Abstract
West Nile virus (WNV) transmission generally involves a mosquito vector and an avian reservoir host, with mammals as incidental hosts. Although most mammalian WNV infections cause low or no morbidity or mortality, tree squirrels are susceptible to WNV-associated neurologic disease with infection prevalence comparable to that in dead birds. Positive species included fox squirrel (Sciurus niger), western gray squirrel (S. griseus), and eastern gray squirrel (S. carolinensis). Kidney tissue (dissected and swabbed), and oropharyngeal (oral) swab samples from tree squirrels submitted by California vector control and rehabilitation agencies were tested by reverse transcription–polymerase chain reaction; cycle threshold values were similar for all three samples, ranging from 21.9 to 26.5. Kidney tissue was more sensitive than oral swabs for detecting WNV in squirrels. Three of 36 live neurologic tree squirrels had viremia approximately 5 log10 plaque-forming units/mL or greater, similar to WNV-infected birds. Tree squirrels are useful in WNV surveillance and provide localized evidence of WNV transmission to mammals.
INTRODUCTION
Transmission of West Nile virus (WNV) generally involves a mosquito vector and an avian reservoir host, with the general paradigm that mammals become infected as incidental or spill-over hosts. Although most WNV infections in mammals either cause low or no morbidity or mortality, some mammals, such as horses or humans, readily develop severe illness. West Nile virus or antibodies to the virus have been detected in many other mammals such as bats, raccoons, rabbits, mice, skunks, opossums, and tree squirrels.1–5 Tree squirrels, in particular, may be highly susceptible to WNV neurologic disease in regions where WNV is newly established. Evidence of WNV infection in tree squirrels in the United States has been reported from at least 11 other states (Arizona, Colorado, Idaho, Illinois, Kansas, Louisiana, Michigan, New York, Ohio, Pennsylvania, and Wyoming).2,4–11
In addition to WNV, at least six other encephalitic viruses have been detected in North American tree squirrels, such as LaCrosse virus (LACV), California virus, St. Louis encephalitis virus, western equine encephalitis virus (WEEV), eastern equine encephalitis virus, and Powassan virus (POWV).12,13 Although most were detected by identification of antibodies to these viruses, thereby indicating previous infection, virus has also been isolated from squirrels infected naturally with WEEV, LACV, and POWV.12,14,15 Infection with WEEV previously was related to squirrel mortality in California.16 These results suggest that tree squirrels may be important in the transmission cycle of these viruses.
Recent accounts indicate that fox squirrels and eastern gray squirrels can develop central nervous system lesions and virus has been detected in kidney, brain, and heart tissue.6,10 Other rodents, such as mice and hamsters, have been experimentally infected with WNV, and function as animal models for research.17,18 It is unclear whether squirrels or other rodents can function as reservoir hosts for WNV. To ascertain the importance of tree squirrels in the transmission cycle of WNV in California, it is essential to first measure its prevalence, intensity of infection, species affected, geographic range, and whether virus is present in blood. If virus is present throughout California in a high proportion of tree squirrels, with a high viremia in circulating blood, this would suggest that squirrels may play a role as competent WNV reservoirs.
MATERIALS AND METHODS
Tree squirrels were reported to the California WNV hotline in conjunction with dead birds. In summer of 2004, the number of reported sick and dead tree squirrels increased dramatically in southern California, coincident with intense WNV activity, thereby prompting us to investigate whether tree squirrels were dying from WNV. Tree squirrels were screened following the same protocol used for birds.19 Squirrels were necropsied at the California Animal Health and Food Safety Laboratories (CAHFS) in a biosafety cabinet, taking precautions to guard against tularemia and plague (e.g., use of double gloves, blunt scissors, avoidance of fleas). In 2004, three samples were taken from each squirrel (kidney tissue, kidney swab, and oral swab) and placed in tissue lysis buffer (Applied Biosystems, Foster City, CA). Kidney tissue was acquired by snipping a 3-mm piece of kidney tissue using blunt scissors and placing in 1 mL of lysis buffer in a 2-mL microcentrifuge tubes; oral and kidney tissue swabs were obtained by swabbing with sterile polyester fiber tipped applicators and swirling swabs in 1 mL of lysis buffer for 1 minute. All three samples were submitted to the Center for Vector-borne Diseases (CVEC) at University of California, Davis and tested by reverse transcription–polymerase chain reaction (RT-PCR) following the protocol outlined by Padgett and others.20 In 2005, only kidney tissue was submitted for testing.
