Abstract
Parallel imaging applied to first-pass, contrast-enhanced cardiac MR can yield greater spatial coverage for a fixed temporal resolution. The method combines rate R = 2 acceleration using TSENSE with shot-to-shot interleaving of two slices. The √R SNR loss is largely compensated for by a longer effective repetition time (TR) and increased flip angle associated with slice interleaving. In this manner, increased spatial coverage is achieved while comparable or better image quality is maintained. Single-heartbeat temporal resolution was accomplished with spatial coverage of eight slices at heart rates up to 71 bpm, six slices up to 95 bpm, and four slices up to 143 bpm. Experiments in normal subjects (N = 6) were performed to assess signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) values. Published 2003 Wiley-Liss, Inc.†
Keywords: MRI, heart, perfusion, contrast agent, SENSE, TSENSE
Coverage of the entire heart during first-pass, contrast-enhanced MRI with single-heartbeat temporal resolution is desirable for quantifying perfusion abnormalities. Current imaging protocols limit our ability to image the entire heart with single-heartbeat temporal resolution, particularly at high heart rates. Multislice coverage can be achieved using fast gradient-recalled echo (FGRE) with echo-train readout and saturation recovery, with a relatively short preparation time (TI) (1). Imaging quality can be improved at the expense of coverage (2) by increasing the TI and the readout flip angle. Alternatively, a notch pulse scheme (3) can be used to increase the effective TI by imaging a slice while another slice is being prepared with a spatially selective presaturation pulse. However, this method may result in artifacts resulting from the flow of saturated blood.
Parallel imaging can be used to reduce the acquisition time of first-pass perfusion images. The straightforward application of parallel imaging reduces imaging time, but at the expense of reduced image quality (i.e., √R SNR loss for acceleration at rate R). The proposed application of accelerated imaging uses the decreased image acquisition time to permit slice interleaving, and yields increased coverage without loss of image quality.
MATERIALS AND METHODS
First-pass, contrast-enhanced imaging using saturation preparation for T1-weighted contrast can be used to image multiple slices per heartbeat. Various imaging parameters have been proposed (1,2) that trade spatial coverage for image quality. Figure 1 illustrates several acquisition strategies. Figure 1a shows the acquisition of a single slice per saturation preparation with relatively short TI. Figure 1b shows a single slice per saturation preparation, with a longer TI for increased T1 contrast and flatter response. The proposed method (Fig. 1c) uses a shot-to-shot, slice-interleaved acquisition with two slices acquired per saturation preparation, which thus reduces the net preparation time. Method 3 uses accelerated imaging to reduce the imaging time for each pair of slices; therefore, the methods all have the same imaging window. Method 3 provides twice the spatial coverage of method 2, using the same TI.
FIG. 1.
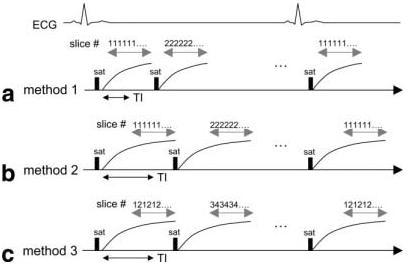
Timing for various acquisition methods: (a) method 1 using single slice per saturation preparation with short TI, (b) method 2 using single slice per saturation preparation with longer TI for flatter response and increased T1 contrast, and (c) method 3 using shot-to-shot slice interleaved acquisition with two slices acquired per saturation preparation and longer TI. Method 3 provides twice the spatial coverage of method 2 using the same TI. Method 3 uses TSENSE acceleration to reduce the imaging time for each pair of slices; therefore, the methods all have the same imaging window.
Imaging time can be reduced by undersampled acquisition with full-FOV reconstruction employing either unaliasing by Fourier encoding the overlaps using the temporal dimension (UNFOLD) (4,5) or parallel imaging methods, such as sensitivity encoding (SENSE) (6). The temporal SENSE (TSENSE) (7) method can be used with interleaved phase-encode acquisition order to adaptively derive or update B1-map estimates, as well as for additional alias artifact suppression.
