Abstract
Concerns have been raised about whether operating microscopes and endoillumination used during ophthalmic surgeries contribute to retinal damage. Despite the recognition that ascorbic acid (vitamin C) helps to protect the eye from light and the abundance of vitamin C in the retina, artificial aqueous humors used during surgery only contain the antioxidant glutathione. To test whether inclusion of antioxidants other than glutathione in surgical solutions might help to preserve retinal integrity, we studied the effects of vitamin C on acute toxicity in isolated rat retinas. Male Sprague-Dawley rats (PND 30 ± 2) were sacrificed for retinal isolation. In the presence or absence of vitamin C (1 or 3 mM), retinas were exposed to 302 nm ultraviolet B (UVB) light for 1 hour and were incubated for a total of 5 hours at 30°C. Retinal damage was assessed by morphological examination and biochemical assay measuring the amount of lactate dehydrogenase (LDH) released from injured cells. In control retinas, LDH release was significantly increased after UVB exposure. The presence of 1 mM vitamin C in the incubation media significantly reduced LDH release during the post-incubation period following UV exposure. No difference was found between 1 mM and 3 mM vitamin C. Microscopic examination revealed that disorganization in the outer nuclear layer after UVB exposure was markedly attenuated by administration of 1 mM vitamin C. One mM vitamin C, a concentration found in the anterior chamber in humans, but not glutathione, prevented phototoxic injury following UV exposure. Although vitamin C itself cannot be used in intraocular irrigating solutions because of adverse interactions with iron released during bleeding, inclusion of antioxidants equivalent to vitamin C should be considered to help protect the retina from intraoperative light toxicity.
Keywords: phototoxicity (light toxicity), vitamin C, isolated rat retina, ex vivo, histology, lactate dehydrogenase (LDH)
Introduction
Light-induced retinal damage is irreversible and results in permanent visual loss, especially when damage involves the macular area. Ocular tissues are more sensitive and vulnerable to light (including ultraviolet light) than other organs (Sliney, 2002). Although light-induced photoreceptor damage has been well described (Noell et al., 1966; Kuwabara and Gorn, 1968), illumination of the ocular fundus is inevitable in clinical settings during ocular examinations and surgeries. As a large number of intraocular surgeries, such as corneal and cataract surgeries and vitrectomies, require direct microscopic illumination of ocular tissue, acute retinal damage has been increasingly observed. The damage is thought to result, at least in part, from the effects of this illumination (Arafat et al., 1994; Kuhn et al., 1991; Michels et al., 1992; Kleinmann et al., 2002). Most experiments studying light-induced injury show retinal damage with continuous illumination over several days (Grignolo et al., 1969; Malik et al., 1986; Edward et al., 1993; Organisciak et al., 2003). However, studying acute retinal damage induced by brief exposure to bright light can help to characterize the damage and to develop strategies for protecting the retina in clinical settings. Although the cornea usually protects the retina from ultraviolet (UV) exposure, the retina can be exposed to short wavelength light via illumination during ocular surgeries.
Free radical scavengers such as glutathione (Winkler and Giblin, 1983), α-tocopherol (vitamin E) (Penn et al., 1987) and ascorbic acid (vitamin C) (Woodford et al., 1983) are thought to serve as components of an endogenous defense system that helps to limit light-induced retinal damage. Vitamin C is also abundant in the retina, where its concentration in guinea pigs and rats is about 1.6 mM (Heath et al., 1961; Woodford et al., 1983). However, intraocular irrigating solutions currently employed during ocular surgery only contain oxidized glutathione as a corneal protectant (Edelhauser et al., 1975, Araie, 1986). Importantly, these surgical solutions do not contain vitamin C, even though human aqueous humor contains high concentrations of the vitamin.
To determine factors contributing to light-induced retinal injury, we developed an acute retinal preparation dissected from rats (Izumi et al., 1995). Advantages of this ex vivo preparation include the ability to control environmental variables such as light exposure, and the ease of studying known concentrations of pharmacological agents. In the present study, we used UVB (302 nm) light to induce acute retinal damage over a short period of exposure. Using morphological and biochemical assays, we assessed the protective actions of vitamin C on acute UVB-induced retinal damage.
Materials and Methods
Animal statement
All animal procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision research and the Washington University Animal Study Committee.
