Abstract
Although glucocorticoids are frequently administered to patients with hormone refractory prostate cancer, their therapeutic effectiveness is limited by the development of glucocorticoid resistance. The molecular mechanisms of glucocorticoid resistance are unknown but are believed to involve neuropeptide growth factors and cytokines. We examined the functional interaction between bombesin and dexamethasone in PC-3 cells and found that bombesin could act as a survival factor by interfering with dexamethasone-mediated growth inhibition. Because glucocorticoids exert their effects through glucocorticoid receptors (GRs), we measured the expression of GRα and GRβ isoforms in the presence of bombesin. Western blotting and real time PCR revealed bombesin induced expression of GRβ, but not GRα. Because GR isoforms are generated by alternative splicing of a common GR gene, we examined the expression of serine-arginine (SR) proteins involved in alternative splicing, and found that the expression of SRp30 was induced by bombesin in PC-3 cells. To characterize the role of SRp30 in splicing of GR isoforms, siRNAs specific to various SRp30 isoforms were transfected into PC-3 cells. We found that suppression of SRp30c expression by siRNA specifically antagonized bombesin’s effect on glucocorticoid-mediated inhibition of PC cells, suggesting that bombesin-induced expression of SRp30c affects GR pre-mRNA splicing, leading to increased GRβ expression and contributing to glucocorticoid resistance in PC cells.
Keywords: Glucocorticoids, glucocorticoid receptors, SRp30 proteins, bombesin, neuropeptides, prostate cancer
1. INTRODUCTION
Glucocorticoid (GC) monotherapy in patients with hormone refractory prostate cancer is of limited utility, resulting in response rates of approximately 20% [1–3]. Such responses, characterized by decreases in PSA, tumor size, and/or pain are typically of short duration [4–8] due to the development of GC resistance. The molecular mechanisms governing GC resistance are unknown.
Glucocorticoids bind glucocortoid receptors (GR) and subsequently exert their anti-tumor effects by facilitating signaling pathways leading to apoptosis or necrosis [9–12]. Human glucocorticoid receptors (hGR), members of the nuclear receptor superfamily, undergo pre-mRNA alternative splicing in exon 9 resulting in two highly homologous receptor isoforms, hGRα and hGRβ. These isoforms are identical through amino acid 727 but differences at their carboxyl-termini account for their differing functions. While GRα functions as a ligand-dependent transcription factor, hGRβ inhibits the transcriptional activity of hGRα in a dose dependent manner [13, 14].
Splicing in eukaryotes occurs in the spliceosome, comprising serine-arginine-rich proteins (SR proteins) and small nuclear ribonucleoprotein particles (snRNPs) [15, 16]. The SR proteins can positively or negatively regulate spliceosome function depending on their precise location, composition and state of modification. Variation in the relative concentrations of splicing factors can simultaneously affect splice-site choice in multiple pre-mRNAs. Changes in levels of various SR proteins occur during tumor progression and correlate with changes in the mRNAs produced by a specific spliceosome [17, 18]. One such SR protein, SRp30 is ubiquitously expressed as multiple subspecies (SRp30 a-c) and exhibits structural similarities with alternative splicing factor SF2/ASF (SRp30a) and SC35 (SRp30b). The SRp30c subspecies may directly contact RNA via two independent RNA recognition motifs that are linked by a glycine-hinge, also binds to CE9 (an intronal element that can repress the 3′ splice site of exon 8 in the hnRNP A1 pre-mRNA) and acts as a repressor of 3′ splice site utilization [19]. SRp30c protein also directs GR splicing in neutrophils [20].
Bombesin is a fourteen amino acid neuropeptide. Bombesin and other members of the bombesin-like polypeptide family are ligands for G protein-coupled receptors (GPCRs) and can influence multiple signaling pathways conducive to PC progression [21, 22]. Bombesin can induce FAK phosphorylation, ligand-independent phosphorylation of the IGF-1 receptor and Akt, and rapid PKCδ degradation [23–25]. More recently we have shown that bombesin augments both androgen receptor (AR) mediated transcription [26], and histone acetyltransferase activity of AR coactivator p300 [27]. Furthermore, bombesin can augment the expression of the angiogenic factors interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF) [28], and induce resistance to etoposide-induced apoptosis [29]. Together these studies suggest that bombesin contributes to PC progression.
