Abstract
Signaling pathways involved in regulating nuclear-cytoplasmic distribution of BRCA1 have not been previously reported. Here, we provide evidence that heregulin β1-induced activation of the Akt pathway increases the nuclear content of BRCA1. First, treatment of T47D breast cancer cells with heregulin β1 results in a two-fold increase in nuclear BRCA1 as assessed by FACS analysis, immunoblotting and immunofluorescence. This heregulin-induced increase in nuclear BRCA1 is blocked by siRNA-mediated down-regulation of Akt. Second, mutation of threonine 509 in BRCA1, the site of Akt phosphorylation, to an alanine, attenuates the ability of heregulin to induce BRCA1 nuclear accumulation. These data suggest that Akt-catalyzed phosphorylation of BRCA1 is required for the heregulin-regulated nuclear concentration of BRCA1. Because most functions ascribed to BRCA1 occur within the nucleus, we postulated that phosphorylation-dependent nuclear accumulation of BRCA1 would result in enhanced nuclear activity, specifically transcriptional activity, of BRCA1. This postulate is affirmed by our observation that the ability of BRCA1 to transactivate GADD45 promoter constructs was enhanced in T47D cells treated with heregulin β1. Furthermore, the heterologous expression of BRCA1 in HCC1937 human breast cancer cells, which have constitutively active Akt, also induces GADD45 promoter activity, whereas the expression of BRCA1 in which threonine 509 has been mutated to an alanine is able to only minimally induce promoter activity. These findings implicate Akt in upstream events leading to BRCA1 nuclear localization and function.
Keywords: BRCA1, heregulin, Akt, phosphorylation, GADD45 promoter
INTRODUCTION
BRCA1 is a tumor suppressor with functions that can be classified as either nuclear or non-nuclear. Its nuclear functions include inhibition of cell growth [1], induction of apoptosis [2], and regulation of the cell cycle [3]; as well as serving as a transcriptional co-activator [4], an E3 ubiquitin ligase [5] and a caretaker in maintaining genomic integrity [6,7]. The known nonnuclear function of BRCA1 is to regulate centrosome duplication [8]. Many of these functions are overlapping. For example, the abilities of BRCA1 to inhibit cell growth, regulate the cell cycle and induce apoptosis are probably due to its transcriptional co-activation activity. BRCA1 induces the expression of genes involved in these processes, including p21WAF/Cip 1 [3] and GADD45 [9], which control the cell cycle, and p53 [10] and Bcl-2 [11], which have roles in apoptosis. The ability of BRCA1 to function in DNA repair processes may be coupled to its ability to act as an E3 ubiquitin ligase. In this context, BRCA1 interacts with BARD1 [BRCA1-associated RING domain 1] to enhance this ligase activity [12] and targets histone H2A/H2AX [13], which colocalizes with BRCA1 at sites of DNA damage. These findings suggest that BRCA1 also can modify chromatin structure, possibly by ubiquitination, as it maintains genomic integrity.
BRCA1 is predominantly a nuclear protein but undergoes nuclear/cytoplasmic shuttling. The nuclear import of BRCA1 has been proposed to be mediated through interaction with importins or with BARD1. BRCA1 interacts with importin α and can be actively imported into the nucleus via the importin α/β pathway. Interaction with importin α can occur through two nuclear localization signals in BRCA1 located at amino acids 503-508 and 606-615 [14], but studies suggest that only the interaction at amino acid residues 503-508 is physiologically important in targeting BRCA1 to the nucleus [15]. The interaction of BARD1 with BRCA1 also results in the translocation of BRCA1 into the nucleus via a piggyback mechanism [16,17]. The nuclear export of BRCA1 occurs via two leucine-rich nuclear export sequences located at amino acid residues 81-99 [17] and 22-30 [18].
BRCA1 is hyperphosphorylated during the late G1 and S phases of the cell cycle and dephosphorylated during early M phase [19]. Protein kinases that phosphorylate BRCA1 include ATM [20], Cds1 (check point kinase) [21], ATR [22] and Akt [23]. Altiok et al. [23] demonstrated that heregulin β1 stimulation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway results in phosphorylation of BRCA1 by Akt on threonine 509, which is adjacent to the nuclear localization signal at residues 503-508 in BRCA1. Importantly, however, the functional significance of the phosphorylation at threonine 509 on the nuclear localization of BRCA1 has not been investigated.
The present studies were undertaken to assess the importance of the Akt pathway in regulating the nuclear content of BRCA1. Our findings reveal that the nuclear localization of BRCA1 is enhanced by activation of the Akt pathway and this nuclear accumulation of BRCA1 is contingent on the availability of threonine 509 for phosphorylation. These studies are the first evidence for a signal transduction pathway modulating BRCA1 nuclear localization. Furthermore, the present findings also affirm the functional importance of regulating BRCA1 subcellular localization.
MATERIALS AND METHODS
Cell culture
All cells were obtained from American Type Culture Collection. T47D and HCC1937 cells were maintained in RPMI 1640 (Invitrogen) with 10% Fetal Clone III (FCS; Hyclone), 1% insulin-transferrin-selenium A (ITSA; Invitrogen) and 1% antibiotic-antimycotic (Invitrogen). HeLa cells were maintained in DMEM with 10% FCS and 1% antibiotic-antimycotic. MCF-7 cells were maintained as previously described [18].
Plasmid constructs and mutagenesis
Construction of pEGFPC1-BRCA1 has been previously described [18]. T509A mutation was generated using the Quik Change XL Site-Directed Mutagenesis kit (Stratagene) using pCRScript-BRCA1 as template. An EcoR I-Kpn I fragment of the T509A mutation was then subcloned into the same sites in pEGFPC1-BRCA1 to yield pEGFPC1-BRCA1T509A. Mutations were confirmed by DNA sequencing. Oligonucleotides corresponding to DNA sequences in the GADD45 promoter were designed as described [11] and cloned into the Kpn I and Bgl II sites of pCAT3 Enhancer plasmid (Promega) to generate GADD45 promoter constructs. Oligonucleotides were generated by Integrated DNA Technologies (Coralville, IA).
