Abstract
Daam1 (Dishevelled-associated activator of morphogenesis-1) is a diaphanous-related formin first studied as a novel dishevelled-binding protein and shown to be crucial for the planar cell polarity (PCP) pathway in Xenopus. Daam1, like other formins, directs nucleation and elongation of new actin filaments using its conserved formin-homology-2 (FH2) domain. Here we report the crystal structure of a large C-terminal fragment of human Daam1 containing the FH2 domain. The structure, determined at 2.25 Å resolution using the SAD phasing method, reveals a “tethered dimer” architecture that is similar to that previously described for the FH2 domain of the yeast formin Bni1, which shares ~21% sequence identity with Daam1. Despite the overall similarity with the dimeric FH2 domain of Bni1 and with a truncated monomeric structure of mDia1, the Daam1 FH2 structure reveals a number of differences in secondary structure elements and in the “lasso/post” dimerization interface that may be functionally important. Most strikingly, the two halves of the crystallographic dimer pack together in a manner that occludes their actin binding surfaces. This “locked” conformation is stabilized by two novel, interacting β-strands formed by the ends of the linkers that connect the two sides of the dimer. The Daam1 FH2 domain has weak actin assembly activity as compared with other mammalian formins, but mutations that disrupt the β-strand lock increase activity ~10-fold to a level comparable to other formins, suggesting that this occluded conformation may represent an autoinhibited conformation of the Daam1 FH2 domain.
Keywords: protein structure, formin, FH2 domain, actin assembly, dishevelled
Introduction
Formins are a large family of proteins that regulate actin filament assembly in response to diverse signals, and they are crucial for a variety of actin-dependent cellular processes including polarized cell growth, vesicular transport and cytokinesis1–4. Characteristic features of this protein family include two commonly shared domains, the Formin-Homology-1 (FH1) and Formin-Homology-2 (FH2) domains5. The FH1 domain, formed by proline-rich stretches, binds profilin as well as SH3 domain containing proteins. The FH2 domain binds actin, and directly nucleates new, unbranched actin filaments6, 7. Additionally, the FH2 domain has been shown to remain associated with the barbed end of the actin filament as it elongates 8–13.
A recent phylogenetic analysis revealed that metazoan FH2 domains segregate into seven subfamilies; three of these groups, Dia(Diaphanous), Daam(Dishevelled-associated activator of morphogenesis) and FRL(formin-related gene in leukocytes) possess some similarities outside of the FH2 domain14, and have been termed “diaphanous-related” formins. They share a common domain structure that includes a GTPase-binding domain (GBD), a diaphanous inhibitory domain (DID), and a coiled-coil (CC) region followed by the FH1, FH2 and C-terminal diaphanous auto-regulatory domain (DAD)2, 15(Figure 1A). These formins are thought to be autoinhibited via intramolecular binding of the N-terminal DID to the C-terminal DAD 16–20. Binding of GTP-bound Rho GTPases to the GBD domain and adjacent DID domain displaces the autoinhibitory interaction, at least partially releasing the FH2 domain to activate actin assembly 17, 19–22.
Figure 1.
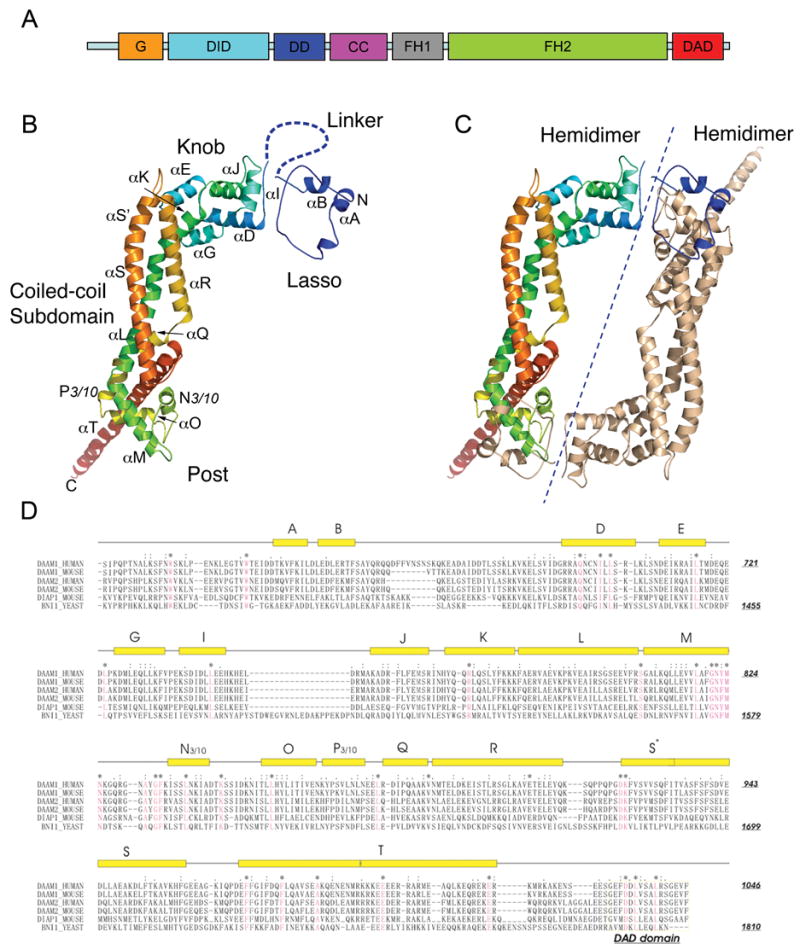
Crystal structure of the Daam1 FH2 domain. (A) The domain structure of human Daam1. (B) Ribbon diagram showing the overall structure of Daam1 FH2 domain. Subdomains including the lasso, the knob, the coiled-coil and the post region are labeled. The invisible linker region is drawn manually with dashed line for the purpose of illustration. The molecule is colored using the visible spectrum (from blue at the N-terminus to red at the C-terminus). (C) Ribbon diagram of the structure of Daam1 FH2 dimer. The dashed line separates the two hemidimers. One molecule is colored the same way as in (B), while the other is colored tan. (D) Sequence alignment and secondary structure of the FH2 and DAD domains. Aligned sequences are from human Daam1, murine Daam1, human Daam2, murine Daam2, murine Dia1 and yeast (S. cerevisiae) Bni1p. Secondary structure elements are shown above the sequences, with rectangles representing helices and thin lines indicating non-helical regions. Conserved residues are colored red. Figures wre prepared using the program PYMOL (Delano, W.L., The PyMol Molecular Graphics System (2002) http://www.pymol.org).