Ex vivo rat retinal preparation
Male Sprague-Dawley rats obtained from Harlan (Indianapolis, IN, USA) at postnatal date (PND) 21 were reared with a cycle of 12 hours white light and 12 hours dim light. At PND 30 ± 2, rats were anesthetized with isoflurane and decapitated. Retinas were dissected using previously described methods (Izumi et al., 1995). Briefly, after enucleation, the lens and vitreous were quickly removed at 4-6°C. The eye cap was cut into 3 pieces and then the retina was gently detached from the retinal pigment epithelium layer. The inferior portion of the isolated retina was discarded because of its lower sensitivity to light (Li et al., 1985). Isolated retinas were incubated in artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, and 10 glucose. ACSF was bubbled continuously with 95% O2- 5% CO2. The osmolarity and pH of aCSF were 287 mmol/Kg H2O and 7.32, respectively. L-ascorbate was the form of vitamin C used in these studies. At 1 mM and 3 mM, the pH of ascorbate-containing aCSF was 7.29 and 7.05, respectively.
UVB exposure
Following dissection, retinal segments were placed on nylon mesh in a beaker containing aCSF and allowed to recover for one hour. Retinal segments were then transferred to glass vials (7 ml) filled with gassed aCSF. The glass vials were placed 5 cm from a UV light source (Model UVLM-28, UVP CA, USA) to expose retinal segments to UVB (302 nm) for 1 hour in the presence or absence of vitamin C (1 or 3 mM). Administration of 0.3 mM oxidized glutathione was also tested in other experiments. The distance was set to induce apparent damage in one hour without changing the temperature of contents in the vials. Retinal tissues in the vials were oriented with the vitreous side up. Vials containing vitamin C were placed closer to the light source by 20% than drug-free vials to adjust the UV dose because we observed that 3mM vitamin C solutions absorbed 34% of 302 nm UVB light. Samples containing glutathione were placed at the same distance as controls because 0.3 mM oxidized glutathione showed little absorbance of 302 nm UVB light. During the exposure period, controls were covered with aluminum foil to prevent UV exposure. The emitted UVB radiation was 640 μW/cm2, as measured by a Dual-Range Light Meter (Fisher Scientific, Pittsburgh, PA). Activity of vitamin C in the vial before and after UVB exposure, measured with a reflectometer (RQflex 10, EMD Chemicals Inc., NJ, USA), revealed no significant change (-5.1%, n = 5). The rate of decomposition of vitamin C with UVB exposure was the same as without UVB.
Incubation for evaluating light-induced damage
After UV exposure, retinal segments were placed in beakers (10 ml) containing fresh aCSF gassed with 95% O2 - 5% CO2 at 30°C for 5 hours. For histological studies, the same concentration of vitamin C used during the UVB exposure was also administered in the post-incubation period. This was done in order to mimic the surgical condition in which there is a slow replacement of intra-operative solutions with endogenous aqueous humor. Some samples were processed for light microscopy to determine morphological damage by UVB. The light intensity throughout the experiment was 700-800 Lux except during the UVB exposure period.
LDH measurements
LDH activity was measured spectrophotometrically from 17 μl samples taken every hour. The activity of LDH was determined by the rate at which its substrate, pyruvate, is reduced to lactate, as monitored by diminished absorbance of the reduced form of nicotinamide adenine dinucleotide (NADH) at 340 nm (Ultrospec 2100 pro UV/Visible Spectrophotometer, Biochrom Ltd., Cambridge, UK). In control studies, 3 mM vitamin C added to LDH standards did not show any significant effect on LDH measurements (n = 5). To control for differences in the size of individual retinal segments, LDH release into the medium was normalized to a percentage of the total LDH value obtained by sonication post-incubation (Model 250 Sonifier, Branson Ultrasonics Corp. CT, Danbury, USA).
For cell lysis, 0.1% Triton X-100 was applied before sonication. There was no effect of 0.1% Triton X-100 on the absorption spectrum at 340 nm.
Histology
Retinal tissues were fixed in phosphate-buffered saline containing 1% paraformaldehyde and 1.5% glutaraldehyde overnight at 4°C. The fixed tissue was rinsed in 0.1% phosphate buffer, placed in 1% buffered osmium tetroxide for 60 minutes, dehydrated with alcohol and toluene, embedded in araldite, cut into sections 1 μm thick, stained with methylene blue and Azure B (Rowley Biochemical Institute, Danvers, MA, USA), and evaluated by light microscopy.
Chemicals
All chemical regents were obtained from Sigma (St. Louis, MO, USA).
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Data were analyzed using analysis of variance (ANOVA). Where the ANOVA P value was significant (< 0.05), pairwise comparisons were analyzed by the Holm-Sidak post hoc test using commercial software (SigmaStat 3.1.1; Systat Software inc., Richmond, CA, USA). P values of < 0.05 were considered significant.