Fluorescence-activated cell sorting
T47D cells (4-5 × 106) were plated in 150 mm cell culture dishes overnight, serum-starved (0% serum) for 48 h and incubated with 10 nM heregulin β1 (Sigma) or an equal volume of PBS with 0.1 % BSA (vehicle) for 90 min at 37ºC. Cells were harvested, centrifuged at 300 × g at 4ºC for 5 min and resuspended in lysis buffer (0.1 M Tris, pH 7.4, 0.1 M NaCl, 3 mM MgCl2, 0.5% NP40). After 30-min incubation on ice, the lysate was centrifuged at 600 × g at 4ºC for 5 min. The nuclear pellet was washed with lysis buffer and centrifuged at 600 × g and washed in PBS. After fixation in 2% paraformaldehyde on ice for 1 h, nuclei were centrifuged at 600 × g, washed twice in 0.1 M glycine-Tris (pH 7.3), once in PBS and then incubated in blocking buffer (5% donkey serum, 1% BSA, and 0.1% Triton X-100 in PBS) for 2 h at 4ºC. Equal numbers of nuclei from each sample were incubated with 1 µg/ml mouse anti-β-galactosidase IgG (Boehringer Mannheim) or 1:100 mouse anti-BRCA1 IgG (Ab-1; Calbiochem) in blocking buffer overnight at 4°C. Nuclei were washed in PBS and incubated in 1:500 dilution of FITC-conjugated donkey anti-mouse IgG in blocking buffer for 45 min at 4ºC. The samples were washed in PBS, incubated in 1 μg/ml RNase H for 15 min at 37ºC and stained in 250 μg/ml propidium iodide. Fluorescence was detected with a Becton-Dickinson FACS Calibur Benchtop Analyzer.
Nuclear-cytoplasmic fractionation and Western blotting
For Western blots of fractionated proteins, nuclear and cytoplasmic fractions were isolated using the Nuclei EZ Prep kit (Sigma). For Western blots of whole cell lysates, cells were lysed in RIPA (10 mM Tris, pH 8.0, 0.14 M NaCl, 0.25% NaN3, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS). Proteins were separated through a 6 or 7% SDS-polyacrylamide gel and transferred to polyvinylidene flouride membrane overnight. Transferred proteins were immunoblotted for BRCA1 (Ab-1; 1:1000), MSH-6 (1:500; BD Biosciences), topoisomerase 1 (1:1000; Santa Cruz), GFP (1:500; Zymed) overnight at 4ºC or for α-tubulin (1:2000; Sigma) at room temperature for 30 min. Membranes were washed in TBST and incubated in peroxidase-conjugated donkey anti-mouse or anti-rabbit IgG (1:10,000; Jackson Immunoresearch). Bands were detected with either Lumiglo (Cell Signaling) or Super Signal West Femto Maximum Sensitivity Substrate (Pierce) chemiluminescence systems. Densitometric analysis was performed using an AlphaImager 2000 Documentation and Analysis System (Alpha Innotech).
Akt kinase activity assay
The activity of Akt was assayed by the ability of immunoprecipitated Akt to phosphorylate GSK-3 in an in vitro kinase assay (Akt Kinase Assay Kit; Cell Signaling) per manufacturer’s protocol. The reactions were terminated by the addition of Laemmli sample buffer and proteins were separated through a 10% SDS polyacrylamide gel. Akt activity was assayed by immunoblotting for phosphorylated GSK-3. Membranes were stripped and reprobed for total GSK-3 (1:1000; Cell Signaling). Total Akt levels were assessed using 1:1000 dilution of Akt antibody (Cell Signaling).
Indirect immunocytochemistry
Cells (5 × 104 cells/well) were plated on 22 mm coverslips in six-well dishes overnight, serum-starved (0% serum) for 48 h and incubated with 10 nM heregulin β1 for 90 min prior to fixation with 2% paraformaldehyde in PBS for 30 min. Cells were washed twice in 0.1 M glycine-Tris, pH 7.3, followed by PBS, incubated in blocking buffer followed by an overnight incubation at 4ºC in primary antibody (Ab-1 or AbC; PharMingen) diluted 1:100 in blocking buffer. Cells were washed in PBS three times and incubated with Cy3-conjugated donkey anti-mouse or anti-rabbit IgG (1:2000) (Jackson Immunoresearch). Nuclei were visualized by 4,6 diamidino-2 phenylindole (DAPI; Sigma). The cells were mounted in Aqua-Polymount (Polysciences, Inc) and fluorescence was analyzed with an Olympus BX41 microscope. Fluorescence intensities were quantified by obtaining the fluorescence of the whole cell and the fluorescence within the nucleus of captured images using the AlphaImager 2000 Documentation system. Cells with a nuclear:total fluorescence ratio ≥0.5 are defined as cells having >50% nuclear fluorescence.
siRNA studies
For Akt siRNA studies, the Signal Silence Akt siRNA kit (Cell Signaling) specific for human Akt was used. For immunofluorescence, T47D cells were plated onto coverslips in 6-well dishes at 50,000 cells/well. Cells were transfected with control siRNA alone or with approximately 20 nM Akt-targeted siRNA using GeneJammer (Stratagene) according to manufacturer’s protocol. Indirect immunocytochemistry for Akt was performed as described above using an antibody against Akt1/2 (1:100; Santa Cruz Biotechnology). Secondary antibody was Cy-3-conjugated donkey anti-rabbit IgG (1:2000; Jackson Immunoresearch). Akt fluorescence was quantified using AlphaImager 2000 Documentation system. Single cells, which had been successfully transfected based on the presence of FITC fluorescence from the control siRNA, within captured images were outlined for quantification of Cy-3 fluorescence.
For Western analysis of Akt expression, cells were plated at 100,000 cells/well. Forty-eight hours post-transfection, cells were lysed in 100 μl/well Laemmli sample buffer and 40 μl of each sample was electrophoresed through a 10% SDS polyacrylamide gel. After transfer to PVDF membrane, Akt was probed using an Akt1/2 antibody (1:1000) overnight at 4ºC and bands detected as described above.
Transient transfections
Transient transfections of pEGFPC1-BRCA1 or pEGFPC1-BRCA1T509A were performed in HeLa and MCF-7 cells using 4 μg DNA and 12 μl GeneJammer. For experiments assessing the heregulin effect, cells were treated with 10 nM heregulin β1 for 90 minutes. Cells were fixed in 2% paraformaldehyde for 30 min, washed in 0.1 M glycine-Tris, pH 7.4 twice and once in PBS for 5 min each. Cells were permeabilized in PBS with 5% normal donkey serum, 1% BSA, and 0.1% Triton X-100 for 30 min. Cells were washed in PBS for 5 min and incubated in 100 μg/ml RNase A (Sigma) in PBS at 37ºC for 20 min. The cells were washed in PBS, incubated in 333 ng/ml propidium iodide in PBS for 2 min, washed in PBS, mounted in AquaPoly Mount, and visualized with an Olympus BX 41 microscope.