Daam1 was first studied as a novel formin protein involved in the planar cell polarity pathway in Xenopus gastrulation23. Habas et al. demonstrated that Daam1 is required for Wnt/Fz signaling in Xenopus gastrulation via direct interactions with both dishevelled and RhoA 23. The “DEP” and “PDZ” domains of dishevelled were demonstrated to bind a C-terminal fragment of Daam1 that spans its FH1, FH2, and DAD domains (residues 490–1078), while RhoA was shown to bind the N-terminal region as in other diaphanous-related formins. In Drosophila, Daam is required for organizing the apical actin cables that define the taenidial fold pattern of the tracheal cuticle, but does not appear to be required for planar cell polarity signaling 24. As with other diaphanous-related formins, Daam1 binds to RhoA and Cdc42 in GTP-dependent manner25.
The formin FH2 domain is the minimal functional unit for the nucleation and elongation of actin filaments7, 8, 26. The crystal structure of the FH2 domain of budding yeast Bni1 revealed the overall architecture of the domain and showed that it forms a stable and flexible ring-shaped dimer27. Analysis of the truncated, monomeric form of the mDia1 FH2 domain of mDia1 revealed a similar rod-shaped core28. Functional analysis of conserved surface residues in these studies identified two actin binding surfaces, one at either end of each rod-shaped subunit27. Co-crystallization of the Bni1 FH2 domain with tetramethyl-rhodamine-labeled actin showed that these conserved patches interact with distinct actin subunits, allowing each of half the FH2 dimer to “bridge” between two or three actin subunits29. Together with functional analysis of formin-mediated actin assembly, these studies suggest a general model of FH2 domain function in which the FH2 dimer can nucleate a new filament by stabilizing an actin dimer (or possibly a higher order oligomer), and processively move with and cap the elongating barbed end via a “stair-stepping” motion made possible by the flexible tethered-dimer construction of the domain9, 11, 13, 27, 30. Essentially, one subunit can move to adopt an “open” configuration to accept a new actin subunit, while the other remains associated in a “closed” configuration31. The adjacent FH1 domain accelerates filament elongation by interacting with profilin-actin complexes and shuttling actin to the barbed end8–10, 31.
Although the overall structure and general mechanism of the FH2 domain are likely conserved, the rates of nucleation and assembly and profilin dependence of FH2 domains vary greatly1, 9. As there are at least 15 distinct formins in mammals2, it is important to understand their respective structural similarities and differences. Here we describe the structure of a fragment of human Daam1 (residues 596–1078) containing the FH2 domain. The Daam1 FH2 domain consists of an N-terminal “lasso” segment, a flexible linker that is largely disordered in the present structure, and a rod-shaped domain formed by three subdomains termed the “knob”, “coiled-coil”, and “post”. As in the Bni1 FH2 domain, the Daam1 domain forms a head-to-tail dimer stabilized by the N-terminal “lasso” segment in each subunit, which wraps around the “post” in the other subunit in the dimer. Although the overall “tethered dimer” architecture is quite similar to that of the yeast Bni1 FH2 domain, we find differences that may be functionally important. In particular, divergence in the lasso/post dimerization interface may prevent heterodimerization of Daam1 with other formins. Additionally, the two halves of the Daam1 dimer pack together in a manner that occludes their actin binding surfaces and is suggestive of an inactive conformation of the FH2 domain. Our structure/function studies of the Daam1 FH2 domain show that indeed the wild-type domain has only very weak actin assembly activity as compared with other mammalian formins (mDia1 and mDia2), but mutations that disrupt the putative autoinhibitory interactions increase actin assembly ~10-fold. These “derepressed” mutants have activity similar to other formins studied to date.
Results and Discussion
Overall structure
The structure reveals the “tethered-dimer” architecture predicted to be characteristic of all FH2 domains27. The Daam1 FH2 domain is composed of five subdomains: the lasso, a flexible linker, a globular knob subdomain, a coiled-coil region and a “post” subdomain with a C-terminal helical extension (Figure 1B). In the dimeric molecule, the two subunits are arranged in a head-to-tail fashion and the lasso of each subunit encircles the post subdomain of the other (Figure 1C). In the present structure, the two subunits are related by crystallographic two-fold symmetry. Although the linker is disordered, we can unambiguously assign the connectivity as that required to form the head-to-tail dimer shown in Figure 1C because the 30-residue linker is not long enough to allow intrasubunit interaction, nor is it long enough to connect the other pairs of subunits related by crystallographic symmetry in this space group (I222). Furthermore, the assigned subunits are intimately associated, with the knob region of one “hemidimer” contacting the post region of the other. The term hemidimer refers to one side of the tethered dimer; it is the rod-like structural unit containing the knob, coiled-coil, and post of one subunit together with the lasso of the other27. The hemidimer has also been functionally termed the “bridge element”, as it contains two actin-binding surfaces and bridges between actin subunits29. The relative orientation of the two hemidimers in the present structure is quite different from that previously observed in Bni1 - the divergent orientation is readily accommodated by rearrangement of the disordered linker segment. This finding is not unexpected, as the “flexible tethering” of the FH2 domain via the linker is thought to allow stair-stepping on the growing barbed-end of the actin filament. Also, the Bni1 FH2 domain has been crystallized with two very different relative orientations of the hemidimers 27.
Comparison with Bni1 and mDia1 FH2 domains
Although the overall FH2 domain architecture is preserved in Daam1, there are considerable differences in secondary structure elements as compared with Bni1 and mDia1. We describe the structure of the Daam1 domain in more detail via comparison with the structure of the Bni1 dimer and a monomeric structure of mDia1 in which the lasso and linker were truncated (at present, there is no reported dimeric mDia1 structure). To facilitate comparison, we use the Bni1 secondary structure nomenclature27. The r.m.s. deviations in Cα carbon atom positions between the Daam1 and Bni1 and mDia1 FH2 domains are 3.6 Å (for 318 Cα atoms) and 3.4 Å (for 314 Cα atoms), respectively.