Results
LDH release
The release of LDH into aCSF was determined in four conditions; control incubation, incubation with 1 mM vitamin C, and incubation after 1 hour of UVB exposure with or without vitamin C. Six retinas were included in each condition. There was a correlation between the amount of LDH released into aCSF and the incubation period of control rat retinas. Co-incubation with 1 mM vitamin C alone had no effect on LDH release. Immediately after 1 hour of UVB exposure, LDH release into aCSF did not differ from controls. However, following 1-hour UVB exposure, release of LDH increased gradually over time, with a significant difference from controls observed over the period of 1 to 5 hours after UVB exposure (**P < 0.01, Holm-Sidak post hoc test compared with aCSF under no illumination). Administration of 1 mM vitamin C during and after UVB exposure suppressed LDH release (*P < 0.01, Holm-Sidak post hoc test compared with UVB alone). Incubation of retinas with 3 mM vitamin C did not provide protection beyond that observed with 1 mM. Data are summarized in Figure 1.
Figure 1. Effects of vitamin C on LDH release after ultraviolet B (UVB) exposure.
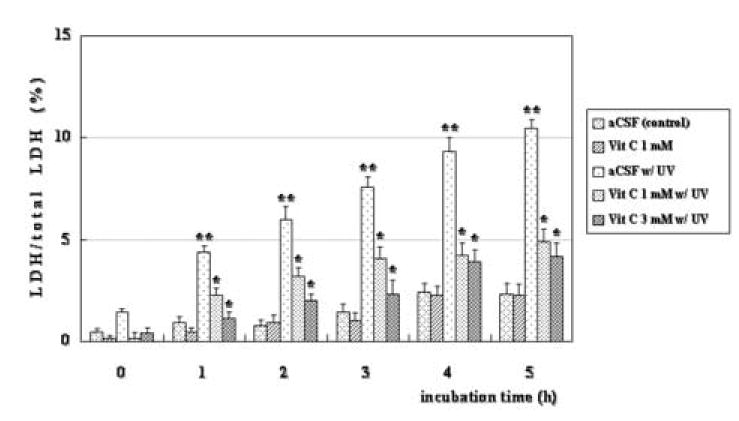
In control retinas, LDH release was minimal for up to 5 hours with or without 1mM vitamin C. Additionally, LDH release was not significant immediately following 1 hour of UVB exposure, but increased over a five-hour postincubation period (n = 6; **P < 0.01, Holm-Sidak post hoc test compared with control without vitamin C). Incubation of retinas with 1 mM and 3 mM vitamin C during and after UVB exposure significantly suppressed LDH release (n = 6; *P < 0.01, Holm-Sidak post hoc test compared with UVB alone).
Histology
Control retinal segments incubated in the dark for 6 hours did not display evidence of histological damage in any retinal layer (n = 6, Fig. 2A). One-hour exposure to UVB caused deterioration of cells in the outer nuclear layer (ONL) (Fig. 2B). These cells displayed pyknotic changes immediately after 1 hour of UVB exposure. During post-incubation for 3 to 5 hours, the damage became more pronounced and severe (Fig. 2E). In these retinas, the outer segments of the photoreceptors (rods) disappeared and columns of photoreceptor cells were totally disrupted 3 hours after UVB exposure. Other retinal layers also exhibited changes suggestive of Müller cell damage 5 hours after UB exposure. Rupture of the outer limiting membrane resulted in a loss of photoreceptor cells from the ONL. Administration of 1mM vitamin C for 1 hour during UVB exposure prevented the histological deterioration (Fig. 2C). The protective effects lasted up to 5 hours after UVB exposure, the longest period studied (Fig. 2F). Only mild changes in the ONL were confirmed at 5 hours incubation after UV exposure. No difference was found between the effects of 1 mM and 3 mM vitamin C (Fig. 2C and 2D, 2F and 2G).
Figure 2. Preservation of morphological integrity by vitamin C after UVB exposure.
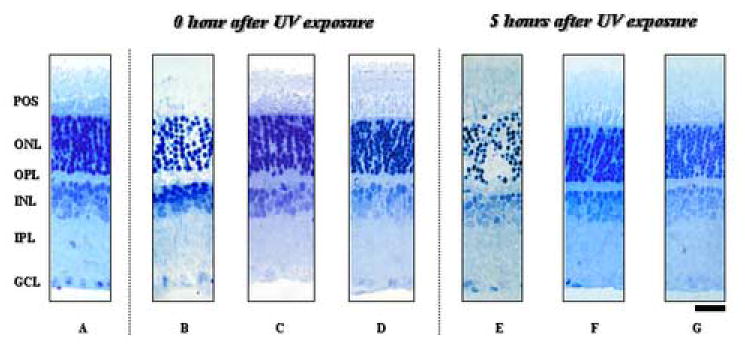
Retinal segments were fixed immediately or 5 hour after UVB exposure for histological evaluation. A control retinal segment incubated with artificial cerebrospinal fluid (aCSF) in the dark for 6 hours did not show any damage (A). Immediately following one-hour UVB exposure, the outer nuclear layer (ONL) exhibited severe damage (B). The acute damage was prevented if retinas were treated with 1 mM or 3 mM vitamin C during UVB exposure (C and D, respectively). Similarly, the damage observed 5 hour after UVB exposure (E) was prevented by administration of 1 mM and 3 mM vitamin C during and after UVB exposure (F and G, respectively). Abbreviations: POS, photoreceptor outer segment; OLM, outer limiting membrane; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar: 50 μm.