From August through October 2005, sick squirrels submitted to two wildlife rehabilitation agencies (Wildlife Care Association, Sacramento, CA, and Lindsay Wildlife Museum, Walnut Creek, CA) were masked with isoflurane and bled by cardiac puncture or by ventral tail metatarsal or saphenous veins prior to being humanely killed. Whole blood (250 μL) was dispensed into 5-mL cryovials containing 1 mL of viral transport medium (VTM20) for a 1:5 dilution of blood. After killing, squirrel carcasses from wildlife rehabilitation agencies were forwarded to CAHFS and tissues were tested as described above. Blood from sick and dying squirrels was tested for antibodies and for live virus. Whole blood in VTM from 36 squirrels was evaluated for virus by standard Vero plaque assay.21 If there was no detectable virus in the blood sample, serum was tested for antibodies against WNV by a plaque reduction neutralization test (PRNT).22 Sera that neutralized 80% of the challenge dose of 100 plaque-forming units (PFU) of the NY99 strain of WNV at a dilution ≥ 1:20 were considered positive.
RESULTS
Characteristics of WNV-infected mammals
Between 2004 and 2005, three tree squirrel species were found dead by the public and subsequently tested positive for WNV in California fox squirrel (Sciurus niger), western gray squirrel (S. griseus), and eastern gray squirrel (S. carolinensis). This is the first record of WNV infection in western gray squirrels. Fox squirrels were the most commonly reported and frequently infected squirrel species, followed by western gray and eastern gray squirrels (Table 1). During summer of 2005, a high proportion of WNV-positive fox squirrels recovered by a rehabilitation center were juveniles ≤ 10 weeks of age (16 of 18 WNV-positive squirrels). Symptoms of WNV infection in sick tree squirrels include head tilt uncoordinated movement, chewing at feet, paralysis, tremors, circling, and lethargy (Padgett KA, unpublished data).6
TABLE 1.
Tree squirrels tested for West Nile virus by RT-PCR in California, 2004–2005*
| Species | No. tested | No. positive | Prevalence, %† |
|---|---|---|---|
| Fox squirrel (Sciurus niger) | 140 | 68 | 48.6 |
| Western gray squirrel (Sciurus griseus) | 64 | 25 | 39.1 |
| Eastern gray squirrel (Sciurus carolinensis) | 17 | 3 | 17.7 |
| Unknown species | 11 | 1 | 9.1 |
| Total | 232 | 97 | 41.8 |
RT-PCR = reverse transcription–polymerase chain reaction.
Prevalence calculated as no. positive/no. tested × 100.
Surveillance of dead squirrels
The prevalence of WNV in dead squirrels mirrored that of dead birds each year: in 2004, 64.5% (49 of 76) of squirrels tested positive compared with 56.4% (3,232 of 5,728) of birds; in 2005, 32.2% (49 of 152) of squirrels tested positive compared with 32.9% (3,046 of 9,263) of birds. A similar pattern appeared to hold for 2006; preliminary results for 2006: 23.2% (32 of 138) of squirrels were positive for WNV thus far this year compared with 24.2% (1,398 of 5,777) of birds. Again, similar to dead birds, the highest prevalence in WNV-positive squirrels was in August of 2004 and 2005 (Figure 1).
FIGURE 1.
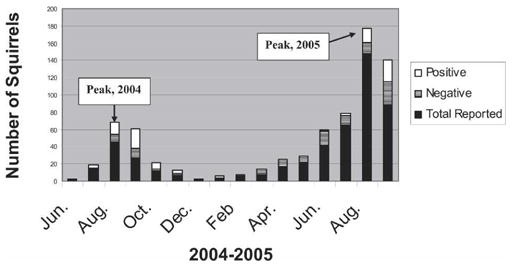
California tree squirrels reported to the West Nile virus (WNV) hotline and tested for WNV, 2004–2005.