The lasso of Daam1 FH2 domain spans residues 602–653, including helices αA and αB. The lasso winds around the protruding helix αM in the post for about 1 1/3 turns. The two tryptophan residues (Trp615 and Trp628) from the lasso insert into hydrophobic pockets in the post in a manner precisely analogous to the corresponding residues in Bni1. However, helices αA and αB in the lasso are approximately one turn shorter than those in Bni1. The ~30 residue linker region (residues 654–682) is unstructured and not visible in the electron density map (Note that there is no helix αC in Daam1 since the linker is not visible).
The greatest differences among the FH2 domains of Daam1, mDia1 and Bni1 lie within the knob subdomain and in its orientation relative to the rest of the domain. In Figures 2A and 2B, we compare the knob subdomains of the three proteins. The knob subdomain of Daam1 closely resembles that of mDia1 (RMSD of 1.8 Å) (Figure 2B), but is more divergent from that of Bni1 (Figure 2A). The Daam1 knob subdomain contains 6 helices (like mDia1), rather than 8 as found in Bni1, as the short αF and αH helices are not formed in Daam1 or in mDia1, despite the fact that there are no deletions in primary sequence relative to Bni1 at these positions (Figure 1D). Additionally, helices αD and αG in Daam1 are both one turn longer than the corresponding helices of Bni1 (Figure 2A). Daam1 also has a much shorter loop connecting helices αI and αJ (termed the “knob loop”) than does Bni1 (Figure 2A). The 25 residue knob loop in Bni1 covers a significant area of the knob outer surface, and also in part defines the orientation of the knob with respect to the rest of the domain via contact with the loop connecting αR an αS in the coiled-coil domain. The short knob loops in Daam1 (and mDia1) do not contact the coiled-coil, and thus these proteins may exhibit more interdomain flexibility. Phylogenetic analysis of FH2 domain has shown that all 14 ascomycota FH2 domains FH2 domains have a long knob loop while the 87 non-ascomycota have short loops2.
Figure 2.
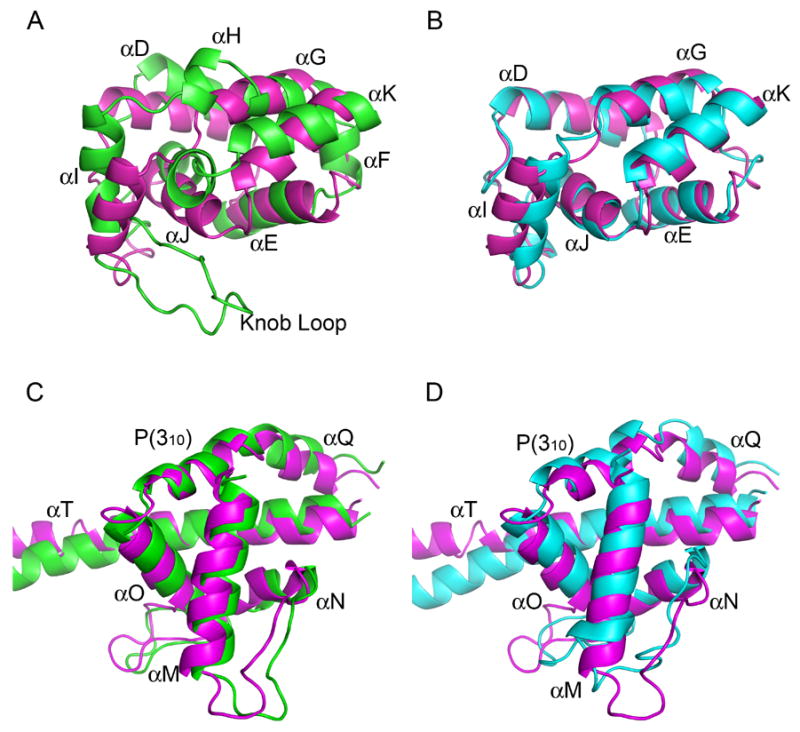
Structure comparisons of the FH2 domain of Daam1 with those of Bni1p and mDia1. (A–B) Comparisons of the knob subdomain of Daam1 and Bni1(A) or mDia1(B). (C–D) Comparisons of the post subdomain of Daam1 and Bni1(C) or mDia1(D). Daam1 is colored in magenta, Bni1 in green while mDia1 in cyan.
The central coiled-coil region, which connects the knob and post subdomains, is quite similar in Daam1, mDia1 and Bni1. The coiled-coil is tightly integrated with the post domain, which is both a site of contact with actin and the point of dimerization via the lasso/post interface. Considering the dual role of the post subdomain, it is not surprising that its structure is well conserved between Daam1 and Bni1. The post is mostly helical, and contains the very highly conserved FH2 signature motif “G-N-Y/F-M-N” which lies within helix αM (Figure 2C and 2D). In the context of the FH2 dimer, the N-terminal lasso encircles helix αM and the adjacent loop (see below). At the C-terminus of the FH2 domain, Daam1 has a longer helix αT than Bni1 and its position is somewhat different, but these differences may not be significant as the length and direction of this long helix may be affected by crystal packing as observed for mDia128. The last 54 residues of the crystallized protein (residues 1025–1078), which includes the DAD domain, are not visible in the electron density map.
The lasso-post dimerization interface
Although the lasso/post dimerization interface in Daam1 is generally similar to that of Bni1, there are interesting differences in interactions and hydrogen bonding networks between the lasso and the highly conserved GN(F/Y)MN sequence motif in the post. For example, in Daam1, Tyr823 in this motif forms hydrogen bonds with the sidechain of Glu646 in the lasso (Figure 3A). In Bni1, this residue is a phenylalanine and this hydrogen bond cannot be formed (Figure 3B). In both Bni1 and Daam1, the first asparagine residue in this motif (Asn822 in Daam1) is at the heart of a hydrogen bond network that stabilizes both the post subdomain and the lasso/post interaction. In both proteins, this asparagine forms two hydrogen bonds with the mainchain atoms of an alanine within the post (Ala1586 in Bni1 and Ala832 in Daam1). However, the interactions between this conserved Asparagine sidechain with the lasso region are very different. In Daam1, the sidechain of Asn822 hydrogen bonds with Asp633 in helix αA of the lasso (Figure 3A), while in Bni1, the corresponding residue (Asn1577) interacts with the hydroxyl group of Tyr1351, which lies at the N-terminus of the lasso, rather than on helix αA (Figure 3B). There are also significant differences in hydrophobic packing. For example, the aromatic sidechain of Phe637 in helix αA of Daam1 occupies the same pocket as the sidechain of Tyr1351 in Bni1, although it lies in a very different position in the primary sequence. Although we can only compare a yeast and mammalian formin here – a dimeric structure of mDia1 is not available - fine differences such as these in the lasso/post interface may in part explain the ability of formins to specifically form unique homodimers, rather than heterodimers, even though the lasso/post dimerization interface is otherwise highly conserved.