In the present study, we report a novel role for bombesin in regulating the pre-mRNA splicing of GR isoforms. Our results demonstrate that bombesin can induce the expression of splicing factor SRp30c which leads to an increase of GRβ expression, thereby diminishing the GC responsiveness of PC cells.
2. MATERIALS AND METHODS
2-1. Materials and chemicals
Bombesin and dexamethasone (DEX) were purchased from Sigma Biotechnology Inc. Small interference (si) RNA of non-targeting siRNA and siRNAs against the a, b and c subspecies of SRp30 proteins were purchased from Dharmacon Inc. (Chicago, IL). Anti-GR α antibody was purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA); and anti-GR β from Affinity Bioreagents (Golden, CO). Anti-actin antibody was obtained from Sigma-Aldrich Inc. (Saint Louis, MO). Antibodies against SR proteins were purchased from ATCC, Inc. The plasmid pSRp30c-c2 (expressing a fusion protein GFP-SRp30c) was kindly provided by Dr. S. Stamm, (Institute of Biochemistry, Erlangen, Germany).
2-2. Cell culture and growth inhibitory assays
PC-3 cells were maintained in RPMI1640 supplemented with 2 mM glutamine, 1% nonessential amino acids, 100 U/ml streptomyocin and penicillin, and 10% FCS. This media was replaced with RPMI 1640 containing 5% charcoal stripped serum (CS) prior to various treatments. Growth assays were performed as described [23, 24]. All growth assays were performed in triplicate on three separate occasions with similar results.
2-3. Western blotting
Cells were lysed in RIPA buffer [10 mM Tris-HCl (pH7.4), 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 1% sodium deoxycholate, 0.1% SDS, 1.2% aprotinin, 5 uM leupeptin, 4 uM antipain, 1 mM phenylmethylsulfonyl fluoride and 0.1 mM Na3VO4]. Twenty to fifty micrograms of protein from each sample was suspended in 2 X laemmli sample buffer, resolved on a 7 or 10 % SDS-PAGE, and transferred to a nitrocellulose membrane. Western blotting was performed as described previously [23, 24] using diluted primary and secondary antibodies. All Western blot experiments were performed at least twice using different cell lysates with similar results.
2-4. Transfection, CAT assays and application of siRNAs
Transfection of CAT reporter vectors was conducted using Lipofectamine (Invitrogen Inc.) as previously described [27, 30]. The experiments were performed in duplicate or triplicate on at least three separate occasions and CAT activities expressed reported relative to untreated controls.
2-5. Real-time quantitative RT-PCR analysis
PCR was performed using the ABI 7000 system and SYBR green I dye (ABI). Thermocycling was carried out in a final volume of 20 μl, containing 2 μl of cDNA sample, 3.5 mM MgCl2, 0.1 μM primers and 10 μl SYBR green I master mix. After a 10 minute initial denaturation at 95°C, the 50 cycle run consisted of a 15 second denaturation step at 95°C and an annealing and extension step at 60°C for 1 min. To avoid amplification of genomic DNA, the forward and reverse primers were designed spanning different exons. The mean of three repeated PCR values was used in the statistical analysis. Results were normalized to β-actin by dividing the individual RT-PCR values by the mean of three repeated β-actin test values of the representative samples to reduce variability between RNA amounts introduced into the RT-PCR reactions. To distinguish the specific PCR products from non-specific products and primer dimers, the melting curve was determined as described previously [31]. Samples were also analyzed by agarose gel electrophoresis to verify that the amplified products exhibited their calculated molecular weights.
2-6. Statistics
The student unpaired t-test (Prism 4 for Windows, GraphPad Software Inc. CA) was used for calculation of p values.
3. RESULTS
3-1. Bombesin interferes with GC induced growth inhibition in PC-3 cells
In vitro studies demonstrate an antiproliferative effect of GCs on human PC cells [32]. To examine whether bombesin, a neuropeptide acting as a survival factor, interferes with GC-induced PC cell growth inhibition, we first performed cell growth assays in the presence of bombesin and DEX in PC-3 cells. Figure 1A demonstrates that treatment of PC-3 cells with DEX concentrations of 0.1, 1, 10 nM and 100 nM led to growth inhibition of 60%, 66%, 60% and 52%, respectively, compared to untreated controls. Treatment with 50 nM bombesin + 10 nM DEX (shaded bar vs. open bar in lane 10, P = 0.011) and 50 nM bombesin + 100 nM DEX (shaded bar vs. open bar, P = 0.013) significantly reduced GC-induced growth inhibition.