The first WNV-positive squirrel tested in California was collected from Los Angeles County at the end of July during the 2004 epidemic; most dead squirrels were reported from this county that year (112 of 255 reports). In 2005, as the epicenter of WNV moved north to the Sacramento area of California, the highest number of dead tree squirrels was reported from northern and central California with Sacramento and Contra Costa counties having the highest number reported (66 and 94, respectively). The number of squirrels reported increased to 493 in 2005 and the number of counties reporting dead squirrels increased from 35 in 2004 to 38 in 2005.
Comparison of sample types
Kidney tissue was the most reliable sample for detecting WNV in squirrels, followed by kidney and oral swabs (Table 2). Of 36 positive tree squirrels tested by all three methods, dissected kidney tissue resulted in only four false-negative results (sensitivity = 89%) and resulted in a mean TaqMan cycle threshold (Ct) value of 26.3. Swabs of kidney tissue detected 22 of 36 positive samples with a Ct value of 28.6. Oral swabs were the least sensitive sample type with only 19 of 36 true positives detected (sensitivity = 52.8%); mean Ct Value was 27.6. Nevertheless, agreement in test results between paired samples of dissected kidney tissue and oral swabs was 68%. There was no significant difference in mean Ct values among sample types (P = 0.17); estimated PFU equivalents for these samples ranged from 3.1 to 3.6 log10 PFU per kidney sample.
TABLE 2.
Comparison among three sample types (kidney tissue, kidney swab, oral swab) tested for West Nile virus (WNV) RNA by RT-PCR in WNV-positive California tree squirrels*
| Sample | No. positive/no. tested | Prevalence, % | Mean Ct value |
|---|---|---|---|
| Kidney tissue | 32/36 | 89 | 26.3 |
| Kidney swab | 22/36 | 61 | 28.6 |
| Oral swab | 19/36 | 53 | 27.6 |
RT-PCR = reverse transcription–polymerase chain reaction; Ct = cycle threshold.
Tests of living squirrels
Of the 36 living tree squirrels with blood tested for WNV by plaque assay, 9 (25%) had a detectable viremia (mean = 3.2 log10PFU/mL, SD = 2.2, range = 0.4–8.0) (Table 3). Of those squirrels that were viremic, three had titers approximately 5 log10PFU/mL or greater, the reputed threshold required to infect California mosquitoes.22 All tree squirrels that were viremic and had carcasses submitted for further testing had RT-PCR–positive kidney tissue (n = 7). Of the 27 tree squirrels that tested negative by plaque assay and were tested by PRNT, 21 (75%) had antibody titers > 1:80, thereby indicating exposure to WNV. Of 21 tree squirrels with antibodies to WNV, 17 were also tested by RT-PCR (kidney tissue) with 12 testing positive for WNV.
TABLE 3.
Viremia and RT-PCR results of West Nile virus–infected California fox squirrels (Sciurus niger), August and September, 2005*
| Animal no. | Viremia† | RT-PCR (kidney tissue) |
|---|---|---|
| 132 | 8.0 | Positive |
| 162 | 4.6 | Positive |
| 181 | 4.5 | Positive |
| 145 | 2.9 | NT |
| 121 | 2.5 | Positive |
| 182 | 2.2 | Positive |
| 184 | 1.9 | Positive |
| 201 | 1.7 | Positive |
| 167 | 0.4 | NT |
RT-PCR = reverse transcription–polymerase chain reaction; NT = not tested.
Of four squirrels that had serial blood collections made prior to killing or death, two were viremic and two were antibody positive only. The first animal who exhibited no neurologic signs had blood drawn four days prior to death because of neurologic signs and death of two cage mates (sib-lings); this blood sample contained 8 log10PFU/mL. A second sample taken three days later when squirrel was ataxic, circling, and dragged its knuckles also was viremic (5.7 log10PFU/mL); this animal died the next day. The second animal was ataxic and had blood drawn two separate days prior to killing. The first sample had a viremia of 1.7 log10PFU/mL and was negative for neutralizing antibodies (< 1:20), the second blood sample taken the day of killing was negative for viremia but neutralizing antibodies were > 1:80 (animal was positive for WNV by RT-PCR of kidney tissue).