Figure 3.
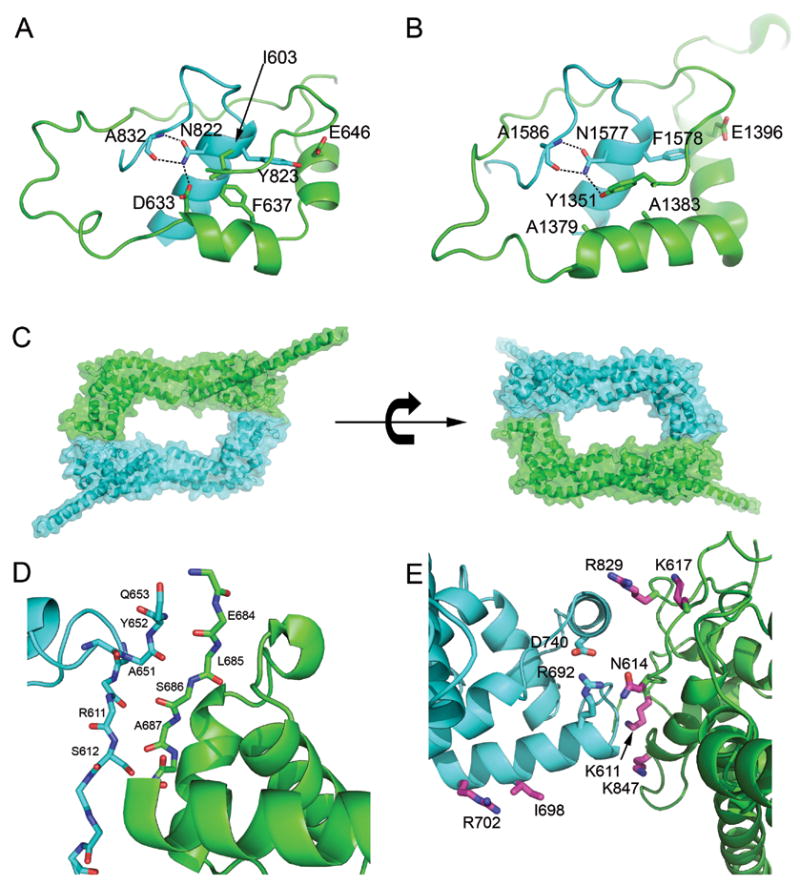
Distinct dimerization interactions in Daam1 and Bni1. Ribbon diagrams of (A) Daam1 and (B) Bni1 lasso/post interface. Conserved residues are labeled and shown in stick models. The lasso is colored in green and the post in cyan in (A–B). (C–E) The inter-hemidimer interface of Daam1 FH2 dimer. (C)Ribbon diagrams of Daam1 FH2 dimer are shown from the top view with one hemidimer colored in green and the other in cyan. The 50% transparent surfaces are also shown with the same color coding to emphasize the inter-hemidimer interface. (D–E) Ribbon diagrams of the inter-hemidimer interface. (D) A novel β-strand interaction formed by the two ends of the linker segment. (E) Residues expected to participate in actin binding are found at the inter-hemidimer interface. These residues are colored magenta and shown in stick form; they correspond to residues found at the actin binding site in the structure of the Bni1/actin complex. Mutation of the equivalent residues in Bni1 and/or mDia1 has been shown to diminish actin assembly activity. In particular, mutation of the residues corresponding to Lys847 and Ile698 completely abolish actin assembly activity in Bni1 (Lys1601 to Asp and Ile1431 to Ala) 27, 29. Note also that we show here that mutation of I698 to alanine abrogates the activity of the Daam1 FH2 domain (see Figure 5). Two residues from Daam1 (R692 and D740), which form a unique salt bridge, are also shown (cyan sidechains).
The actin-binding surfaces
Analysis of the Bni1 FH2 domain and its complex with actin revealed two actin binding patches on each side of the dimer, one on the knob subdomain and one on the post subdomain (which is formed by residues from the lasso and post together) 27, 29. Residues that are most important for actin binding (as judged by previous structure-function studies) are mostly well-conserved in Daam1, despite differences in nearby residues that are also in the interface. The knob and lasso/post regions of Daam1 are shown superimposed on the Bni1/actin complex in Figure 4. In the knob subdomain, the highly conserved FH2 residue Ile698 (equivalent to Ile1431 in Bni1) is located at the center of the interface, where it contacts a hydrophobic pocket in the actin cleft (Figure 4A). This isoleucine is at the center of helix αD, which is almost 1 turn longer in Daam1 than in Bni1. Mutation of Ile698 to alanine completely abolishes the actin assembly activity of Daam1 (see following section and Fig. 5G), as did mutation of Ile1431 in Bni127, 29. Nearby residue His1434 in Bni1 is also important for actin binding; the superposition suggests that Arg702 in Daam1 may make the equivalent interaction, even though it is displaced by one position in the primary sequence (Figure 4A). The second actin binding surface, a composite surface formed by the lasso and post, is also well conserved (Figure 4B). Daam1 residues Lys611 (in the lasso) and Lys847 (in the post) are positioned to make similar interactions with actin as the equivalent residues in Bni1 (Lys1359 and Lys1601 respectively).
Figure 4.