Choramphenicol acetyltransferase activity assays
T47D cells were transfected with 2 µg of GADD45 promoter constructs and 0.2 µg of β-galactosidase plasmid [24] in serum-free media. Forty-eight h later, the cells were treated with vehicle or heregulin β1 for 90 min. After harvesting, β-galactosidase and CAT assays were performed as previously described [18,24].
HCC1937 cells were transfected with 2 μg pEGFPC1 or 4 μg wildtype or T509A mutant BRCA1 construct using GeneJammer. Cells were grown in the presence of G418 to select for transfected cells. Fluorescence analysis was performed as described above. For CAT assays, cells were plated at 100,000 cells/well and transfected with 2 μg GADD45 promoter constructs and 1 µg β-galactosidase plasmid. Forty-eight hours later, cells were harvested and assayed for β-galactosidase and CAT activities.
Statistical analysis
Where applicable, data were analyzed by a paired Student’s t-test or ANOVA using GraphPad Prism 4 (GraphPad) software.
RESULTS
Heregulin β1 enhances nuclear accumulation of BRCA1
Based on the report that Akt phosphorylates threonine 509 of BRCA1 [23] and the observation that this residue is adjacent to the functional nuclear localization signal at amino acids 503-508, we surmised that activation of the phosphatidylinositol 3-kinase/Akt pathway may influence the nuclear level of BRCA1. To test this postulate, we compared the nuclear content of BRCA1 in T47D cells after exposure to heregulin β1, an agent previously demonstrated to activate the Akt pathway [25], to the nuclear content of BRCA1 in vehicle-treated cells. T47D cells were chosen for this assessment because the initial studies demonstrating the heregulin-induced, Akt-mediated phosphorylation of BRCA1 were performed with this cell line [23]. For these studies, cells were serum-starved for 48 h, at which time approximately 80% of the cells were arrested in G1 phase of the cell cycle (data not shown). Synchronized cells were treated with vehicle or heregulin β1 for 90 min, harvested and thereafter, the nuclei isolated. Nuclei were fixed and incubated with a mouse antibody against BRCA1 or against β-galactosidase (negative control). Intact nuclei (Fig. 1A) were analyzed for FITC intensity by flow cytometry. We found that heregulin β1 treatment increased the nuclear fluorescence intensity of BRCA1, as shown in Fig. 1B and C. Panel (i) of Fig. 1B depicts the staining intensity of the antibody against BRCA1 in nuclei isolated from vehicle-treated cells and demonstrates that slightly greater than 50% of the nuclei exhibited higher than background levels (above the horizontal line) of FITC staining. Panel (ii) demonstrates that nearly all the nuclei isolated from cells treated with heregulin β1 immunostained for BRCA1. Furthermore, these data reveal an approximate 2.3-fold increase in overall fluorescence intensity (Fig. 1C) in nuclei isolated from cells treated with heregulin β1 when compared to the intensity in nuclei isolated from cells treated with vehicle. Panel (iii) of Fig. 1B depicts the staining intensity of the antibody against β-galactosidase, a protein that is not expressed in the nucleus of mammalian cells. The absence of β-galactosidase staining in the nuclei indicates that there is little, if any, nonspecific binding of the secondary antibody. Fig. 1D demonstrates that there is no difference in the steady-state level of total BRCA1 protein in vehicle or heregulin-treated cells.
Fig. 1. Heregulin β1 increases the nuclear content of BRCA1 in T47 D human breast cancer cells.
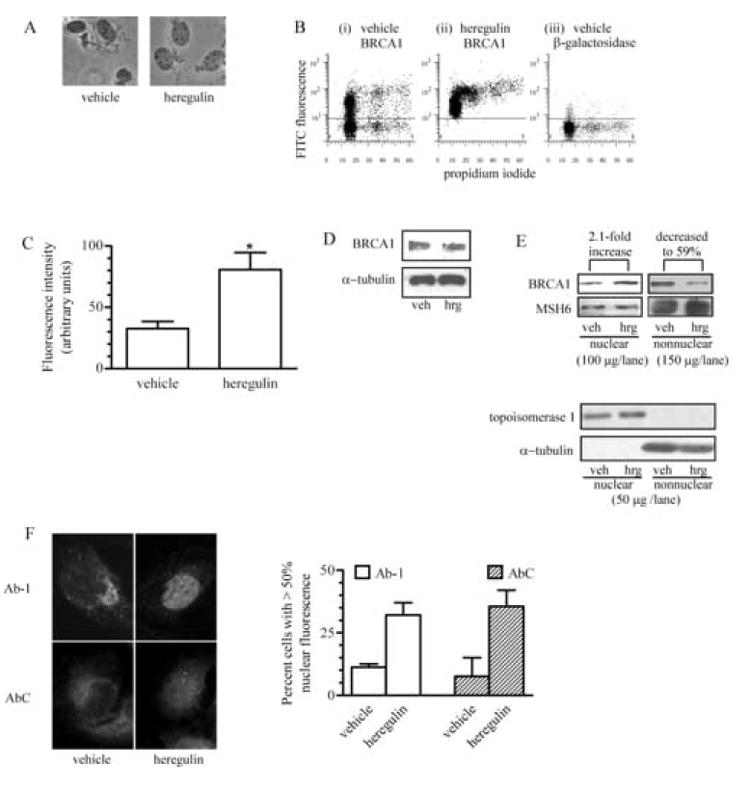
A, Nuclei were isolated from vehicle-treated or 10 nM heregulin-treated T47D cells, stained with trypan blue and visualized by light microscopy to assess nuclear integrity. Magnification, 400x. B, Flow cytometry panels depicting the FITC fluorescence in nuclei isolated from vehicle-treated (i) or 10 nM heregulin-treated (ii) cells and stained for BRCA1 or the fluorescence in nuclei isolated from vehicle-treated cells and stained for β-galactosidase (iii), which was used as a control for nonspecific signal. The y-axis plots the log of the FITC fluorescence and the x-axis is the linear fluorescence for the propidium iodide staining of the nuclei. Each dot represents a single nucleus. The number of nuclei with detectable levels of FITC staining (area above the line) for β-galactosidase was extremely low (~5%). C, FITC fluorescence intensities in nuclei isolated from vehicle or heregulin-treated cells and immunostained for BRCA1. Values shown are arbitrary units. (*, p<0.05; n=3; mean ± s.e.m.). D, Immunoblot of BRCA1 levels in whole cell lysates of T47D cells treated with vehicle or 10 nM heregulin β1 for 90 min. Equal loading was determined by immunoblotting for α-tubulin. E, Immunoblots of nuclear and nonnuclear fractions of T47D cells treated with vehicle or 10 nM heregulin β1. 100 µg nuclear and 150 µg nonnuclear protein were electrophoresed on the same gel, transferred and immunoblotted with Ab-1. Different film exposures were required to obtain nuclear and nonnuclear images. Membranes were stripped and probed for MSH6, which was used to assess loading variability. 50 µg of each sample was also electrophoresed on another gel and immunoblotted for topoisomerase 1 and α-tubulin. F, Immunofluorescence of BRCA1 in T47D cells. Serum-starved T47D cells treated with vehicle or 10 nM heregulin β1 for 90 min were immunostained with either antibody Ab-1 or AbC. Quantification of fluorescence was obtained by analyzing captured images with AlphaImager 2000 software. The fluorescence within a single cell and the fluorescence within the nucleus (as assessed by DAPI) were outlined individually and fluorescence intensity values obtained. Cells with a nuclear:total fluorescence ratio > 0.5 were classified as having >50% nuclear fluorescence. At least 99 cells/sample from two experiments were evaluated. (mean ± s.d.) Magnification, 400x.