DISCUSSION
Three tree squirrel species were found to be susceptible to WNV in all regions of California and were useful for WNV surveillance, with prevalence and peak of WNV infection mirroring that of dead birds. Moreover, unlike birds, tree squirrels are terrestrial peridomestic animals, thus, highly localized information on WNV-transmission can be acquired by including squirrels in a WNV surveillance program. Squirrels also can be useful for WNV surveillance in areas where large corvid populations are absent or where bird numbers have decreased significantly because of WNV or other factors.
In other areas of the United States where WNV infection has been noted in squirrels, the two species involved are fox squirrels and eastern gray squirrels, both non-native species to California. Although this is the first report of WNV in western gray squirrels, other western states have noted mortality in this species (Drew M, Idaho Fish and Game, unpublished data). Although many of the squirrels submitted during the summer of 2005 were ≤ 10 weeks old, it is unclear whether juvenile fox squirrels are more susceptible than adults or whether this simply reflects timing of second litter for this species (i.e., generally July in California).
Kidney tissue was a better sample to use for WNV RT-PCR testing than oral swabs because oral swabs missed up to 50% of true-positive samples. Nevertheless, oral swabs are an alternative sampling method to consider when necropsy is not possible, with the benefit of being easier and safer than necropsy and sampling of internal tissues (e.g., kidney, brain, spleen). Although RT-PCR detects abundance of viral RNA and not live virus, the finding that there was no difference in Ct values among positive oral tissues compared with kidney tissues suggests that a similar amount of virus is sampled by each method provided that the squirrel secretes WNV into the oral cavity. It does appear that WNV-infected tree squirrels, irregardless of sampling method, have less virus at death than WNV-infected American crows (oral swab and/or kidney tissue); crows tested at CVEC using identical methods had mean Ct values 10 units lower (or 2 orders of magnitude more PFU equivalents) than that of tree squirrels.20
In addition to providing a sampling method for WNV, these results indicated that at least half of all tree squirrels sampled shed WNV in their oral secretions. A recent study detected WNV RNA by plaque assay in oral, fecal, and urine samples of experimentally infected fox squirrels.5 These results suggest the potential for contact transmission of WNV among cagemates or siblings, similar to that seen by birds;23 empirical evidence from rehabilitation centers supports this premise (Padgett K, unpublished data). Furthermore, these results indicate additional care should be made when rehabilitating squirrels, such as avoiding squirrel bites by wearing leather gloves, autoclaving cage material, and separate housing for squirrels with neurologic signs.
Some squirrels naturally infected with WNV had sufficient virus in their blood to be able to potentially infect mosquitoes (approximately 5 log10PFU/mL). The viremia of one tree squirrel in this study (8 log10PFU/mL) was in the range of many passerine birds that have been implicated as competent reservoirs of WNV.23 There is some concern that if mammals such as tree squirrels and lagomorphs (i.e., rodents/hares) can serve as reservoirs, they may infect mammalophagic mosquito species.24 For example, a secondary transmission cycle has been documented for WEEV that involved Aedes melanimon and lagomorphs;25 in this case, the jump from birds to mammals as a reservoir and day-biting mammalophagic mosquitoes as the vector markedly increases risk of transmission of WEEV to humans and horses. Of note, one of 11 California lagomorphs (black-tailed jackrabbit) tested for WNV was positive (Padgett K, unpublished data).
Although the role of tree squirrels in the WNV transmission cycle is uncertain, tree squirrels have been shown to be important for WNV surveillance in California. Results from our study indicate that squirrels sick or dying during the summer months with neurologic symptoms have a high likelihood of testing positive for WNV determined either by detection of antibodies or virus in blood, kidney tissue, or oral secretions. Further studies directed at reservoir competence of tree squirrels using Culex and Aedes mosquito species are warranted to assess potential risk to human health.