The damage induced by UVB was not completely homogenous throughout the retina (Fig 3A-C). Thus, in sliced sections we measured the length of damaged and undamaged areas separately. After exposure to UV light, only 10.6 ± 6.1% (n = 3) of control retinas incubated with aCSF was preserved, whereas 90.8 ± 4.8% and 85.4 ± 8.8% of retinas treated with vitamin C 1 mM and 3 mM were preserved, respectively (Table 1; P < 0.01 for both 1 and 3 mM vitamin C). We also examined lower concentrations of vitamin C, but found no protection at concentrations below 1 mM. Data are summarized in Table 1.
Figure 3. The appearance of whole retinas after UVB exposure.
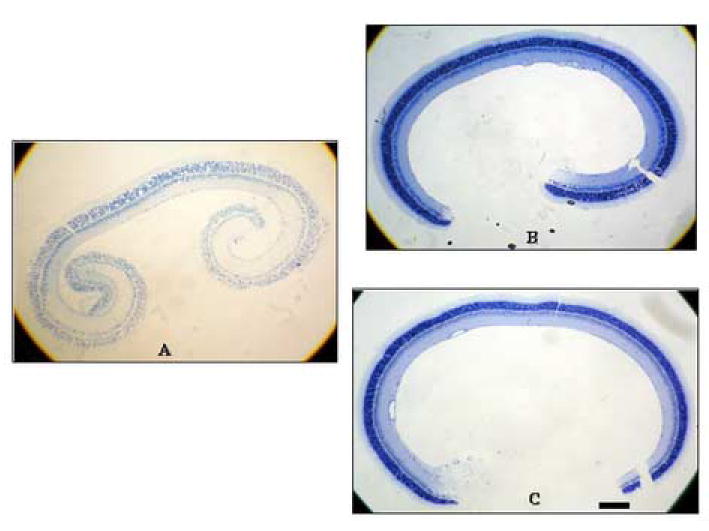
The outer nuclear layer (ONL) was selectively damaged 5 hours after exposure to UVB for one hour (A). Note that the damage was ubiquitous over the retina. Treatment of retinas with 1mM and 3 mM vitamin C during and after UVB exposure prevented the damage (B and C, respectively). Scale bar: 200 μm.
Table 1.
Effects of vitamin C concentration on ultraviolet B (UVB)-mediated retinal damage
| Control | 0.03 mM | 0.1 mM | 0.3 mM | 1 mM | 3 mM |
|---|---|---|---|---|---|
| 10.6 ± 6.1 | 13.1 ± 2.1 | 14.8 ± 1.7 | 21.6 ± 4.6 | 90.8 ± 4.8* | 85.4 ± 8.8* |
| (%) |
Each value represents the ratio of the intact part of the retina to the whole length of the retina. Data are expressed as mean ± SEM (n = 3; * P < 0.01, ANOVA followed by post hoc Holm-Sidak test compared with control).
The protective effects of vitamin C were not mimicked by glutathione, an agent routinely included in surgical solutions. Retinas incubated with aCSF containing glutathione at 0.3 mM, a concentration found in commercially available solutions, showed no protective effects at 0 or 5 hour after UVB exposure (Fig 4A-C).
Figure 4. The effect of oxidized glutathione on UVB-mediated retinal damage.
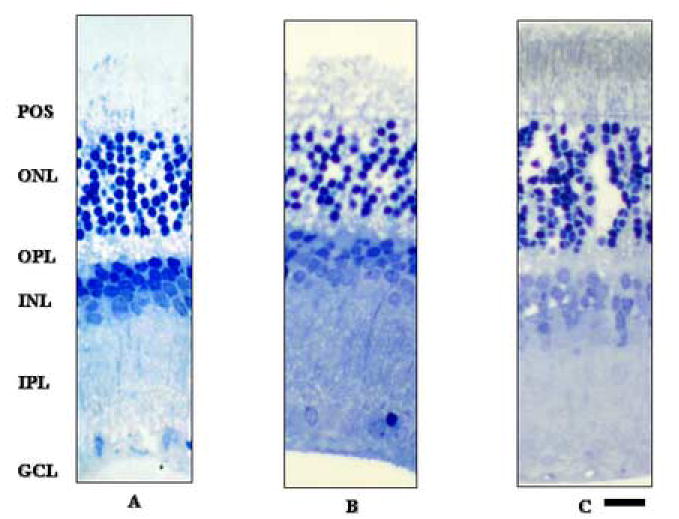
Control retina showed severe damage that was largely localized to the outer nuclear layer (A). Retinas incubated with 0.3 mM oxidized glutathione did not demonstrate any neuroprotection 0 or 5 hours after UVB exposure (B and C, respectively). Abbreviations are the same as used in Figure 2. Scale bar: 50 μm.