Figure 1. Bombesin interferes with GC induced growth inhibition in PC-3 cells.
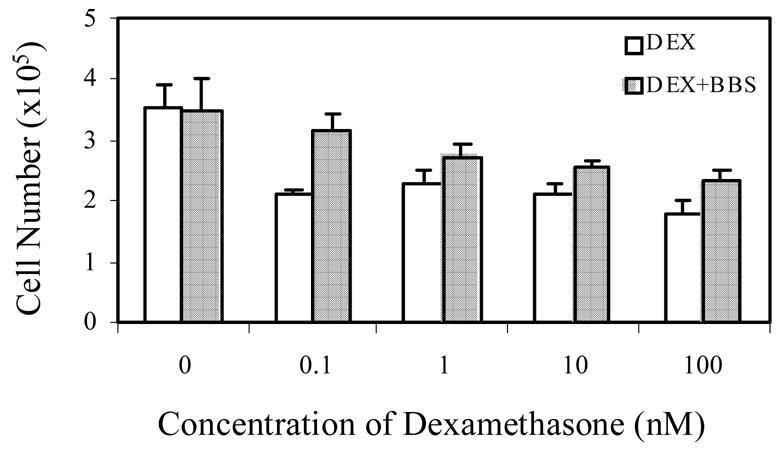
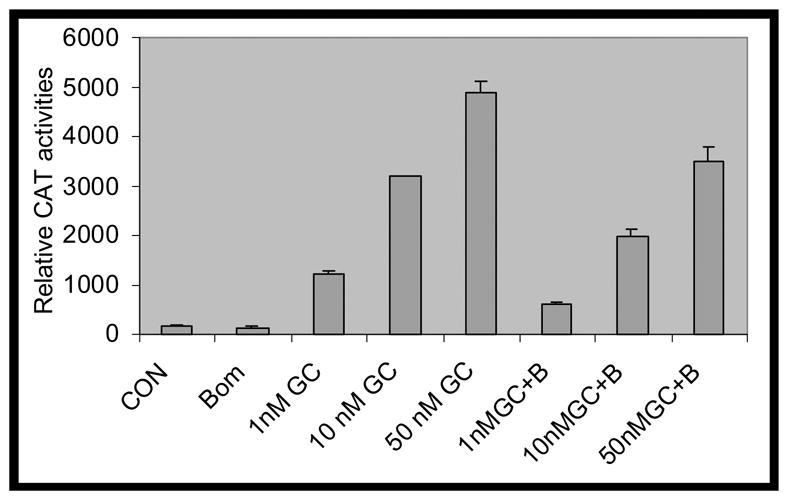
A. PC-3 cells were grown in RPMI1640 containing 5% CS. Cells were treated daily with increasing concentrations of Dexamethasone (DEX), either in the absence (white bars) or in the presence of 50 nM bombesin (BBS) (dark bars). After 4 days, cells were harvested and counted in a hemocytometer. Cell viability was determined by dye exclusion after Trypan Blue staining. Error bars indicated. P<0.02. In lane 0, the open bar denotes vehicle treatment (equal amount of ethanol and distilled water) for GC and bombesin and the shaded bar the vehicle treatment (equal amount of ethanol) plus bombesin alone. B. GRE-tkCAT, a plasmid with glucocorticoid receptor responsive element (GRE) in front of thymidine kinase (tk) promoter and CAT reporter gene, was transfected into PC-3 cells by lipofectamine method. Twenty four hours after transfection, medium were replaced by RPMI1640 containing 5% CS and treated without (CON) (lane 1) or with 50 nM bombesin alone (BOM) (lane 2), various concentrations of DEX (1 nM, 10 nM and 50 nM) (lanes 3, 4, 5) and in combination with 50 nM bombesin (1 nM GC + B, 10 nM GC + B and 50 nM GC + B) (lanes 6, 7 and 8) for 18 hours. Measurements of CAT activities were carried out and relative CAT activities were calculated by comparing the values of various treatments vs. control. Error bar indicated. P<0.01.