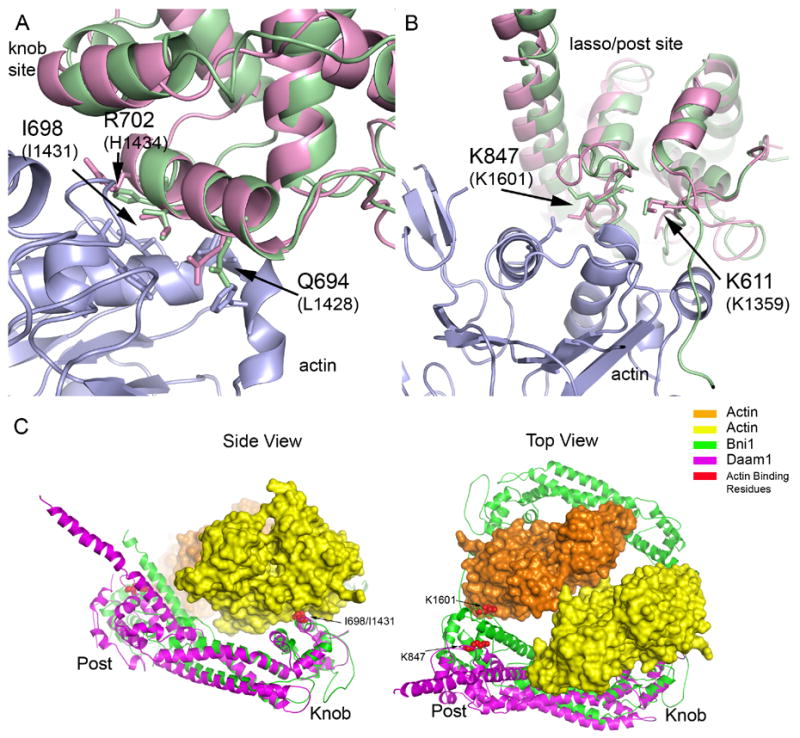
Insights into interactions with actin via comparisons with the structure of the Bni1/actin complex (A–B) The Daam1 FH2 structure is superimposed on the Bni1/actin complex (PDB ID 1Y64) in the regions of the knob actin-binding site (A) and the lasso/post binding site (B). The actin is colored blue, Daam1 magenta and Bni1 green. Selected residues that are known to be important for actin assembly by Bni1 are shown in stick form and labeled. (C) Overall views of the Daam1 FH2 domain (magenta) superimposed on the Bni1/actin complex (1Y64). Two actin subunits (yellow and orange) and two Bni1 FH2 domains (green) from the Bni1/actin structure are shown; this configuration may represent a “strained” intermediate in FH2-mediated assembly or actin filaments29. The sidechains of key actin binding residues are shown in CPK form and are colored red (Ile698 and Lys847 in Daam1; Ile1431 and Lys1601 in Bni1). The superposition was carried out using the knob subdomain only. Note that while the knob and post sites independently superimpose well on the actin complex (panels A and B), both cannot be simultaneously brought into register with actin due to a different relative orientation of the knob subdomain. Bringing the knob into register (side view) leaves the actin binding residues in the laso/post region displaced from actin by ~17 Å (best seen in the Top view).
Figure 5.
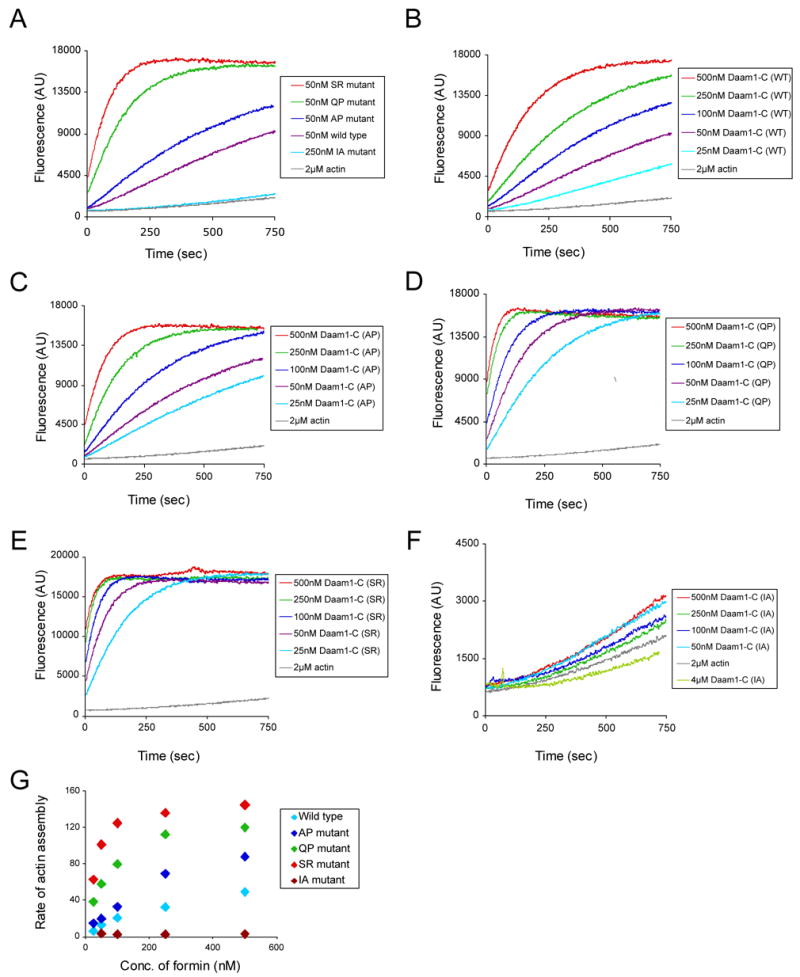
Actin assembly activities of wild type and mutant Daam1 FH2-C fragments. (A) Purified Daam1 polypeptides were directly compared at one concentration (50 nM) for their effects on assembly of 2 μM monomeric actin (5% pyrene-labeled). (B–F) Each purified Daam1 polypeptide was tested over a range of concentrations for its effects on assembly of 2 μM monomeric actin (5% pyrene-labeled). (G) Concentration-dependent effects of wild type and mutant Daam1 polypeptides on the rate of actin assembly. Rates were determined from the slopes of the actin assembly curves in B–F.