The ability of heregulin β1 to induce nuclear accumulation of BRCA1 also was evaluated via immunoblotting of nuclear and nonnuclear fractions of T47D cells. These data, shown in Fig. 1E, revealed an increase in nuclear BRCA1 of 2.1-fold in heregulin-treated cells relative to the level in vehicle-treated cells. Furthermore, there was a concomitant 41% decrease in nonnuclear BRCA1 levels. These values were standardized for loading variability with MSH6 expression, which did not change appreciably between samples in our experiments. To demonstrate that the nuclear and nonnuclear fractions were free of proteins from the other fraction, 50 µg protein from the same fractions were immunoblotted for topoisomerase 1, a nuclear protein marker, and α-tubulin, a nonnuclear protein marker. Our data demonstrate the absence of contaminating proteins from one fraction in the other.
Indirect immunocytochemistry (Fig. 1F) with two different BRCA1 antibodies also was used to substantiate our results. Use of the Ab-1 antibody, generated against the N-terminal 304 amino acids of BRCA1, and use of AbC, generated against amino acid residues 768-793 of BRCA1, revealed a low level of immunofluorescence within the nuclear compartment of vehicle-treated cells. The predominant staining occurred in a small, concentrated area outside the nucleus. In the heregulin-treated cells, there was loss of the fluorescence concentrated outside the nucleus and an increase in nuclear fluorescence. Quantification of the percentage of cells with predominantly (>50%) nuclear fluorescence revealed similar results with each antibody, indicating an approximate 3-fold increase in cells with predominantly nuclear BRCA1. Thus, the heregulin-associated increase in nuclear BRCA1 fluorescence in Figs. 1B-1F is evidence for increased nuclear accumulation of BRCA1 in response to treatment with heregulin β1.
Activation of Akt influences BRCA1 subcellular localization
Altiok et al. [23] have demonstrated that it is activation of Akt that results in phosphorylation of BRCA1 in response to heregulin β1. Heregulin β1 binds to ErbB2/ErbB3 heterodimers, which serve as the receptor for activation of the PI3K/Akt pathway [25]. To test if this pathway is involved in the heregulin-induced increase in the nuclear content of BRCA1, we used two different experimental approaches. First, if Akt is involved, its level of activity should increase when T47D cells were treated with heregulin β1. We established that by using an in vitro kinase assay in which glycogen synthase kinase 3 (GSK-3) was the substrate. The results shown in Fig. 2 demonstrate a 2.3-fold increase in phospho-GSK-3 in cells treated with heregulin β1 when compared to the level in cells treated with vehicle, indicative of an increase in Akt activity in heregulin-treated cells.
Fig. 2. Heregulin β1 activates Akt.
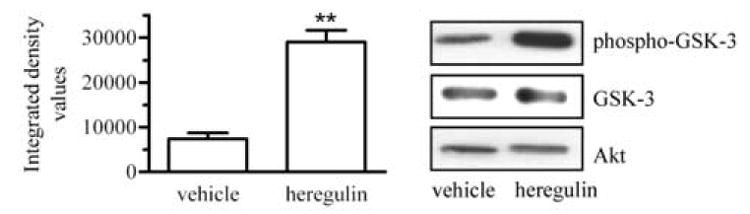
Phosphorylated Akt was immunoprecipitated from whole cell lysates of T47D cells treated with vehicle or with 10 nM heregulin β1 for 90 min. Akt activity was measured by an in vitro Akt kinase assay using GSK-3 as substrate and Western blot analysis for phospho-GSK using an anti-phospho-GSK-3 antibody. Membranes were also probed for total GSK-3 and Akt. Densitometric values for phospho-GSK-3 were normalized to total GSK-3 levels. (*, p<0.05; n=4; mean ± s.e.m.).
Second, we utilized small interfering RNA (siRNA) to knock down the expression level of Akt. Cells were transfected with a fluorescein-conjugated control siRNA alone or with Akt-targeted siRNA and immunostained for Akt. Transfected cells exhibiting green fluorescence were evaluated for Akt expression on a per-cell basis. Cells transfected with the control and Akt siRNAs exhibited an approximate 65% decrease in Akt fluorescence compared to that detected in cells transfected with control siRNA alone (Fig. 3A). This decrease was also evidenced in an immunoblot comparison of Akt levels in untransfected cells, cells transfected with control siRNA or with Akt-targeted siRNA (Fig. 3B). As shown in Fig. 3C, cells transfected with control siRNA and treated with heregulin exhibited a strong nuclear fluorescence pattern for BRCA1. This was not observed in cells transfected with Akt siRNA and treated with heregulin β1. Rather, these cells exhibited the strong concentration of BRCA1 outside the nucleus as seen in Fig. 1F. Quantification of the data revealed that in control siRNA-transfected cells, heregulin β1 increased the percentage of cells with predominantly nuclear fluorescence by ~2.5-fold. In the presence of Akt-targeted siRNA, heregulin β1 was unable to induce an increase in nuclear BRCA1. These findings suggest that there is a reciprocal relationship between Akt expression and the ability of heregulin to result in BRCA1 accumulation within the nucleus.