The function of GC is mediated by cognate GRs. To examine if bombesin countered GC-induced growth inhibition by interfering with GR activity, we determined the effects of bombesin on CAT activities in PC-3 cells transiently transfected with the plasmid GRE-tk-CAT, containing a glucocorticoid responsive element (GRE) upstream of the thymidine kinase (tk) promoter. As shown in Figure 1B, treatment with 50 nM bombesin and DEX led to decreased CAT activities of 40% (1 nM DEX), 38% (10 nM DEX) and 30% (50 nM DEX) compared to GC treatments alone.
3-2. Bombesin augments mRNA and protein levels of GRβ
Glucocorticoid signaling is mediated by both the active isoform GRα and dominant negative isoform GRβ. The ratio between GRα and GRβ levels determines GC-induced reporter gene expression in vitro [33]. To examine whether the regulatory effects of bombesin on GC-induced CAT activities result from altered splicing of GR, we measured the effect of bombesin on GRα and GRβ mRNA and protein levels. Figure 2B showed that following bombesin treatment, GRβ mRNA increased 3.2 (lanes 4h vs. 0h, P = 0.009) and 1.7 fold relative to control (lanes 18h vs. 0h, P = 0.008) at 4 and 18 hours, respectively, whereas little change was detected in GRα mRNAs (1.3 and 0.8 fold relative to the control, Lanes 4h vs. 0h, P = 0.52, and lanes 18h vs. 0h, P = 0.62, respectively). The increase in GRβ mRNA paralleled an increase in GRβ protein at 18 hours (Figure 2C). The increased expression of GRβ mRNA and protein suggested that bombesin treatment specifically altered the splicing of the GRβ gene.
Figure 2. Bombesin induced GRβ on both protein and RNA levels in PC-3 cells.
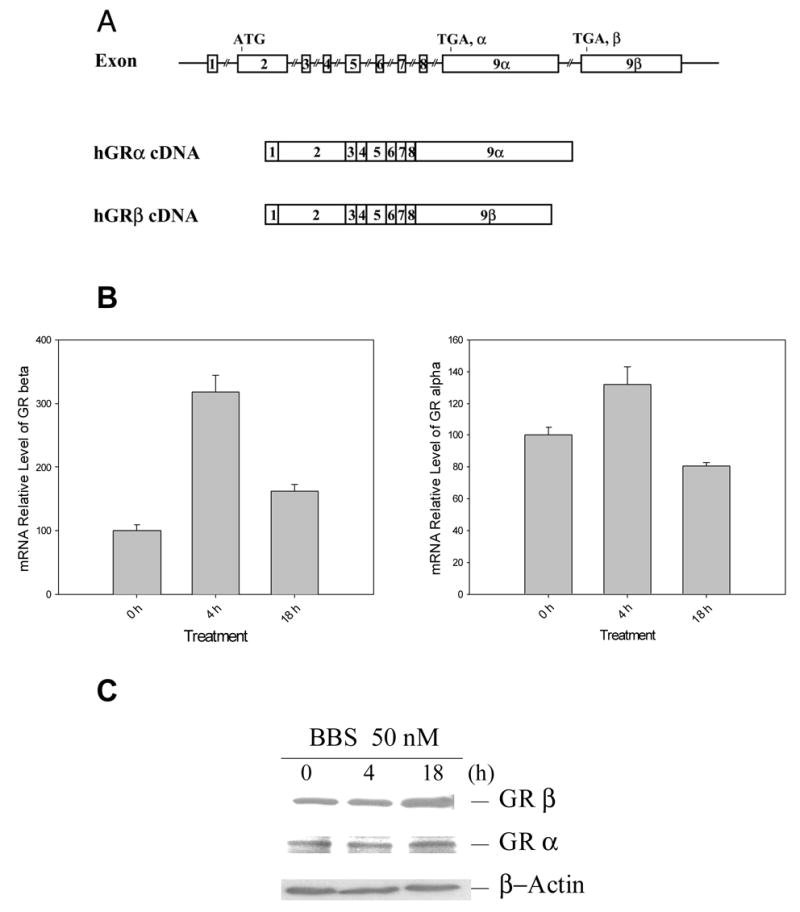
A. Schematic illustrating that alternative splicing of exons 9α and 9β results in two isoforms (GRα and GRβ) from GR gene [46]. B. Message RNA levels of GRα and GRβ in the presence of 50 nM bombesin. The RNAs prepared from PC-3 cells with bombesin treatment at various time points (0, 4h and 18h) were subjected to real time RT-PCR measurement. PCR was performed using primers specific to GR α and GRβ and normalized by that of β-actin. C. Western analysis of GR α and β proteins in the presence of bombesin. PC-3 cells were treated with 50 nM bombesin at 0, 4 and 18 hours (lanes C, 4 and 18). Blots were detected with anti-GRα, anti-GRβ and anti β-actin antibodies.