Although the knob and lasso/post binding surfaces in the Daam1 structure independently superimpose well with those of Bni1 in complex with actin, both cannot be simultaneously superimposed due to divergence in the relative orientation of the knob subdomains. Comparison of the Daam1 and Bni1 FH2 domains using difference distance matrix analysis with the program DDMP (http://www.csb.yale.edu/userguides/datamanip/ddmp/ddmp_descrip.html) confirms the similarity in individual subdomains, and further reveals that the knob subdomain in Daam1 takes different orientation relative to the coiled-coil and lasso/post regions (data not shown). Thus superposition of the Daam1 and Bni1 using their knob subdomains leaves key actin binding residues in the lasso/post region separated by ~ 17 Å (see Figure 4C).
What are the implications of this observation for understanding the mechanism of actin assembly? The knob and lasso/post surfaces in the Bni1/actin complex interact with two different actin subunits, which are arranged in a “filament-like” orientation. In this “strained” conformation, the two actin subunits are related by a 180° crystallographic rotation (and ~27 Å translation), rather than the 166° rotation seen in the Holmes model of F-actin32. This strained conformation may represent an intermediate in actin assembly. The difference in the relative disposition of the two actin biding surfaces of Daam1, as compared with those of Bni1, implies that either i) there must be sufficient flexibility in the Daam1 hemidimer so that it can bend to adopt the conformation of Bni1, or ii) that the specific 180° actin orientation seen in the Bni1/actin complex is not critical for the mechanism of assembly, or iii) that both flexibility in the FH2 domain and divergence in the actin orientations may be accommodated in actin assembly. The third possibility seems most likely, because long, rod-shaped domains like the FH2 domain often have some intrinsic flexibility and because the precise 180° rotation seen in the Bni1 complex may have been enforced by crystallographic symmetry.
The actin-binding surface is occluded in the Daam1 FH2 dimer
In order to function, the actin binding surfaces of Daam1 must obviously be exposed. Additionally, the stair-stepping movements expected to accompany filament elongation is thought to inherently require the “floppy” tethering of the two sides of the dimer; in the active state one does not expect a defined orientation of one hemidimer with respect to the other. The Daam1 FH2 domain maintains the tethered-dimer architecture of Bni1, and indeed the linker segments connecting the hemidimers are mostly unstructured. However, it is interesting to note that there is also a significant interface between the hemidimers in the present structure, and that this interface occludes the actin binding surfaces on both hemidimers. In this crystallographic dimer, the knob subdomain of each subunit packs closely against the lasso/post region of the other, burying a combined surface area of ~1300 Å2 (Figure 3C). This particular orientation of the two sides of the dimer appears to be stabilized by a novel β-strand interaction formed by the two ends of the linker segment (Figure 3D). Residues 651–653 at one end of the linker form a short β-strand parallel to residues 683-686 at the opposite end. The remainder of the linker (residues 654–682) is unstructured (Figure 1B). The residues involved in the β-strand are conserved in Daam proteins, but are not more generally conserved among diaphanous-related formins. Structural and mutagenesis studies of the actin binding surfaces of Bni1 identified a number of residues in the knob and lasso/post sites that participate in actin assembly 27, 29. Most of the corresponding residues in Daam1 are within or near the inter-hemidimer interface (Figure 3E). Thus we hypothesize that the mode of interaction we observe between the two hemidimers in the present structure could be relevant to regulation of Daam1, perhaps representing a “locked” conformation in which the actin assembly activity is repressed because the actin binding surfaces are occluded. Consistent with this idea, we find that the actin-assembly activity of the Daam1 C-terminal fragment is much lower than that of other mammalian formins that have been characterized to date, including both mDia1 and mDia2 (see supplementary figure S2).
In order to determine whether this β-strand interface indeed maintains the Daam1 FH2 domain in a low-activity state in vitro, we introduced several mutations designed to disrupt the interface and compared the activity of the mutants with the wild-type. We mutated residues Ala651 and Q653 to proline in order to disrupt the novel β-strand (mutants AP and QP). We also mutated Ser612 to arginine (SR). This mutation will abolish the inter-hemidimer hydrogen bond formed by the sidechain hydroxyl group of Ser612, and can also be expected to preclude formation of the interaction due to the steric clash of the bulky arginine sidechain with knob region of the apposed subunit. These mutations are not expected to affect binding to actin, based on their positions in the structure. The actin assembly activities of the wild type and mutant Daam1 FH2 domains are compared in Figure 5. While the activity of the mutants varied considerably, all were more active that the wild-type. The SR mutant, which showed the highest activity, was approximately 10-fold more active than the wild type at a concentration of 50 nM (Figure 5G, also compare the activity of 50 nM SR in Fig. 5A with that of 500 nM WT in Fig. 5B). The QP mutant showed more than 5-fold higher activity than the wild-type (Figure 5G, also compare the activity of 50 nM QP in Fig. 5A with that of 250 nM WT in Fig. 5B). While the activity of wild-type Daam1 is lower than that observed for other formins, the activated mutants have activity well within the range of other mammalian formins. We conclude that the β-strand interactions do indeed maintain the Daam1 FH2 domain in a low activity conformation in solution, and that mutations that disrupt the inhibitory interface activate the domain.
We also tested whether Ile698 in the knob region (equivalent to Ile1431 in Bni1) was required for actin assembly by Daam1. As expected based on analysis of the same mutation in Bni1 and mDia1, we find that mutation of Ile698 to alanine (IA) effectively abolishes the actin assembly activity of Daam1 (Fig. 5F and 5G).
Concluding remarks
The structure described here provides only the second view of a complete, dimeric formin FH2 domain, and it reveals a locked, inactive conformation stabilized by β-strand interactions between the hemidimers. Sequence and structural comparisons of the Daam1 FH2 structure with Bni1 and other FH2 domains also shows that the overall structure of the lasso/post dimerization motif is preserved, but that key differences in dimer contacts may facilitate homodimerization over heterodimerization. Furthermore, we find that the actin binding surfaces in the knob and post regions of Daam1 closely resemble those of Bni1, and that the key residues are conserved. However, we find that small differences in the relative orientation in the knob subdomain of Daam1 would preclude it from interacting with actin in exactly the manner observed in the structure of the Bni1/actin complex. This finding raises the question of whether the precise 180° relative orientation of actin subunits in the Bni1/actin structure is an actual intermediate in assembly of actin filaments by formins. Further investigation will be required to answer this question and to better understand the extent to which flexibility in the formin FH2 domains may play a role in their regulation or in actin assembly. It is possible for example, that the orientation of the knob domain in the present structure is altered by formation of the locked dimer, and that its release would yield a conformation more similar to that in Bni1.