The acquisition order of the slices can also be interleaved spatially (i.e., for four slices, the slices can be acquired with shot-to-shot interleaving 131313 and 242424). Furthermore, the effective TR is increased by a factor of 2, which allows the use of an increased readout flip angle. The √R SNR loss from accelerated imaging is largely compensated for by this increased flip angle (8,9). A longer preparation time (TI) can be used to further improve the image contrast and the point spread function (PSF). Slice interleaving combined with accelerated imaging maintains the same overall image acquisition window.
In this study, imaging was performed on a GE 1.5T CV/i scanner, using a multishot echo-planar imaging fast gradient-recalled echo (EPI-FGRE) sequence with the following parameters: echo-train length = 4, TR = 6.9 ms, bandwidth = ±125 k, and 10% trigger window. The acquisition matrix was 128 × 80 with typically a 40 × 25 cm2 FOV, producing a nominal resolution of 3.1 × 3.1 mm2 and 8-mm slice thickness. Three methods were compared: method 1 (as described in Ref. 1); method 2 (method 1 modified for improved image quality); and method 3, with slice interleaving and R = 2 TSENSE acceleration. The variable parameters are listed in Table 1. TI is defined at the center of the k-space acquisition rather than at the first readout, as in Refs. 1 and 2. A modified center-out k-space acquisition order (1) that acquires the central lines with the first echo was used for all methods. The TE for the first echo was approximately 1.6 ms for all methods, with interecho spacing of 0.75 ms. The effective TR for method 3 with slice interleaving was 13.8 ms, which permitted an increased flip angle. The number of short-axis slices acquired per heartbeat was a function of heart rate. The maximum number of slices that could be acquired for each method, assuming a 10% trigger window, is shown in Fig. 2.
Table 1.
Variable Parameters for Three Methods
| Method 1 | Method 2 | Method 3 | |
|---|---|---|---|
| Prep flip angle | 70° | 90° | 90° |
| Readout flip angle | 10° | 20° | 30° |
| Prep time, TI (ms)a | 80 | 120 | 120 |
| Effective TR (ms) | 6.9 | 6.9 | 13.8 |
| Acceleration factor | 1 | 1 | 2 |
Defined as time to center of k-space.
FIG. 2.
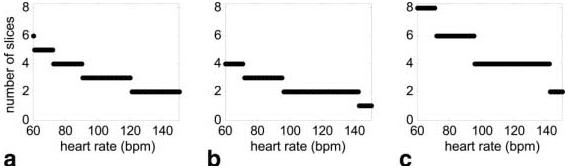
Spatial coverage (number of slices) vs. heart rate for (a) method 1 using TI = 80 ms, (b) method 2 using TI = 120 ms, and (c) method 3 using TI = 120 ms and TSENSE acceleration combined with slice interleaving (assuming a 10% trigger window in each case).
The initial image for each slice was used as a reference for B1-map estimation, and did not have any saturation preparation. A fixed 10° readout flip angle was used for the initial reference image for all of the methods, which could also be used to normalize surface coil intensity variation. A standard GE four-element cardiac surface coil array was used. The subjects in this study (N = 6) were normal, healthy volunteers, who provided informed consent in accordance with an NIH-approved protocol. Images were acquired for 40 heartbeats beginning approximately 5 s prior to the administration of a single-dose bolus (0.1 mmol/kg) of contrast agent (Gadopentetate Dimeglumine; Berlex Magnevist) at 5 ml/s, followed by a saline flush (20 ml at 5 ml/s). The contrast agent was administered intravenously in the left antecubital vein.
Data were acquired using all three methods for each volunteer. Care was taken to use the same coil positioning and slice orientation for the three exams conducted on different days. The data were acquired over a 5-week period, and the order of the three methods varied. Reconstructions were performed using both TSENSE and UNFOLD. UNFOLD temporal filtering used 80% of the available bandwidth (1 dB) using a low-latency temporal filter design (10). The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) measurements were made in six equiangular segments of the left ventricle (LV) (short-axis slice). The measured post-enhancement SNR was calculated using an average of four image frames starting at the time of the peak contrast enhancement for myocardium. The precontrast SNR was calculated using an average of two frames preceding the bolus injection.