3-3. Induction of SRp30 proteins by bombesin
SR proteins are major components of the spliceosome and play a key role in pre-mRNA splicing of steroid receptors and other proteins [34]. We therefore assessed whether bombesin altered SR protein expression in PC-3 cells. Western blot analysis using the antibody (16H3, ATCC, CRL-2385) which recognizes SRp20, SRp30, SRp40, SRp55 and SRp75 proteins [34] revealed that SRp30 protein expression but not that of other SR proteins was augmented at 18 hours following treatment with 50 nM bombesin (Figure 3, lanes bombesin vs. control). A kinetic analysis showed that the expression of SRp30 protein was induced by bombesin at 18 hours. These data indicate that SRp30, like GRβ, is induced by bombesin treatment of PC-3 cells.
Figure 3. Induction of SRp30 protein by bombesin in PC-3 cells.
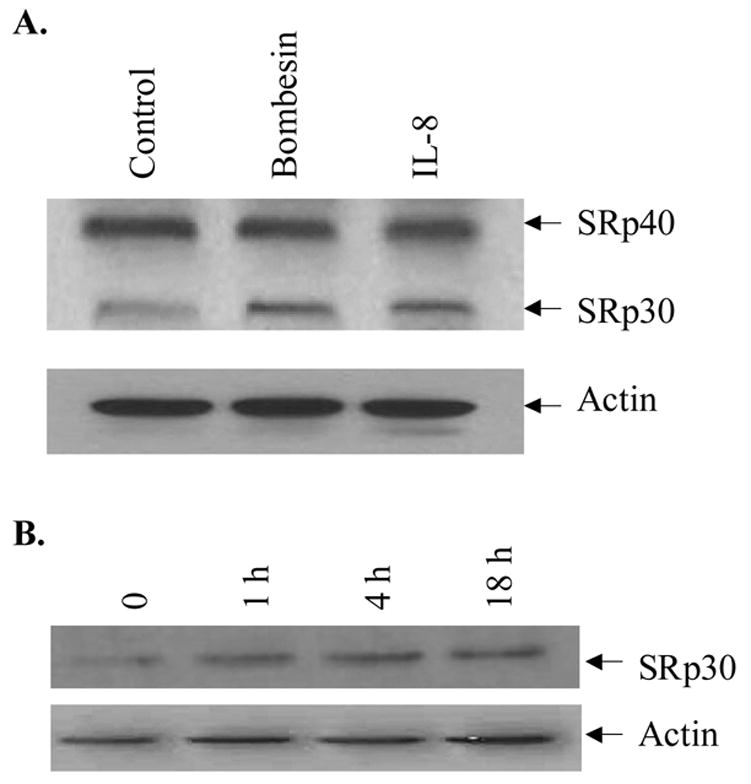
A. PC-3 cells cultured in RPMI 1640 containing 5% CS medium were treated without (lane Control) or with 50 nM bombesin (lane Bombesin) and IL-8 (0.25 ug/ml, positive control) (lane IL-8). Western blots were probed with anti-SR proteins and anti-actin antibodies. Arrows show SRp30 and another SR protein (SRp40). B. Time course of SRp30 protein induction by bombesin in PC-3 cells. Cells treated with 50 nM bombesin at various times (0, 1h, 4h and 18 h) were lysed and subjected to Western analysis. Blots were probed with anti-SR proteins and anti- β-actin antibodies.
3-4. SRp30c is responsible for aberrant GRβ splicing induced by bombesin
Xu, et al. reported that SRp30 protein directs alternative splicing of GR pre-mRNA to GRβ in neutrophils [20]. The expression of SRp30 induced by bombesin suggests SRp30 may be responsible for the aberrant splicing of GR isoforms by bombesin. SRp30 comprises three subspecies: SRp30a (SF2/ASF), SRp30b (SC35) and SRp30c. To identify the subspecies potentially responsible for the altered splicing of GRβ, we conducted quantitative real time reverse transcriptase PCR, quantifying their relative expression in PC-3 cells. All subspecies were detected with SRP30c being the most abundant (Figure 4A). This expression pattern differed markedly from that observed in neutrophils, where SRp30c was the only detectable subspecies [20].