All diaphanous-related formins, including Daam1, are likely to employ a conserved regulatory apparatus involving intramolecular engagement of the C-terminal DAD motif by the N-terminal regulatory region that can be released by Rho-family GTPases to promote activation. However, it is also clear that individual formins can employ distinct additional regulatory mechanisms that adapt them to their unique cell-biological roles4. Such formin-specific regulatory mechanisms are ripe for further study. In particular, the manner in which dishevelled activates Daam1 remains unclear, although it is known to bind to a C-terminal fragment containing the FH2 domain. Daam1 has also been shown to collaborate with the Src tyrosine kinase in addition to Rho-GTPases in regulating actin dynamics25.
While our structure/function analysis indicates that the locked conformation that we observe in the crystal structure does indeed repress Daam1 FH2 activity in vitro, the relevance of the interaction in a cellular context remains to be investigated, as does the means by which it could be disrupted to promote activation. In this regard, it is tempting to speculate that interaction with dishevelled could disrupt the β-strand lock, or that perhaps phosphorylation of one of the tyrosine or serine residues in or near the interface (e.g. Tyr652 or Ser686) could promote its release. The present work will provide a structural foundation for further studies designed to address these questions in vivo.
Materials and Methods
Protein Expression & purification
A DNA fragment encoding amino acids 596–1078 of human Daam1 was amplified by PCR and subcloned into a modified pET vector containing an amino-terminal His6 tag with tobacco etch virus (TEV) cleavage site. The S612R, A651P, Q653P and I698A mutations were introduced into this construct using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, California, United State) according to the manufacture’s protocol. All of the constructs were confirmed by DNA sequencing. The proteins were purified by metal-affinity chromatography on a Ni-chelating column, and the N-terminal His6-tag was removed by digestion with TEV protease. The cleaved protein was further purified by anion-ion exchange (MonoQ) and gel filtration (Superdex S200) chromatography. Gel filtration was carried out in storage buffer (30mM Tris, pH 8.0, 150mM NaCl and 1mM TCEP). The Daam1 FH2-C protein elutes from a gel filtration column as expected for a stable dimer and the purified protein was concentrated to 6–8mg/ml and stored at −70°C in storage buffer.
Crystallization and Structure determination
Crystals of the Daam1 FH2 domain (residue 596–1078) were obtained by the hanging drop method using 6–8 mg/ml protein mixed in a 1:1 volume ratio with the precipitation solution containing 1.8M sodium/potassium phosphate, pH 6.2. Crystals formed in 2 days and grew to full size over 1–2 weeks at 20°C and were harvested in a solution containing 1.9M sodium/potassium phosphate and 20% glycerol and flash-frozen in liquid nitrogen. A mercury derivative was prepared by soaking crystals in 1 mM methyl mercury nitrate for 2 hours. Diffraction data were recorded to 2.25Å resolution from a single, frozen native crystal with space group I222 and unit cell parameters a=101.1Å, b=100.7Å and c=148.8Å. A SAD (Single-wavelength Anomalous Dispersion) dataset to 2.6 Å resolution was collected at the mercury peak wavelength from the methyl-mercury derivative. All diffraction data (see Table 1) were collected at the NE-CAT beamline 24-ID at the Advanced Photon Source, Argonne National Laboratory, and were processed using the program XDS33.
Table 1.
Crystal structure determination statistics
| Native | MeHg derivative | |
|---|---|---|
| Resolution (Å) | 30-2.25 | 30-2.60 |
| Space group | I222 | I222 |
| Unit cell (Å) | a=101.1 b=100.7 c= 148.8 | a= 108.3 b=104.4 c=152.9 |
| Wavelength (Å) | 1.008 | 1.008 |
| Molecules/a.s.u | 1 | 1 |
| Reflections (total/unique) | 116451/25001a | 114504/45540 |
| Completeness (%) | 99.9a | 88.4 |
| Riso/Ranom (%)b | 6.7(32.6) | 9.4(51.3)/9.8(24.2) |
| I/sigmab | 14.2(2.9) | 6.9(2.6) |
| Phasing power (centric/acentric) | 0.61/0.42 | |
| Rcullis(anomalous) | 0.96 | |
| FOM(Figure of Merit) | 0.16 | |
| Number of sites | 4 | |
| Refinement statistics | ||
| Resolution range (Å) | 20-2.25 | |
| Water molecules | 78 | |
| Rcryst/Rfree (%) | 21.8/25.0 | |
| Rmsdc bond length/angles | 0.009 Å/1.30º | |
| Ramachandran Plot | ||
| most favored (%) | 92.1 | |
| additionally allowed (%) | 7.4 | |
| generously allowed (%) | 0.5 | |
| disallowed (%) | 0 |
Completeness is reported within an ellipsoid of resolution extending to 3.25 along a* and 2.25 in the b* and c* directions. Only these data were used for final refinement due to marked anisotropy.
Values in parentheses indicate the statistics in the last shell.
Deviations from ideality.
Attempts to determine the structure by molecular replacement were not successful, likely due to low sequence identities with available structures (30% sequence identity with mDia1, 21% with Bni1) and to flexibility within the FH2 domain. Therefore the structure was determined by SAD phasing using the methyl mercury derivative (Table 1). The electron density map was improved with solvent flattening in CNS34; the initial partial models were obtained by Resolve35 and the complete atomic model was built using the program Coot36 and O37. The structure was initially refined using the program CNS34 against native dataset at 2.25Å. Due to the significant anisotropy of diffraction (3.25 Å along a* axis while 2.25 Å at the perpendicular plane), further refinement with Refmac38 was performed using a data set filtered to include only the reflections contained within an ellipsoid of revolution with axes corresponding to the observed diffraction limits in the a* (3.25 Å) and b* and c* plane (2.25 Å). The marked anisotropy of this data set was confirmed using the anisotropic diffraction server39. The final model contains 393 residues and 78 water molecules and has been refined to an R value of 21.5% (Rfree = 25.0%). The C-terminal tail region, including the DAD domain (residues 1025–1078), and the linker segment (residues 654–682) were not observed in the electron density map and are presumed to be disordered. A representative portion of the electron density map is shown in supplemental figure S1.