RESULTS
Image reconstruction for the accelerated imaging case (method 3) was performed using TSENSE with and without UNFOLD temporal filtering, as well as UNFOLD alone, for comparison of artifact suppression performance. UNFOLD alone is sensitive to breathing-motion artifacts (11). The combination of TSENSE and UNFOLD filtering provided the greatest artifact suppression and was the most robust approach. Figure 3 shows example short-axis images for a single slice acquired using method 3 and reconstructed with UNFOLD alone (top row), TSENSE alone (middle row), and combined TSENSE and UNFOLD (bottom row). The three cases (columns) illustrate various artifact mechanisms. The case shown in the left column illustrates the time of peak right ventricle (RV) contrast enhancement. The reconstruction using UNFOLD alone has an FOV/2 artifact of the RV due to the rapid change in contrast. The case shown in the center column illustrates an artifact in the UNFOLD-alone reconstruction due to breathing motion toward the end of acquisition. The case shown in the right column illustrates a frame with a residual alias artifact for the TSENSE-alone reconstruction. The images for this case are magnified (zoomed) in order to show the more subtle artifact. This artifact is caused by EPI distortion, which leads to errors in the sensitivity map estimated in vivo using the TSENSE method.
FIG. 3.
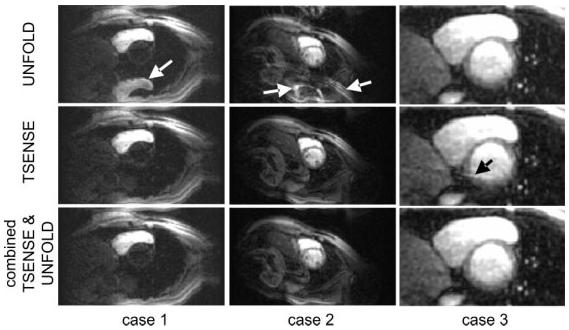
Illustration of artifacts for three cases using various reconstruction methods. Case 1 illustrates an UNFOLD artifact due to dynamic contrast enhancement. Case 2 illustrates an UNFOLD artifact due to breathing motion. Case 3 illustrates a residual TSENSE artifact due to EPI distortion-related errors in in vivo sensitivity map estimates.
Figure 4 shows example short-axis images for a single slice acquired using the three methods. All images are scaled for equal noise standard deviation and are displayed using the same window level. The parameters of method 2 yielded images of improved quality compared to method 1, but at the expense of spatial coverage. The image quality achieved with method 3 is comparable to that obtained with method 2, and has twice the spatial coverage. The measured SNRs (for six subjects and two slice locations) of myocardium post-enhancement for each method are shown in Fig. 5a and b for the anteroseptal and posterior segments, respectively, corresponding to segments with the maximum and minimum SNR due to variation in surface coil intensity. The measured CNR (post-enhancement minus pre-enhancement SNR) of the myocardium is shown in Fig. 5c and d for the anteroseptal and posterior segments, respectively. The mean SNR for all subjects for method 2 is 1.35 ± 0.1 (N = 6 segments) times that for method 1, and the mean SNR for all subjects for method 3 is 1.65 ± 0.1 times that for method 1.
FIG. 4.
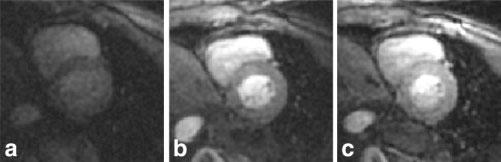
Example first-pass contrast-enhanced images postcontrast for (a) method 1 using TI = 80 ms with five-slice coverage at heart rate ∼ 60 bpm, (b) method 2 using TI = 120 ms with four-slice coverage at heart rate ∼ 60 bpm, and (c) method 3 using TI = 120 ms with eight-slice coverage at heart rate ∼ 60 bpm, reconstructed using TSENSE with UNFOLD temporal filtering.
FIG. 5.