Fig. 3. Nuclear localization of BRCA1 is dependent on the expression of Akt.
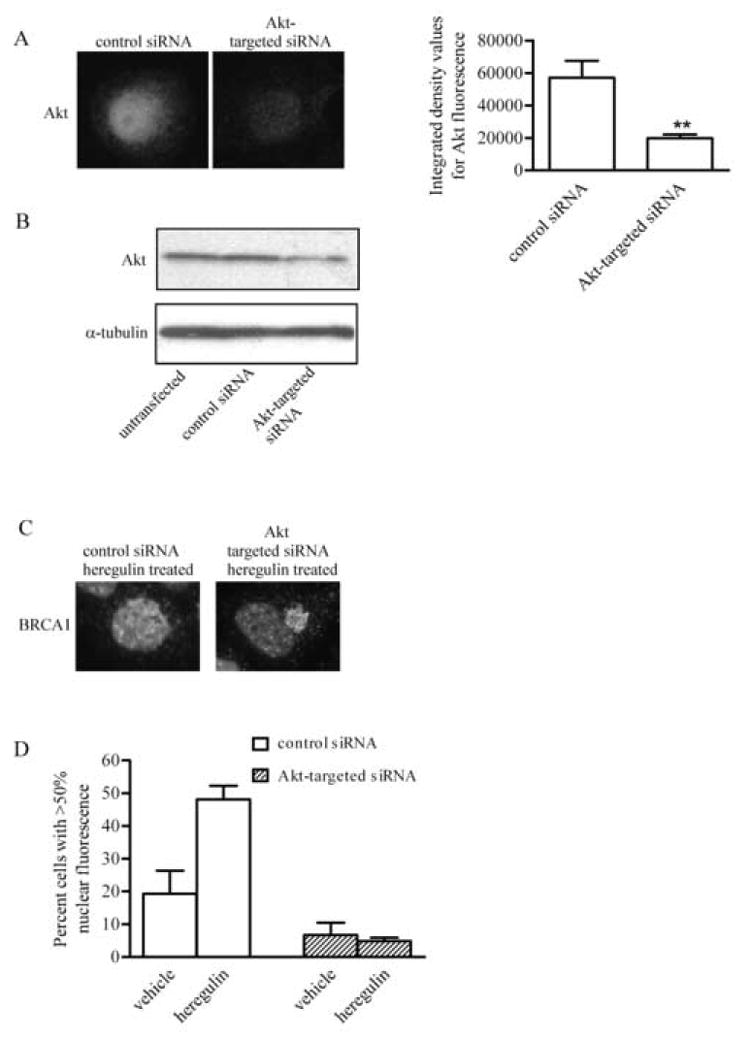
A, T47D cells were transiently transfected with a fluorescein-conjugated control siRNA with or without siRNA targeted for Akt provided in the Signal Silence Akt siRNA kit. 48 h post transfection, cells were immunostained for Akt. Pictures show representative cells transfected with the control siRNA alone or with Akt-targeted siRNA. Graph demonstrates the average integrated density values (IDV) of fluorescence intensity for Akt. IDV was determined by analyzing single transfected cells within captured images with AlphaImager 2000 software. Transfected cells were detected based on the presence of fluorescein of the control siRNA. Values represent mean ± s.e.m. of fluorescence in cells from at least eight microscopic fields per sample. B, T47D cells or cells transfected with ~20 nM control siRNA or Akt-targeted siRNA were lysed and equal volumes subjected to immunoblotting for Akt. Membrane was probed for α-tubulin to control for loading variability. C, T47D cells were transiently transfected with a control siRNA alone or with siRNA targeted for Akt. Cells were treated with 10 nM heregulin β1 and immunocytochemistry was performed to detect BRCA1. Pictures are representative images of BRCA1 localization in cells transfected with control siRNA alone or with Akt-targeted siRNA and treated with 10 nM heregulin β1. D, Quantification of cells with >50% nuclear fluorescence from samples transfected with control siRNA alone or with Akt-targeted siRNA in the absence or presence of heregulin. At least 88 cells from two experiments were scored for each group. (mean ± s.d.) Magnification, 400x.
Threonine 509 is necessary for the heregulin-stimulated accumulation of BRCA1 into the nucleus
The amino acid residue in BRCA1 that is phosphorylated by Akt is threonine 509 [23]. To assess whether the availability of threonine 509 for phosphorylation is necessary for accumulation of BRCA1 into the nucleus, we evaluated the intracellular distribution of GFP-tagged BRCA1, as well as GFP-tagged BRCA1 in which threonine 509 had been mutated to an alanine (BRCA1T509A; Fig. 5). cDNAs were transfected into HeLa or MCF-7. Transfected cells were scored as having a BRCA1 fluorescence pattern that was either nuclear or localized between both the nucleus and cytoplasm. Fig. 4A shows representative cells transfected with each construct and fluorescence scored as nuclear or nuclear/cytoplasmic. GFP-tagged BRCA1 was localized predominantly in the nucleus in approximately 25-35% of the cells in each cell line (Fig. 4B). Mutant BRCA1 localized similarly. Administration of heregulin β1 resulted in an ~2-fold increase in the percent of cells with nuclear only fluorescence. However, heregulin β1 was unable to induce nuclear acumulation of T509A mutant BRCA1 (Fig. 4C). These data suggest that factors other than Akt phosphorylation of BRCA1 at threonine 509 also are involved in nuclear localization. However, the data also support the interpretation that the heregulin-induced accumulation of BRCA1 is dependent on Akt phosphorylation of threonine 509.
Fig. 5. Heregulin β1 enhances BRCA1 transcriptional activity.