Acknowledgments
We thank Marzieh Shafii, Ian Holser, Julian Saputo, Jessica Jennings, Karen Ehnert, the staff of the Lindsay Museum, and the staff of the Wildlife Care Association for excellent technical assistance. In addition, we are grateful to the staff of the WNV hotline and all the California vector control districts that submitted squirrels and other mammals for testing: Alameda County Mosquito Abatement District (MAD), Burney Basin MAD, Butte County Mosquito Vector Control District (MVCD), Consolidated MAD, Contra Costa County MVCD, El Dorado County Vector Control (VC), Fresno MVCD, Greater Los Angeles Vector Control District (VCD), Marin-Sonoma MVCD, Napa County MAD, Orange County VCD, Placer County MAD, Sacramento-Yolo MVCD, San Bernardino County VCP, San Mateo County MAD, San Joaquin County MVCD, Santa Clara County VCD, Santa Cruz County MVCD, Shasta County MVCD, Tehama County MVCD, Tuolumne County Environmental Health (EH), and Ventura County EH.
Footnotes
Reprint requests: Kerry A. Padgett, Vector-Borne Disease Section, California Department of Health Services, 850 Marina Bay Parkway, Richmond, CA 94804, Telephone: 510-412-6252, E-mail: kpadgett@dhs.ca.gov.
Financial support: This research was supported by the Centers for Disease Control and Prevention, as part of grant U50/CCU923677-02 (Epidemiology and Laboratory Capacity for Infectious Diseases) and grant Number RO1-AI55607 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health to William K. Reisen.
References
- 1.Anderson JF, Vossbrinck CR, Andreadis TG, Iton A, Beckwith WH, Mayo DR. Characterization of West Nile virus from five species of mosquitoes, nine species of birds, and one mammal. Ann N Y Acad Sci. 2001;951:328–331. doi: 10.1111/j.1749-6632.2001.tb02709.x. [DOI] [PubMed] [Google Scholar]
- 2.Marfin AA, Petersen LR, Eidson M, Miller J, Hadler J, Farello C, Werner B, Campbell GL, Layton M, Smith P, Bresnitz E, Cartter M, Scaletta J, Obiri G, Bunning M, Craven RC, Roehrig JT, Julian KG, Hinten SR, Gubler DJ the ArboNET Cooperative Surveillance Group. Widespread West Nile virus activity, eastern United States, 2000. Emerg Infect Dis. 2001;7:730–735. doi: 10.3201/eid0704.010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- 4.Root JJ, Hall JS, Mclean RG, Marlenee NL, Beaty BJ, Gansowski J, Clark L. Serologic evidence of exposure of wild mammals to flavivirus in the central and eastern United States. Am J Trop Med Hyg. 2005;72:622–630. [PubMed] [Google Scholar]
- 5.Root JJ, Oesterle PT, Nemeth NM, Klenk K, Gould DH, McLean RG, Clark L, Hall JS. Experimental infection of fox squirrels (Sciurus niger) with West Nile virus. Am J Trop Med Hyg. 2006;75:697–701. [PubMed] [Google Scholar]
- 6.Kiupel M, Simmons HA, Fitzgerald SD, Wise A, Sikarskie JG, Cooley TM, Hollamby SR, Maes R. West Nile virus infection in eastern fox squirrels (Sciurus niger) Vet Pathol. 2003;40:703–707. doi: 10.1354/vp.40-6-703. [DOI] [PubMed] [Google Scholar]
- 7.West Nile virus activity—United States, November 3–8, 2004. MMWR Morb Mortal Wkly Rep. 2004 Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5344a7.htm. [PubMed]
- 8.West Nile virus activity—2005. MMWR Morb Mortal Wkly Rep. 2005 Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5441a7.htm. [PubMed]
- 9.West Nile virus activity—United States January 1–August 15, 2006. MMWR Morb Mortal Wkly Rep. 2006 Avilable from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5532a3.htm. [PubMed]