Discussion
In animal experiments, light-induced retinal damage is characterized by degeneration in the ONL (Noell et al., 1966; Schmidt and Zuclich, 1980; Li et al., 1985; Zhang et al., 2004). Consistent with these findings, we observed that exposure of rat retinal segments to UVB selectively damaged photoreceptor cells over a one-hour period of exposure. This finding indicates that the isolated retinal preparation can serve as a useful ex vivo model for studying light-induced damage and the effects of putative protective agents. In the present study, we found that vitamin C, an endogenous antioxidant found in aqueous humors, blocked UVB-induced retinal damage.
Previously, we reported that isolated retinal segments from rats exhibit intact morphology for up to 20 hours when incubated under control conditions (Izumi et al., 1995). We also demonstrated that aCSF and intraoperative irrigating solutions used in surgery are adequate for retinal incubation (Tokuda et al., 2004). The ability to use multiple segments from the same eye during these studies enables precise comparisons among different conditions during light exposure.
Most UV light below 290 nm wavelength is filtered by the cornea (Merriam et al., 2000). The transmittance of the lens varies according to developmental age and the young human lens passes a small window of UVB light to the retina (Barker et al., 1991). Thus, some parts of the UV spectrum above 290 nm can reach the retina, especially in individuals with aphakic eyes. In rat retinas, blue light is reported to induce severe disruption (Wu et al., 1999; Grimm et al., 2001), whereas Ham et al. (1982) demonstrated that retinas of aphakic monkeys were about 6 times more sensitive to near-ultraviolet than blue light. Some commercially available fiberoptic instruments used for retinal surgery include spectra below 400 nm (van den Biesen et al. 2000). UVB induces DNA damage (Lisby et al. 2005) and activates cyclooxygenase-2 (COX-2), which has recently been shown to induce epidermal neoplasms (Pentland et al., 1999; An et al., 2002). Based on these observations, we used UVB light to induce acute retinal damage. In the ex vivo rat retinal preparation, UVB caused morphological deterioration of the retina in a relatively short time. Initially, the damage was restricted to photoreceptor cells, and was characterized by pyknotic nuclei and the disappearance of outer segments of photoreceptor cells. This damage became more pronounced over several hours after cessation of UVB exposure.
The histological retinal damage induced by UV light varied from region to region. This could reflect local differences in the susceptibility of the retina to light, but is more likely to result from diffused light reflection within the curved retinal surface. To account for this, we evaluated overall morphological changes by measuring the ratio of the intact regions of the retina to the whole length of the retina after UVB exposure. There was a significant difference between control retinas and retinas treated with 1 mM and 3 mM vitamin C, although there was no statistical difference between the two concentrations of vitamin C.
The LDH assay is an established method for quantitatively evaluating neuronal toxicity in ex vivo or in vitro systems. However, it has been shown that LDH release is delayed after an ischemic insult in an ex vivo chick retinal preparation (Romano et al., 1995). Similarly, in our rat retinal preparation, morphological damage induced by kainate administration or an ischemic insult precedes LDH release (Izumi et al., 1995, 2003). In the present study, morphological changes were apparent immediately following one hour UVB exposure even though LDH release was minimal at that point. LDH levels gradually increased during the post-incubation period following UV exposure, suggesting that extracellular release of intracellular contents is delayed relative to the onset of histological injury. It is possible, however, that the damage induced by one hour of UVB exposure is insufficient to cause enough LDH release to be detected enzymatically.
Although the mechanisms underlying light-induced retinal injury are not fully understood, this damage is widely believed to result from free radical formation following intracellular calcium overload (Feeney and Berman, 1976; Noell, 1980; Li et al., 1993). Both enzymatic (e.g. catalase, glutathione) and non-enzymatic (e.g. vitamin A, C, E, melanin) endogenous antioxidants exert protective effects on photochemical damage in the retina (Beatty et al., 2000; Boulton et al., 2001). Among these endogenous agents, vitamin C is particularly important because it is water-soluble. Vitamin C serves as an essential cofactor for enzymes in several metabolic pathways. Vitamin C also prevents systemic phototoxicity, a common feature observed in some types of porphyria that results from the production of porphyrin-derived free radicals (Bohm et al., 2001). Vitamin C also prevents systemic phototoxicity in tin-protoporphyrin (SnPP)-treated suckling rats (Keino et al., 1993). Although most animals can synthesize vitamin C from glucose, humans must acquire the vitamin from dietary sources because they lack gluconolactone oxidase, the enzyme required for ascorbate biosynthesis. Prolonged deprivation of vitamin C impairs post-translational hydroxylation of collagen and causes scurvy. Intake of foods rich in vitamin C is reported to decrease the risk of cardiovascular disease (Marchioli, 1999) and to prevent some forms of cancer (Prasad et al., 1979; Park et al., 1980).