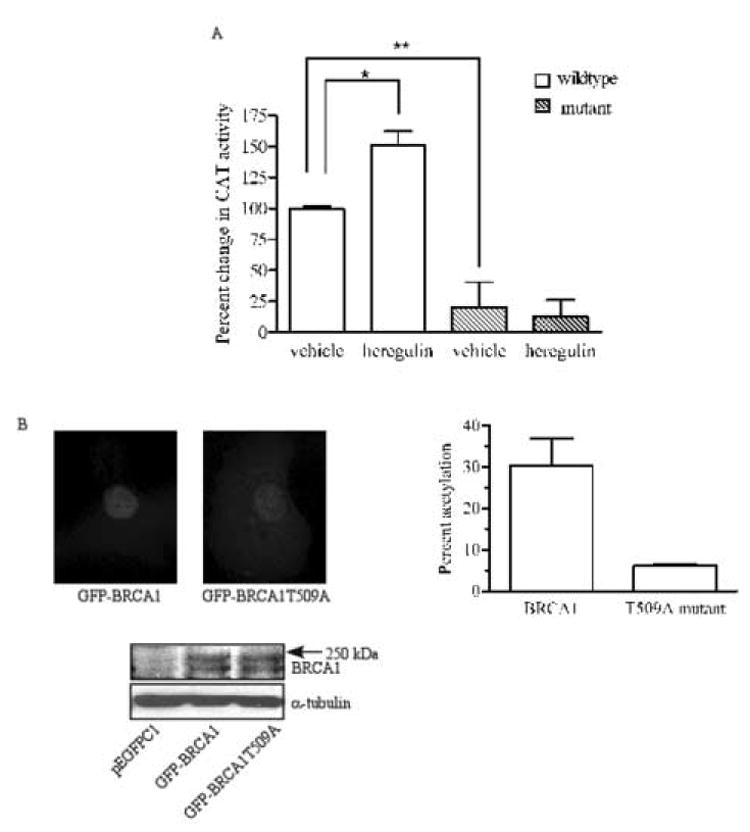
T47D cells were transfected with a wildtype GADD45 promoter construct or constructs in which BRCA1 binding sites were mutated. Cells were treated with vehicle or 10 nM heregulin β1 for 90 min, harvested, and lysed. The lysates were analyzed for β-galactosidase activity to standardize for transfection efficiency and CAT activity as a measure of transcriptional activity. Results shown are percent changes in acetylation when compared to that of vehicle-treated cells transfected with the wildtype construct. (n=3, mean ± S.E.; *p<0.05; **p<0.01). B, HCC1937 cells were stably transfected with pEGFPC1, pEGFPC1-BRCA1 or pEGFPC1-BRCA1T509A and then transiently transfected with GADD45 promoter and β-galactosidase constructs. Pictures are representative images of nuclear expression of GFP-tagged wildtype BRCA1 and nuclear/cytoplasmic distribution of GFP-tagged mutant BRCA1. For Western blot analysis of GFP-tagged protein expression, 50 μg protein from whole cell lysates of HCC1937 cells transfected with pEGFPC1, pEGFPC1-BRCA1 or pEGFPC1-BRCA1T509A were analyzed. The blot shown demonstrates bands detected by an antibody against GFP. Graph shows percent acetylation in cells expressing wildtype or mutant BRCA1 after acetylation in cells tranfected with empty vector had been subtracted. (mean ± s.d. of two experiments).
Fig. 4. Mutagenesis of threonine 509 to an alanine reduces the ability of heregulin to induce nuclear localization of BRCA1.
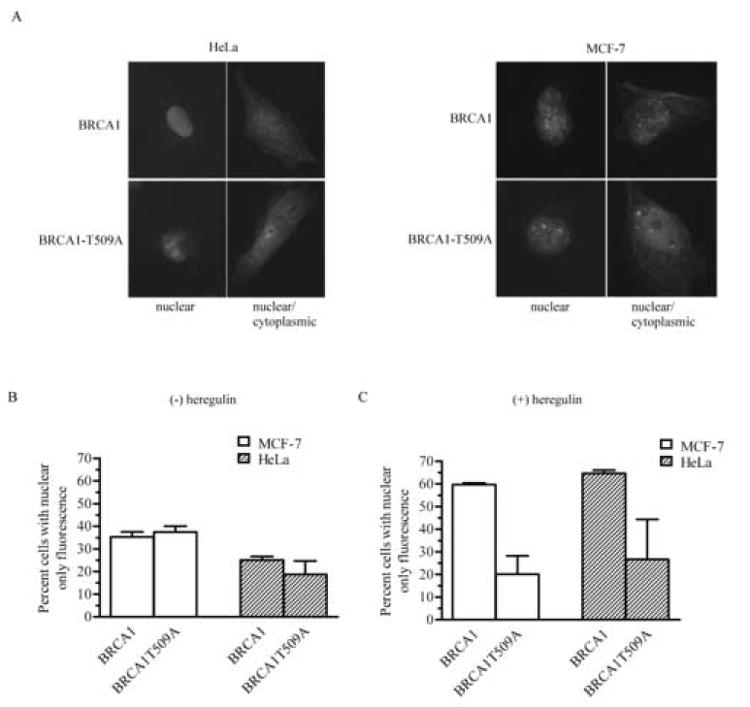
A, pEGFPC1-BRCA1 or pEGFPC1-BRCA1T509A expression in HeLa or MCF-7 cells was visualized by fluorescence microscopy after the cells were fixed and permeabilized. Nuclei were detected with propidium iodide (not shown). Data shown are representative of 2 separate transfections. Photographs are representative of cells with either a nuclear or nuclear/cytoplasmic fluorescence pattern. Magnification, 400x. B, Quantitative assessment of cells expressing wildtype or T509A mutant BRCA1. C, Quantitative assessment of cell expressing wildtype or T509A mutant BRCA1 in the presence of 10 nM heregulin β1. For B and C, at least 100 cells/experiment from 2 experiments were evaluated for each group. (mean ± s.d.).
Heregulin β1 enhances BRCA1 transcriptional activity
We explored whether the heregulin β1-induced increase in nuclear BRCA1 coincided with an increase in its transcriptional activity. Since the BRCA1 binding sites within the GADD45 promoter have been characterized, we chose GADD45 promoter constructs, linked to chloramphenicol acetyl transferase (CAT) as a reporter, to evaluate the ability of heregulin β1 to regulate BRCA1 transcriptional activity. BRCA1 binds to two OCT-1 motifs and one CAAT motif within the GADD45 promoter [11]. The level of BRCA1-transactivated GADD45 promoter activity, revealed as CAT catalytic activity, was increased by 150% in cells treated with heregulin β1 relative to the promoter activity in cells treated with vehicle (Fig. 5A). Using GADD45 promoter constructs in which the CAAT motif and one OCT-1 motif were mutated resulted in significantly reduced levels of CAT activity in vehicle-treated cells and this activity was not stimulated by heregulin β1. These findings, which are consistent with the interpretation that both the baseline and stimulated levels of promoter activity are due to BRCA1, demonstrate that the increase in nuclear BRCA1 provoked by heregulin-treatment and via an Akt-dependent pathway coincides with an increase in BRCA1-driven gene transcription, which was used as a measure of one of BRCA1’s nuclear functions.