Figure 4. Suppression of GRβ expression by siRNAs of SRp30 proteins.
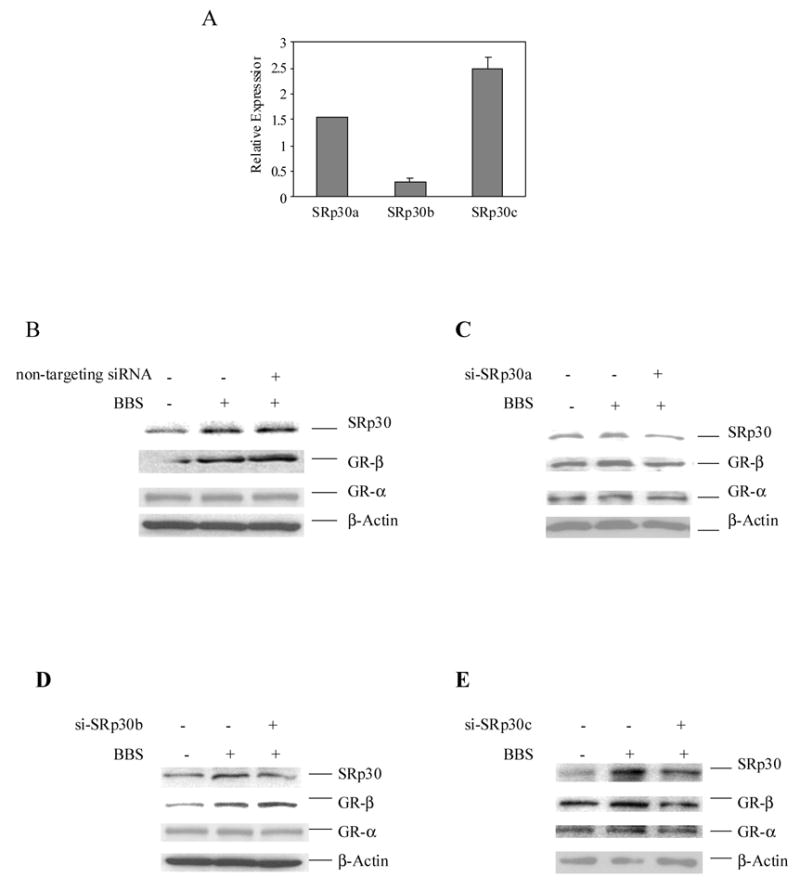
A. Real time PCR showing expression of a b and c subspecies of SRp30 proteins in PC-3 cells. Message RNA levels of SRp30 a, b and c subspecies were measured by real time PCR as described in Methods. Data were expressed as mean ± S.E. B, C, D and E. The effects of siRNAs of a, b and c subspecies of SRp30 proteins and non-targeting siRNA on expressions of GRα and GRβ proteins. Small interference RNAs of a, b an, c subspecies of SRp30 protein and non-targeting siRNA (Dharmaco Inc.) were transfected into PC-3 cells by lipofactamine method. Twenty four hours after transfection cells were treated with or without 50 nM bombesin. Cell lysates were resolved on 4–20% SDS PAGE and the blots were detected with antibodies of GRα, GRβ, SR proteins and β actin respectively.
To assess the relative contribution of the various SRp30 subspecies in modulating GR splicing in PC-3 cells, we introduced siRNAs of SRp30a, SRp30b and SRp30c into PC-3 cells to suppress their expression (Figure 4 B-E), and assessed GR splicing following bombesin treatment. Alternative splicing of GR pre-mRNA to GRβ was not affected with treatment of non-targeting siRNA, nor was it affected with siRNAs specifically targeting SRp30a and b proteins (panels GRβ, Figure 4, B-D). However, targeting of SRp30c with a specific siRNA resulted in a significant reduction in GRβ production at 18 hours after transfection (panel GRβ, Figure 4E), whereas GRα production was not affected (panels GRα, Figure 4, B-E). These data suggest that in the presence of bombesin, SRp30c mediates the alternative splicing of GR pre-mRNA, leadint to the increased generation of GRβ mRNA.