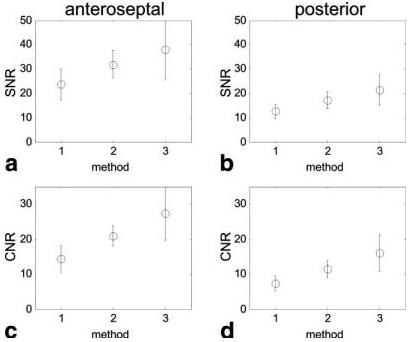
Measured SNR (mean ± SD, N = 6) of myocardium post-enhancement for each method (see Table 1) for (a) the anteroseptal segment and (b) the posterior segment, and measured CNR (post-enhancement minus pre-enhancement SNR) of myocardium (mean ± SD, N = 6) for (c) the anteroseptal segment and (d) the posterior segment.
DISCUSSION
The proposed method using shot-to-shot slice interleaving with acceleration, based on the TSENSE parallel imaging method, provides greater spatial coverage (number of slices per heartbeat) with improved image quality. Both methods 2 and 3 use a 90° saturation preparation, which should reduce the beat-to-beat signal intensity variation due to arrhythmias or inaccurate gating as compared to method 1, which uses a 70° preparation pulse. Furthermore, methods 2 and 3 (with longer TI) acquire images during a flatter portion of the saturation recovery response, thereby reducing distortion of the PSF.
Method 3 has approximately 20% improved SNR compared to method 2. This agrees closely with the expected gain based on the increase in contrast difference due to the longer TR and greater readout flip angle, and loss in SNR by factor g√Re (where g represents the so-called SENSE g-factor (6), and Re is the effective acceleration, which includes the effect of the UNFOLD temporal filter). The estimated improvement in contrast (signal difference) between methods 2 and 3 is approximately 1.7:1, while the SNR loss (g√Re) is approximately 1.3–1.5 using the UNFOLD noise equivalent bandwidth of 0.8, and the SENSE g-factor is 1–1.2 using the four-coil array. The estimated improvement is based on simulated transient magnetization response using values of T1 = 100–800 ms and T2 = 50 ms. With the use of eight-coil systems, the R = 2 SENSE g-factor is expected to be on the order of 1.1 or less.
The image quality produced by the proposed method is limited by both EPI ghosts and SNR. EPI ghosts also limit the degree of artifact suppression in the TSENSE reconstruction due to errors in the TSENSE autocalibrating reference image (in vivo B1-map estimates) caused by EPI distortion. These residual TSENSE artifacts are further suppressed by means of low-pass temporal filtering (UNFOLD). The proposed method can be applied to GRE acquisition without EPI at reduced acquisition efficiency. For example, a GRE acquisition with single-echo readout will have approximately TR = 3 ms, and therefore 3 ms per echo, as compared to the EPI case with ETL = 4, with 6.9/4 = 1.7 ms per echo. In this case, using the same imaging parameters, the imaging time would be 240 ms for an interleaved rate 2 acquisition, and 120 ms with straightforward application of SENSE without slice interleaving. The imaging time for an interleaved acquisition is too long without EPI, and causes motion blur and artifacts. Acquisitions obtained using SENSE with non-EPI GRE will not have EPI ghosts, but will have √2 SNR loss due to accelerated acquisition. Higher-rate SENSE acceleration may be used to further reduce the imaging time. For example, R = 4 TSENSE with slice interleaving of two slices would reduce the imaging time from 240 ms to 120 ms in the above example using non-EPI GRE. The SNR with rate 4 acceleration and interleaving of two slices would be reduced even further.
Temporal resolution is an important factor because it affects the quantitative analysis of time intensity curves. The temporal resolution of the proposed method using the UNFOLD temporal filter with 80% bandwidth slightly degrades the effective resolution. The effective temporal resolution is 1.25 heartbeats. Low-pass filters are often characterized by their transient response to an impulse or step input (10). For this application, the ramp response is a more appropriate performance measure for modeling the first-pass contrast enhancement of the myocardium. Figure 6 compares the time intensity curves for the LV region and myocardium (posterior segment) for the TSENSE method without (solid lines) and with (dotted lines) UNFOLD temporal filtering. In this particular example, excellent TSENSE artifact suppression was achieved without UNFOLD temporal filtering, and thus a direct means of comparison was obtained. The temporal filter introduces a small (unimportant) latency and a slight overshoot. In cases with little or no EPI ghosting distortion, the TSENSE artifact suppression is adequate and the UNFOLD temporal filtering may be eliminated.