- 10.Heinz-Taheny KM, Andrews JJ, Kinsel MJ, Pessier AP, Pinkerton ME, Lemberger KY, Novak RJ, Dizikes GJ, Edwards E, Komar N. West Nile virus infection in free-ranging squirrels in Illinois. J Vet Diagn Invest. 2004;16:186–190. doi: 10.1177/104063870401600302. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich G, Montenieri JA, Panella NA, Langevin S, Lasater SE, Klenk K, Kile JC, Komar N. Serologic evidence of West Nile virus infection in free-ranging mammals, Slidell, Louisiana, 2002. Vector-Borne Zoon Dis. 2005;5:288–292. doi: 10.1089/vbz.2005.5.288. [DOI] [PubMed] [Google Scholar]
- 12.Milby MM, Reeves WC. Natural infection in vertebrate hosts other than man. In: Reeves WC, editor. Epidemiology and Control of Mosquito-Borne Arboviruses in California, 1943–1987. Sacramento CA: California Mosquito and Vector Control Association; 1990. pp. 26–65. [Google Scholar]
- 13.Day JF, Stark LM, Zhang J, Ramsey AM, Scott TW. Antibodies to arthropod-borne encephalitis viruses in small mammals form southern Florida. J Wild Dis. 1996;32:431–436. doi: 10.7589/0090-3558-32.3.431. [DOI] [PubMed] [Google Scholar]
- 14.Timoney P. Powassan virus infection in the grey squirrel. Acta Virol. 1971;15:429. [PubMed] [Google Scholar]
- 15.Ksiazek TG, Yuill TM. Viremia and antibody response to LaCrosse virus in sentinel gray squirrels (Sciuris carolinensis) and chipmunks (Tamias striatus) Am J Trop Med Hyg. 1977;26:815–821. doi: 10.4269/ajtmh.1977.26.815. [DOI] [PubMed] [Google Scholar]
- 16.Lennette EH, Ota MI, Dobbs ME, Browne AS. Isolation of western equine encephalomyelitis from naturally-infected squirrels in California. Am J Hyg. 1956;64:276–280. doi: 10.1093/oxfordjournals.aje.a119840. [DOI] [PubMed] [Google Scholar]
- 17.Xiao S, Guzman H, Zhang H, Travassos da Rosa APA, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann N Y Acad Sci. 2001;951:84–93. doi: 10.1111/j.1749-6632.2001.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 19.McCaughey K, Miles S, Woods L, Chiles R, Hom A, Kramer V, Jay-Russell M, Sun B, Reisen W, Scott T, Hui L, Steinlein D, Castro M, Houchin A, Husted S. The California West Nile virus dead bird surveillance program; Presented at the Seventy-First Annual Conference of the Mosquito and Vector Control Association of California; Palm Springs, CA. 2003. [Google Scholar]
- 20.Padgett KA, Cahoon-Young B, Carney R, Woods L, Read D, Husted S, Kramer V. Field and laboratory evaluation of diagnostic assays for detecting West Nile virus in oropharyngeal swabs from California wild birds. Vector Borne Zoonotic Dis. 2006;6:183–191. doi: 10.1089/vbz.2006.6.183. [DOI] [PubMed] [Google Scholar]
- 21.Campbell GL, Eldridge BF, Hardy JL, Reeves WC, Jessup DA, Presser SB. Prevalence of neutralizing antibodies against California and Bunyamwera serogroup viruses in deer from mountainous areas of California. Am J Trop Med Hyg. 1989;40:428–437. doi: 10.4269/ajtmh.1989.40.428. [DOI] [PubMed] [Google Scholar]
- 22.Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 23.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiawsirisup S, Platt KB, Tucker BJ, Rowley WA. Eastern cottontail rabbits (Sylvilagus floridanus) develop West Nile virus sufficient to infect select mosquito species. Vector Borne Zoonotic Dis. 2005;5:342–350. doi: 10.1089/vbz.2005.5.342. [DOI] [PubMed] [Google Scholar]
- 25.Hardy JL. The ecology of western equine encephalomyelitis virus in the central valley of California, 1945–1985. Am J Trop Med Hyg. 1987;37:18s–32s. doi: 10.4269/ajtmh.1987.37.18s. [DOI] [PubMed] [Google Scholar]