3-5. SRp30c affects bombesin-mediated interference of GC action in MMTV-CAT
To confirm the functional significance of SRp30c expression in the GR pathway, we next tested the SRp30 isoform siRNAs on expression of the hormone-responsive MMTV promoter using PC-3 cells transfected with a plasmid containing an MMTV promoter upstream of a CAT reporter gene. As shown in Figure 5A, DEX-induced CAT activities were reduced to 42% in the presence of 50 nM bombesin compared to treatment with DEX alone (lanes 4 vs. 3) similar to that observed for the GRE-tk-CAT vector (as described in Figure 1A). While siRNAs to SRp30a and b did not affect CAT activities (38% and 45%, Figure 5A, lanes 5 and 6 vs. 3), transfection of SRp30c siRNA effectively abolished bombesin inhibition of MMTV-CAT activity induced by DEX (Figure 5A, lanes 7 vs. 3). Prostate cancer cells transfected with the vector pSRp30c-c2 overexpressed SRp30c as a GFP fusion protein (Figure 5B) and exhibited lower CAT activities in both GC and bombesin + GC treated cells (Figure 5C), when compared to that of control vector expressing GFP (45%, lane 7 vs. lane 3, P =0.009, and 44%, lane 8 vs. lane 4, P =0.008). These results suggest that the overexpression of SRp30c led to elevated GRβ expression and resulted in decreased GR reporter activity. In contrast, no SRP30c dependent changes in CAT activity were detected between between treated and untreated cells (Figure 5C, lanes 5 vs. 1 and lanes 6 vs. 2). Taken together, the data demonstrate that elimination (or enhancement) of SRp30c expression can regulate the splicing of GRβ to antagonize (or augment) the effect of bombesin on GC action.
Figure 5. The effects of SRp30c on CAT activities of MMTVCAT in the presence of bomebisn and GC.
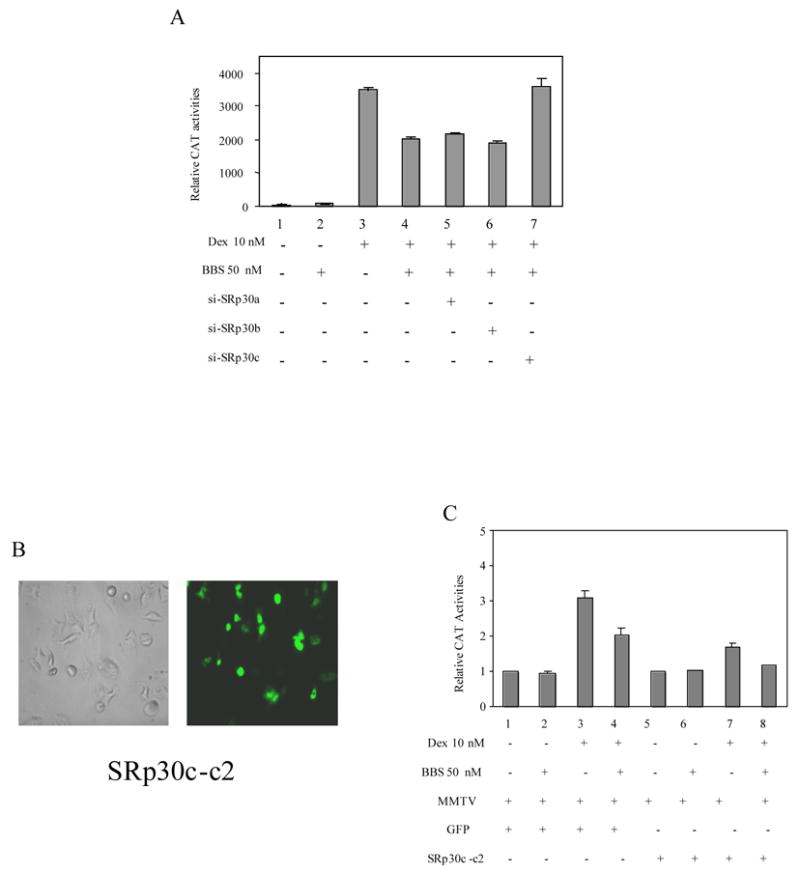
A. The effects of siRNAs of SRp30a, SRp30b and SRp30c on CAT activities of MMTVCAT. PC-3 cells were transfected without (lanes 1–4) or with siRNAs of SRp30a, SRp30b and SRp30c (lanes 5, 6 and 7). Twenty four hours later, treatments without (lane 1) or with 50 nM bombesin (lane 2), 10 nM DEX (lane 3) and 50 nM bombesin + 10 nM DEX (lanes 4–7) were performed and the resultant CAT activities were measured as described before. B. GFP fluorescence of transfected pSRp30c-c2 PC3 demonstrating the overexpression of GFP-SRp30c. C. The effect of overexpression of SRp30c on CAT activities. Lanes 1- 4 were mock-transfected cells and lanes 5–8 were cells transfected with pSRp30c-c2. Treatments without (lanes 1 & 5) or with 50 nM bombesin (lanes 2 & 6), 10 nM DEX (lanes 3 & 7) and 50 nM bombesin + 10 nM DEX (lanes 4 & 8) were performed and CAT activities measured.