FIG. 6.
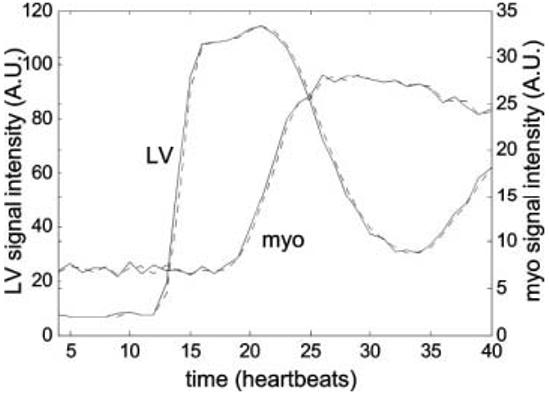
Time intensity curves for LV and myocardium contrast enhancement comparing the TSENSE method without (solid) and with (dotted) UNFOLD temporal filtering, showing nearly identical responses.
Steady-state free precession (SSFP) (or fast imaging with steady precession (FISP)) imaging with short TR may also be used with saturation recovery to produce high-quality images without resorting to an echo-train readout. However, a full comparison of the FISP and FGRE methods remains to be done.
CONCLUSIONS
It has been demonstrated that slice interleaving with TSENSE achieves twice the spatial coverage compared to other techniques, without reducing image quality. Higher acceleration factors may be practical with the use of a greater number of coils, although the SENSE g-factor will increase.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- 1.Ding S, Wolff SD, Epstein FH. Improved coverage in dynamic contrast-enhanced cardiac MRI using interleaved gradient-echo EPI. Magn Reson Med. 1998;39:514–519. doi: 10.1002/mrm.1910390403. [DOI] [PubMed] [Google Scholar]
- 2.Bertschinger KM, Nanz D, Buechi M, Luescher TF, Marincek B, von Schulthess GK, Schwitter J. Magnetic resonance myocardial first-pass perfusion imaging: parameter optimization for signal response and cardiac coverage. J Magn Reson Imaging. 2001;14:556–562. doi: 10.1002/jmri.1219. [DOI] [PubMed] [Google Scholar]
- 3.Slavin GS, Wolff SD, Gupta SN, Foo TK. First-pass myocardial perfusion MR imaging with interleaved notched saturation: feasibility study. Radiology. 2001;219:258–263. doi: 10.1148/radiology.219.1.r01mr35258. [DOI] [PubMed] [Google Scholar]
- 4.Madore B, Glover GH, Pelc NJ. Unaliasing by Fourier encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn Reson Med. 1999;42:813–828. doi: 10.1002/(sici)1522-2594(199911)42:5<813::aid-mrm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Epstein FH, Kellman P, McVeigh ER. First-pass cardiac MRI using UNFOLD. Radiology. 2000;217(Suppl):379. [Google Scholar]
- 6.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 7.Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE) Magn Reson Med. 2001;45:846–852. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 8.Reeder SB, McVeigh ER. The effect of high speed gradients on fast gradient echo imaging. Magn Reson Med. 1994;32:612–621. doi: 10.1002/mrm.1910320510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzka DA, Epstein FH, McVeigh ER. Improved SNR in breath-hold cardiac cine imaging by slice interleaving; Proceedings of the 8th Annual Meeting of ISMRM; Denver. 2000. p. 1529. [Google Scholar]
- 10.Kellman P, Epstein FH, McVeigh ER. Low latency temporal filter design for real time interventional MR imaging using UNFOLD. Magn Reson Med. 2000;44:933–939. doi: 10.1002/1522-2594(200012)44:6<933::aid-mrm15>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Bella EVR, Wu YJ, Alexander AL, Parker DL, Green D, McGann CJ. Comparison of temporal filtering methods for dynamic contrast MRI myocardial perfusion studies. Magn Reson Med. 2003;49:895–902. doi: 10.1002/mrm.10439. [DOI] [PubMed] [Google Scholar]