We also compared the ability of wildtype and T509A mutant BRCA1 to induce GADD45 promoter activity. HCC1937 cells were stably transfected with either empty plasmid or with plasmid containing wildtype or T509A mutant BRCA1. HCC1937 cells were chosen because they express truncated, nonfunctional BRCA1 and because they also have constitutively elevated levels of Akt activity due to loss of PTEN [26]. Analysis of the localization of these proteins revealed that ~90% of cells expressing wildtype BRCA1 had nuclear only fluorescence, whereas only 55% of cells expressing mutant BRCA1 had nuclear only fluorescence. The remaining cells displayed fluorescence in both the nucleus and cytoplasm. Fig. 5B shows the nuclear fluorescence of wildtype and the nuclear/cytoplasmic fluorescence of mutant BRCA1. Western blot analysis revealed that the GFP-tagged proteins expressed by pEGFPC1-BRCA1 and pEGFPC1-BRCA1T509A migrated as a doublet that electrophoresed slightly faster than the 250kDa marker. These bands were not detectable in cells transfected with the empty vector. The presence of a doublet likely represents hyper- and hypo-phosphorylated forms of the protein as has been reported for endogenous BRCA1 in other cell lines [19]. The level of CAT activity in cells expressing wildtype BRCA1 was 30% above that in cells transfected with the empty vector. However, the T509A mutant was able to induce only a 5% increase in promoter activity. These data further support our interpretation that Akt-induced nuclear accumulation of BRCA1 coincides with BRCA1 nuclear function.
DISCUSSION
Although previous studies have demonstrated that BRCA1 can shuttle between the nucleus and the cytoplasm via nuclear import and export systems [14,17,18], none have identified a signaling pathway that regulates the nuclear/cytoplasmic distribution of BRCA1. In this study, we demonstrate that BRCA1 accumulates in the nucleus after Akt activation and this accumulation is dependent on the availability of a phosphorylation site at amino acid residue 509 of BRCA1. In wildtype BRCA1, residue 509 is a threonine which serves as the site for Akt-catalyzed phosphorylation. Our findings further reveal that the heregulin-stimulated increase in nuclear BRCA1 correlates with an increase in BRCA1 transcriptional activity.
Previous studies have demonstrated that phosphorylation of BRCA1 regulates its intranuclear localization and function in response to DNA repair signals [27]. BRCA1 is phosphorylated by ATM and ATR at a number of serine/threonine residues in response to cellular DNA damage [20,22], and this phosphorylation appears to be important for the function of BRCA1 during repair of damaged DNA. ATM, ATR and Chk2 [21] also are responsible for regulating the function of BRCA1 in cell cycle checkpoint control, albeit at different phases of the cell cycle [28-30]. However, no previous reports have demonstrated a role for phosphorylation in regulating the nuclear/cytoplasmic distribution of BRCA1.
The translocation of proteins into the nucleus after activation of Akt or its upstream activator, PI3K, has been reported for several other proteins. For example, inhibition of PI3K has been shown to impair the nuclear translocation of both cdk2 [31] and cdk4 [32]. In a similar manner, the overexpression of PTEN, a phosphatase that acts on inositol phospholipids generated by PI3K, prevented cyclin D1 from localizing to the nucleus during the G1 to S cell cycle transition [33], an effect overridden by the introduction of constitutively active Akt. Our results extend such phenomena to BRCA1 localization. Thus, suppression of Akt expression attenuates heregulin-induced relocation of BRCA1 to the nucleus. Although the critical phosphorylation occurs at threonine 509 for heregulin-induced nuclear accumulation of BRCA1, our data also suggest that BRCA1 can enter the nucleus in the absence of this phosphorylation since the T509A mutation did not completely eliminate the presence of the mutant BRCA1 within the nucleus. These findings suggest that the mechanism by which BRCA1 enters or is retained in the nucleus is still intact in BRCA1-T509A-expressing cells; however, the efficiency of nuclear import or retention has been decreased by the lack of phosphorylation at threonine 509. Alternatively, BRCA1-T509A enters the nucleus or is retained there via another mechanism that is less efficient than, but independent of, Akt-directed phosphorylation of threonine 509.
The present findings that heregulin β1 treatment of breast cancer cells results in Akt-mediated nuclear translocation of BRCA1 suggest multiple foci for dysregulation of this pathway in cancer initiation or progression. The heregulin β1 receptor, an ErbB2/ErbB3 heterodimer, is overexpressed in breast cancer, whereas BRCA1 expression is reduced. Long-term phosphorylation of BRCA1 by heregulin-induced activation of PI3K/Akt has been reported to decrease expression of BRCA1 at both the mRNA and protein levels [34]. Thus, it is possible that Akt-dependent BRCA1 phosphorylation and nuclear localization ultimately may account for the down-regulation of BRCA1 expression occurring in some breast cancers.