The retina is constantly exposed to reactive oxygen species generated by light exposure, and millimolar concentrations of vitamin C (1.04-1.07 mM) are found in human aqueous humor (Taylor et al., 1991). In guinea pig, vitamin C levels are higher in the neural retina than in the aqueous humor. Following extensive light exposure, the level of vitamin C in the retina is diminished (Woodford et al., 1983). Moreover, the fact that diurnal mammals have a high concentration of vitamin C in their aqueous humor but nocturnal mammals do not (Koskela et al., 1989) suggests a significant role for vitamin C in protecting diurnal animals from sunlight. Interestingly, light-induced retinal damage may be more pronounced in scorbutic than control monkeys (Tso, 1987), again suggesting the importance of vitamin C as a retinal protectant.
Artificial aqueous humors currently used for intraocular surgeries contain glutathione for protection of the corneal endothelium (Edelhauser et al., 1975, Araie, 1986), but do not routinely contain vitamin C or other antioxidants. In the present study, oxidized glutathione at the same concentration found in commercially available solutions used for ophthalmic surgery did not protect against UVB-induced retinal damage. During intraocular surgeries, the retina is intensively exposed to illumination. Interestingly, Postel et al. (1998)reported cases of iatrogenic phototoxicity following ophthalmic surgery. Most patients were asymptomatic because the damaged area spared the macular region. Some patients, however, had scar tissue in the macula and developed choroidal neovascularization resulting in severe visual disturbances. The ophthalmology literature further suggests that, although poor visual outcomes after intraocular surgery occur in a minority of cases, the problem may be much more prevalent than previously recognized. Considering the risk of phototoxicity, van den Biesen et al. (2000) warned that the safe exposure time of commercial endoillumination is less than 11 minutes, a time frame that is unrealistic in vitreoretinal surgery. Complicating this is the fact that the detached retina is even more sensitive to light (Zilis et al., 1991). These observations, coupled with the results of the present study, suggest that supplying effective free radical scavengers other than glutathione in surgical solutions may be beneficial for preventing light-induced retinal damage during intraocular surgery, especially vitrectomy, and for maintaining the physiological integrity of the retina for an extended period following surgery. The importance of carefully considering the content of surgical solutions is magnified by the fact that following vitrectomy the retina is exposed to the ophthalmic solution for 24 hours or more, given the time it takes to replenish the 4.0 ml vitreous cavity with endogenous aqueous humor that is produced at a rate of 3.04 μl/min (Tsukamoto and Larsson, 2004). Vitamin C itself is not appropriate for use in surgical solutions. In the presence of ferrous ions released during bleeding, vitamin C participates in the Fenton reaction (Fisher and Naughton, 2004) and this results in the production of toxic hydroxyl radicals. Inclusion of alternative free radical scavengers in surgical solutions, however, may help to preserve retinal integrity following intraocular surgery.
Conclusion
The ex vivo acute retinal preparation is useful for assessing UVB-induced retinal damage. Using this model, we found that 1 mM vitamin C, a level found in human aqueous humors, prevented UVB-induced retinal damage, suggesting the importance of vitamin C as an antioxidant in preventing light-induced retinal damage during intraocular surgeries. Using this ex vivo model, further studies with blue and white lights should help to clarify the potential role of vitamin C.
