acad Sci USA. 1995;92:11751–11755. doi: 10.1073/pnas.92.25.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams KL, Tucker JB, White G, Weiss DS, et al. Lactone modulation of the gamma-aminobutyric acid A receptor: evidence for a positive modulatory site. Mol Pharmacol. 1997;52:14–119. doi: 10.1124/mol.52.1.114. [DOI] [PubMed] [Google Scholar]
- 112.Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric beta3 GABA(A) receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 113.Yagle M, Martin MW, de Fiebre CM, de Fiebre NC, Drewe JA, Dilon GH. (3H)Etynylbicycloorthobenzoate ((3H)EBOB) binding in recombinant GABAa receptors. NeuroToxicol. 2003;24:817–824. doi: 10.1016/S0161-813X(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 114.Yakushiji T, Tokutomi N, Akaike N, Carpenter DO. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987;22:1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]
- 115.Yoon KW, Covey DF, Rothman SM. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang HG, Lee HJ, Rocheleau T, ffrench-Constant RH, Jackson MB. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric acid receptors. Mol Pharmacol. 1995;48:835–840. [PubMed] [Google Scholar]
- 117.Zhorov BS, Bregestovski PD. Chloride channels of glycine and GABA receptors with blockers: Monte Carlo minimization and structure-activity relationships. Biophys J. 2000;78:1786–1803. doi: 10.1016/S0006-3495(00)76729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