Acknowledgments
We thank Drs. Ifeanyi Arinze and Lee Limbird for helpful comments. Experiments/data analyses were performed in part through the use of the Flow Cytometry Special Resource Center (Veterans’ Administration Medical Center, Nashville, TN) and the DNA Core Facility (Meharry Medical College). We acknowledge the following support from the National Institutes of Health: Research Initiative for Scientific Enhancement (RISE) Grant 2R25 GM59994 (CVH, LDF), NIH-NCRR supported Research Centers in Minority Institutions (RCMI) Grant 2G12RR03032 (MET), National Cancer Institute K01 CA89494 (MET), National Institutes of General Medical Sciences F31 GM069036 (CVH) and Heart, Lung and Blood 5 T32 HL 07735-09 (CVH, LDF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing his early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 2.Shao N, Chai YL, Shyam E, Reddy P, Rao VN. Induction of apoptosis by the tumor suppressor protein BRCA1. Oncogene. 1996;13:1–7. [PubMed] [Google Scholar]
- 3.Somasundaram K, Zhang H, Zeng YX, Houvras Y, Peng Y, Zhang H, Wu GS, Licht J, Weber BL, El-Deiry WS. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 4.Chapman MS, Verma IM. Transcriptional activation by BRCA1. Nature. 1996;382:678–679. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- 5.Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J Biol Chem. 2003;278:34743–34746. doi: 10.1074/jbc.C300249200. [DOI] [PubMed] [Google Scholar]
- 6.Deng CX, Scott F. Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation. Oncogene. 2000;19:1059–1064. doi: 10.1038/sj.onc.1203269. [DOI] [PubMed] [Google Scholar]
- 7.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 8.Deng CX. Roles of BRCA1 in centrosome duplication. Oncogene. 2002;21:6222–6227. doi: 10.1038/sj.onc.1205713. [DOI] [PubMed] [Google Scholar]
- 9.Fan S, Wang JA, Yuan RQ, Ma YX, Erdos MR, Brody LC, Goldberg ID, Rosen EM. BRCA1 as a potential human prostate tumor suppressor: modulation of proliferation, damage responses and expression of cell regulatory proteins. Oncogene. 1998;16:3069–3082. doi: 10.1038/sj.onc.1202116. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi T, Monteiro AN, August A, Aaronsom SA, Hanafusa H. BRCA1 regulates p53-dependent gene expression. Proc Natl Acad Sci USA. 1998;95:2302–2306. doi: 10.1073/pnas.95.5.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan W, Jin S, Tong T, Zhao H, Fan F, Antimore MJ, Rajasekaran B, Wu M, Zhan Q. BRCA1 regulates GADD45 through its interactions with the OCT-1 and CAAT motifs. J Biol Chem. 2002;277:8016–8017. doi: 10.1074/jbc.M110225200. [DOI] [PubMed] [Google Scholar]
- 12.Chen A, Kleiman FE, Manley JL, Ouchi T, Pan ZQ. Autoubiquitination of the BRCA1-BARD1 RING ubiquitin ligase. J Biol Chem. 2002;277:22085–22092. doi: 10.1074/jbc.M201252200. [DOI] [PubMed] [Google Scholar]
- 13.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen CF, Li S, Chen Y, Chen OL, Sharp ZD, Lee WH. The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the nuclear transport signal receptor. J Biol Chem. 1996;271:32863–32868. doi: 10.1074/jbc.271.51.32863. [DOI] [PubMed] [Google Scholar]
- 15.Thakur S, Zhang HB, Peng Y, Le H, Carroll B, Ward T, Yao J, Farid LM, Couch FJ, Wilson RB, Weber BL. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997;17:444–452. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbro M, Rodriguez JA, Baer R, Henderson BR. BARD1 induces BRCA1 intranuclear foci formation by increasing RING-dependent BRCA1 nuclear import and inhibiting BRCA1 nuclear export. J Biol Chem. 2002;277:21315–21324. doi: 10.1074/jbc.M200769200. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez JA, Henderson BR. Identification of a functional nuclear export sequence in BRCA1. J Biol Chem. 2000;275:38589–38596. doi: 10.1074/jbc.M003851200. [DOI] [PubMed] [Google Scholar]
- 18.Thompson ME, Robinson-Benion CL, Holt JT. An amino-terminal motif functions as a second nuclear export sequence in BRCA1. J Biol Chem. 2005;280:21854–21857. doi: 10.1074/jbc.M502676200. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JE, Smith M, Tonkinson JL, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–809. [PubMed] [Google Scholar]
- 20.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 21.Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 22.Chen J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60:5037–5039. [PubMed] [Google Scholar]
- 23.Altiok S, Batt D, Altiok N, Papautsky A, Downard J, Roberts TM, Avraham H. Heregulin induces phosphorylation of BRCA1 through phosphatidylinositol 3-Kinase/AKT in breast cancer cells. J Biol Chem. 1999;274:32274–32278. doi: 10.1074/jbc.274.45.32274. [DOI] [PubMed] [Google Scholar]
- 24.Kamata N, Jotte RM, Holt JT. Myristylation alters DNA-binding activity and transactivation of FBR (gag-fos) protein. Mol Cell Biol. 1991;11:765–772. doi: 10.1128/mcb.11.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson KM, Streuli CH, Anderson NG. Autocrine signalling through erbB receptors promotes constitutive activation of protein kinase B/Akt in breast cancer cell lines. Breast Can Res Treatment. 2003;81:117–128. doi: 10.1023/A:1025765215765. [DOI] [PubMed] [Google Scholar]
- 26.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 27.Scully R, Chen J, Ochs RL, Keegen K, Hoekstra M, Feunteun J, Livingston DM. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 28.Ouchi M, Fujiuchi N, Sasai K, Ktayama H, Minamishima YA, Ongusal PP, Deng C, Sen S, Lee SW, Ouchi T. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J Biol Chem. 2004;279:19643–19648. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- 29.Okada S, Ouchi T. Cell cycle differences in DNA damage-induced BRCA1 phosphorylation affect its subcellular localization. J Biol Chem. 2003;278:2015–2020. doi: 10.1074/jbc.M208685200. [DOI] [PubMed] [Google Scholar]
- 30.Feng Z, Kachnic L, Zhang J, Powell SN, Xia F. DNA damage induces p53-dependent BRCA1 nuclear export. J Biol Chem. 2004;279:28574–28578. doi: 10.1074/jbc.M404137200. [DOI] [PubMed] [Google Scholar]
- 31.Keenan SM, Bellone C, Baldassare JJ. Cyclin-dependent kinase 2 nucleocytoplasmic translocation is regulated by extracellular regulated kinase. J Biol Chem. 2001;276:22404–22409. doi: 10.1074/jbc.M100409200. [DOI] [PubMed] [Google Scholar]
- 32.Lee HT, Kay EP. Regulatory role of PI 3-kinase on expression of Cdk4 and p27, nuclear localization of Cdk4, and phosphorylation of p27 in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2003;44:1521–1528. doi: 10.1167/iovs.02-0637. [DOI] [PubMed] [Google Scholar]
- 33.Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol. 2003;23:6139–6149. doi: 10.1128/MCB.23.17.6139-6149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miralem T, Avraham HK. Extracellular matrix enhances heregulin-dependent BRCA1 phosphorylation and suppresses BRCA1 expression through its C terminus. Mol Cell Biol. 2003;23:579–593. doi: 10.1128/MCB.23.2.579-593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