Acknowledgments
Supported by grant AG18434 and the Bantly Foundation. The assistance of Joann Labruyere is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Arafat AF, Dutton GN, Wykes WN. Subclinical operating microscope retinopathy: the use of static perimetry in its detection. Eye. 1994;8:467–472. doi: 10.1038/eye.1994.111. [DOI] [PubMed] [Google Scholar]
- Araie M. Barrier function of corneal endothelium and the intraocular irrigating solutions. Arch Ophthalmol. 1986;104:435–438. doi: 10.1001/archopht.1986.01050150137045. [DOI] [PubMed] [Google Scholar]
- Barker FM, Brainard GC, Dayhaw-Barker P. Transmittance of the human lens as a function of age. Invest Ophthalmol Vis Sci. 1991;32S:1083. [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Böhm F, Edge R, Foley S, Lange L, Truscott TG. Antioxidant inhibition of porphyrin-induced cellular phototoxicity. J Photochem Photobiol B. 2001;65:177–183. doi: 10.1016/s1011-1344(01)00259-7. [DOI] [PubMed] [Google Scholar]
- Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Edelhauser HF, Van Horn DL, Hyndiuk RA, Schultz RO. Intraocular irrigating solutions. Their effect on the corneal endothelium. Arch Ophthalmol. 1975;93:648–657. doi: 10.1001/archopht.1975.01010020614011. [DOI] [PubMed] [Google Scholar]
- Edward DP, Lim K, Sawaguchi S, Tso MO. An immunohistochemical study of opsin in photoreceptor cells following light-induced retinal degeneration in the rat. Graefes Arch Clin Exp Ophthalmol. 1993;231:289–294. doi: 10.1007/BF00919107. [DOI] [PubMed] [Google Scholar]
- Feeney L, Berman ER. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol. 1976;15:789–792. [PubMed] [Google Scholar]
- Fisher AE, Naughton DP. Iron supplements: the quick fix with long-term consequences. Nutr J. 2004;16:2. doi: 10.1186/1475-2891-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignolo A, Orzalesi N, Castellazzo R, Vittone P. Retinal damage by visible light in albino rats. An electron microscope study. Ophthalmologica. 1969;157:43–59. doi: 10.1159/000305619. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Williams T, Rol P, Hafezi F, Reme C. Rhodopsin-mediated blue-light damage to the rat retina: effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci. 2001;42:497–505. [PubMed] [Google Scholar]
- Ham WT, Jr, Mueller HA, Ruffolo JJ, Jr, Guerry D, 3rd, Guerry RK. Action spectrum for retinal injury from near-ultraviolet radiation in the aphakic monkey. Am J Ophthalmol. 1982;93:299–306. doi: 10.1016/0002-9394(82)90529-3. [DOI] [PubMed] [Google Scholar]
- Heath H, Beck TC, Rutter AC. Biochemical changes in aphakia. Vision Res. 1961;1:274–286. [Google Scholar]
- Izumi Y, Benz AM, Kirby CO, Labruyere J, Zorumski CF, Price MT, Olney JW. An ex vivo rat retinal preparation for excitotoxicity studies. J Neurosci Methods. 1995;60:219–225. doi: 10.1016/0165-0270(95)00015-m. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hammerman SB, Kirby CO, Benz AM, Olney JW, Zorumski CF. Involvement of glutamate in ischemic neurodegeneration in isolated retina. Vis Neurosci. 2003;20:97–107. doi: 10.1017/s0952523803202017. [DOI] [PubMed] [Google Scholar]
- Keino H, Mimura S, Nagae H, Banno T, Kashiwamata S. Protection by L-ascorbic acid against phototoxicity in tin-protoporphyrin-treated suckling rats. Biol Neonate. 1993;63:183–190. doi: 10.1159/000243930. [DOI] [PubMed] [Google Scholar]
- Kleinmann G, Hoffman P, Schechtman E, Pollack A. Microscope-induced retinal phototoxicity in cataract surgery of short duration. Ophthalmology. 2002;109:334–338. doi: 10.1016/s0161-6420(01)00924-1. [DOI] [PubMed] [Google Scholar]
- Koskela TK, Reiss GR, Brubaker RF, Ellefson RD. Is the high concentration of ascorbic acid in the eye an adaptation to intense solar irradiation? Invest Ophthalmol Vis Sci. 1989;30:2265–2267. [PubMed] [Google Scholar]
- Kuhn F, Morris R, Massey M. Photic retinal injury from endoillumination during vitrectomy. Am J Ophthalmol. 1991;111:42–46. doi: 10.1016/s0002-9394(14)76894-1. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Gorn RA. Retinal damage by visible light. An electron microscopic study. Arch Ophthalmol. 1968;79:69–78. doi: 10.1001/archopht.1968.03850040071019. [DOI] [PubMed] [Google Scholar]
- Li J, Edward DP, Lam TT, Tso MO. Amelioration of retinal photic injury by a combination of flunarizine and dimethylthiourea. Exp Eye Res. 1993;56:71–78. doi: 10.1006/exer.1993.1010. [DOI] [PubMed] [Google Scholar]
- Li ZY, Tso MO, Wang HM, Organisciak DT. Amelioration of photic injury in rat retina by ascorbic acid: a histopathologic study. Invest Ophthalmol Vis Sci. 1985;26:1589–1598. [PubMed] [Google Scholar]