Actin assembly assays
Rabbit skeletal muscle was purified 40 and labeled with pyrenyliodoacetamide as described 41, 42. Monomeric rabbit skeletal muscle actin was prepared by gel filtration using a Sephacryl S-200 column (GE heatthcare) equilibrated in G-buffer (10 mM Tris (pH 8.0), 0.2 mM ATP, 0.2 mM 0.2 mM DTT). Actin assembly was CaCl2, andmeasured in 60 μl reactions. Gel-filtered monomeric actin (final concentration 2 μM, 5% pyrene-labeled) was converted to Mg-ATP-actin for 2 min immediately before use in reactions as described 43. To initiate assembly, Mg-ATP-actin was mixed with 15 μl of buffer or HEKG5 proteins in and added immediately to 3 μl of 20x initiation HEKG5mix (40 mM MgCl2, 10 mM ATP, 1 M KCl). Pyrene fluorescence was monitored over time at 25 °C at an excitation of 365 nm and emission of 407 nm in a fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ). Rates of actin assembly were calculated from the slopes of the assembly curves at 50% polymerization.
Protein Data Bank accession code
The atomic coordinates and structural factors have been deposited in the Protein Data Bank with the accession code 2J1D.
Supplementary Material
Acknowledgments
We thank John Wallingford and Xi He for providing the Daam1 cDNA, Azin Nezami for helpful discussions. The x-ray diffraction data were collected at NE-CAT beamline ID24 at the Advanced Photon Source, Argonne National Labs. This work was supported in part by a NIH grant GM071834 (MJE). MJE is the recipient of a Scholar Award from the Leukemia and Lymphoma Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kovar DR. Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol. 2006;18:11–7. doi: 10.1016/j.ceb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Higgs HN. Formin proteins: a domain-based approach. Trends Biochem Sci. 2005;30:342–53. doi: 10.1016/j.tibs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Goode BL, Eck MJ. Mechanism and Function of Formins in the Control of Actin Assembly. Annual Review of Biochemistry in press. 2007 doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 5.Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, Ohya Y, Takai Y. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. Embo J. 1996;15:6060–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat Cell Biol. 2002;4:626–31. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 7.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–5. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 8.Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 2004;303:2007–10. doi: 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- 9.Kovar DR, Pollard TD. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc Natl Acad Sci U S A. 2004;101:14725–30. doi: 10.1073/pnas.0405902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–29. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond SH, Evangelista M, Boone C, Yang C, Dar AC, Sicheri F, Forkey J, Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr Biol. 2003;13:1820–3. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Kozlov MM, Bershadsky AD. Processive capping by formin suggests a force-driven mechanism of actin polymerization. J Cell Biol. 2004;167:1011–7. doi: 10.1083/jcb.200410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16:1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zigmond SH. Formin-induced nucleation of actin filaments. Curr Opin Cell Biol. 2004;16:99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. Embo J. 2005;24:4176–87. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–81. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–30. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 20.Nezami AG, Poy F, Eck MJ. Structure of the autoinhibitory switch in formin mDia1. Structure. 2006;14:257–63. doi: 10.1016/j.str.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–8. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe N, Higashida C. Formins: processive cappers of growing actin filaments. Exp Cell Res. 2004;301:16–22. doi: 10.1016/j.yexcr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 24.Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133:957–66. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- 25.Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312:2180–94. doi: 10.1016/j.yexcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Pring M, Evangelista M, Boone C, Yang C, Zigmond SH. Mechanism of formin-induced nucleation of actin filaments. Biochemistry. 2003;42:486–96. doi: 10.1021/bi026520j. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–23. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- 28.Shimada A, Nyitrai M, Vetter IR, Kuhlmann D, Bugyi B, Narumiya S, Geeves MA, Wittinghofer A. The core FH2 domain of diaphanous-related formins is an elongated actin binding protein that inhibits polymerization. Mol Cell. 2004;13:511–22. doi: 10.1016/s1097-2765(04)00059-0. [DOI] [PubMed] [Google Scholar]
- 29.Otomo T, Tomchick DR, Otomo C, Panchal SC, Machius M, Rosen MK. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433:488–94. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- 30.Harris ES, Li F, Higgs HN. The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J Biol Chem. 2004;279:20076–87. doi: 10.1074/jbc.M312718200. [DOI] [PubMed] [Google Scholar]
- 31.Vavylonis D, Kovar DR, O’Shaughnessy B, Pollard TD. Model of formin-associated actin filament elongation. Mol Cell. 2006;21:455–66. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–9. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 33.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr. 1993;26:795–800. [Google Scholar]
- 34.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 35.Terwilliger TC. Improving macromolecular atomic models at moderate resolution by automated iterative model building, statistical density modification and refinement. Acta Crystallogr D Biol Crystallogr. 2003;59:1174–82. doi: 10.1107/S0907444903009922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 37.Jones TAaKM. Electron-density map interpretation. Methods in Enzymology. 1997;277:173–208. doi: 10.1016/s0076-6879(97)77012-5. [DOI] [PubMed] [Google Scholar]
- 38.Murshudov GN, Vagin AA, Lebedev A, Wilson KS, Dodson EJ. Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr D Biol Crystallogr. 1999;55:247–55. doi: 10.1107/S090744499801405X. [DOI] [PubMed] [Google Scholar]
- 39.Strong M, Sawaya MR, Wang S, Phillips M, Cascio D, Eisenberg D. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006;103:8060–5. doi: 10.1073/pnas.0602606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–71. [PubMed] [Google Scholar]
- 41.Higgs HN, Pollard TD. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem. 1999;274:32531–4. doi: 10.1074/jbc.274.46.32531. [DOI] [PubMed] [Google Scholar]
- 42.Pollard TD. Purification of a high molecular weight actin filament gelation protein from Acanthamoeba that shares antigenic determinants with vertebrate spectrins. J Cell Biol. 1984;99:1970–80. doi: 10.1083/jcb.99.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovar DR, Kuhn JR, Tichy AL, Pollard TD. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol. 2003;161:875–87. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