- Lisby S, Gniadecki R, Wulf HC. UV-induced DNA damage in human keratinocytes: quantitation and correlation with long-term survival. Exp Dermatol. 2005;14:349–355. doi: 10.1111/j.0906-6705.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- Malik S, Cohen D, Meyer E, Perlman I. Light damage in the developing retina of the albino rat: an electroretinographic study. Invest Ophthalmol Vis Sci. 1986;27:164–167. [PubMed] [Google Scholar]
- Marchioli R. Antioxidant vitamins and prevention of cardiovascular disease: laboratory, epidemiological and clinical trial data. Pharmacol Res. 1999;40:227–238. doi: 10.1006/phrs.1999.0480. [DOI] [PubMed] [Google Scholar]
- Merriam JC, Löfgren S, Michael R, Söderberg P, Dillon J, Zheng L, Ayala M. An action spectrum for UV-B radiation and the rat lens. Invest Ophthalmol Vis Sci. 2000;41:2642–2647. [PubMed] [Google Scholar]
- Michels M, Lewis H, Abrams GW, Han DP, Mieler WF, Neitz J. Macular phototoxicity caused by fiberoptic endoillumination during pars plana vitrectomy. Am J Ophthalmol. 1992;114:287–296. doi: 10.1016/s0002-9394(14)71792-1. [DOI] [PubMed] [Google Scholar]
- Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- Noell WK. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 1980;20:1163–1171. doi: 10.1016/0042-6989(80)90055-3. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Invest Ophthalmol Vis Sci. 2003;44:486–492. doi: 10.1167/iovs.02-0708. [DOI] [PubMed] [Google Scholar]
- Park CH, Amare M, Savin MA, Hoogstraten B. Growth suppression of human leukemic cells in vitro by L-ascorbic acid. Cancer Res. 1980;40:1062–1065. [PubMed] [Google Scholar]
- Penn JS, Naash MI, Anderson RE. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987;44:779–788. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- Postel EA, Pulido JS, Byrnes GA, Heier J, Waterhouse W, Han DP, Mieler WF, Guse C, Wipplinger W. Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol. 1998;116:753–757. doi: 10.1001/archopht.116.6.753. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Sinha PK, Ramanujam M, Sakamoto A. Sodium ascorbate potentiates the growth inhibitory effect of certain agents on neuroblastoma cells in culture. Proc Natl Acad Sci U S A. 1979;76:829–832. doi: 10.1073/pnas.76.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Price MT, Olney JW. Delayed excitotoxic neurodegeneration induced by excitatory amino acid agonists in isolated retina. J Neurochem. 1995;65:59–67. doi: 10.1046/j.1471-4159.1995.65010059.x. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Zuclich JA. Retinal lesions due to ultraviolet laser exposure. Invest Ophthalmol Vis Sci. 1980;19:1166–1175. [PubMed] [Google Scholar]
- Sliney DH. How light reaches the eye and its components. Int J Toxicol. 2002;21:501–509. doi: 10.1080/10915810290169927. [DOI] [PubMed] [Google Scholar]
- Taylor A, Jacques PF, Nadler D, Morrow F, Sulsky SI, Shepard D. Relationship in humans between ascorbic acid consumption and levels of total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr Eye Res. 1991;10:751–759. doi: 10.3109/02713689109013869. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Tsukamoto T, Fujisawa S, Matsubara M. Evaluation of toxicity due to vital stains in isolated rat retinas. Acta Ophthalmol Scand. 2004;82:189–194. doi: 10.1111/j.1600-0420.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- Tso MO. Retinal photic injury in normal and scorbutic monkeys. Trans Am Ophthalmol Soc. 1987;85:498–556. [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Larsson LI. Aqueous humor flow in normal human eyes treated with brimonidine and dorzolamide, alone and in combination. Arch Ophthalmol. 2004;122:190–193. doi: 10.1001/archopht.122.2.190. [DOI] [PubMed] [Google Scholar]
- van den Biesen PR, Berenschot T, Verdaasdonk RM, van Weelden H, van Norren D. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol. 2000;84:1372–1375. doi: 10.1136/bjo.84.12.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Giblin FJ. Glutathione oxidation in retina: effects on biochemical and electrical activities. Exp Eye Res. 1983;36:287–297. doi: 10.1016/0014-4835(83)90013-1. [DOI] [PubMed] [Google Scholar]
- Woodford BJ, Tso MO, Lam KW. Reduced and oxidized ascorbates in guinea pig retina under normal and light-exposed conditions. Invest Ophthalmol Vis Sci. 1983;24:862–867. [PubMed] [Google Scholar]
- Wu J, Seregard S, Spangberg B, Oskarsson M, Chen E. Blue light induced apoptosis in rat retina. Eye. 1999;13:577–583. doi: 10.1038/eye.1999.142. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2753–2759. doi: 10.1167/iovs.03-1344. [DOI] [PubMed] [Google Scholar]
- Zilis JD, Machemer R. Light damage in detached retina. Am J Ophthalmol. 1991;111:47–50. doi: 10.1016/s0002-9394(14)76895-3. [DOI] [PubMed] [Google Scholar]