3-6. Suppression of SRp30c expression eliminates bombesin’s antagonism of GC action on PC-3 cell growth
The above results demonstrate that SRp30c plays a key role on bombesin-mediated interference of GC induced CAT activities in vitro. To verify whether this is the case in vivo, we examined the effects of siRNA of SRp30c on growth of PC-3 cells in the presence of GC and bombesin. As shown in Figure 6, after transfection of control siRNA (non-targeting siRNA) into PC-3 cells (lanes 1–4), inclusion of 50 nM bombesin together with 100 nM DEX resulted in a 22% increase in cell number (lanes 4 vs. 3, P = 0.0135) relative to treatment with 100 nM DEX alone. When siRNA directed against SRp30c was transfected, the increase in cell number following bombesin treatment was significantly reduced to 16% (lanes 5 vs. 4, P = 0.0374), showing that suppression of SRp30c expression using a specific siRNA attenuates bombesin’s antagonism of GC action in PC cells. Together these data indicate that SRp30c subspecies plays an important role in bombesin-mediated survival effect in the presence of GC in PC cells.
Figure 6. The effect of SRp30c siRNA on growth of PC-3 cells in the presence of GC and bombesin.
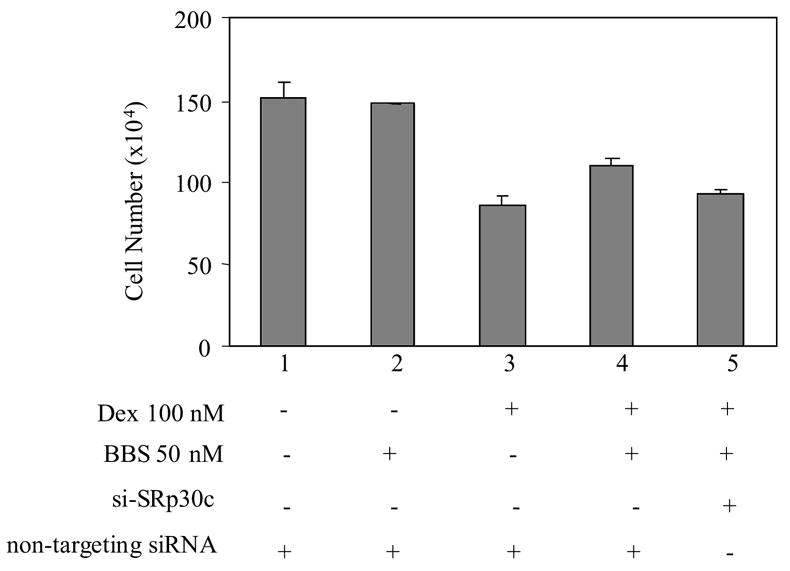
SiRNAs of non-targeting (lanes 1–4) or SRp30c protein were transfected into PC-3 cells. Twenty-four hours later, treatments without (lane 1) or with 50 nM bombesin (lane 2), 10 nM DEX (lane 3), and 50 nM bombesin + 10 nM DEX (lanes 4 & 5) were carried out and cell growth was measured the same as described in Figure 1. Y axis: survival cell numbers. X axis: various treatments. P values were calculated by ANOVA. Error bars indicated.
4. DISCUSSION
GCs have been used in clinical oncology for more than three decades and have been an integral part of endocrine treatment for advanced PC. However the molecular mechanisms underlying the development of GC resistance are poorly understood. In this study we report that the survival factor bombesin can reverse GC action (Figure 7). We demonstrate that: 1) bombesin interferes with the inhibitory effects of GCs on proliferation of PC cells; 2) bombesin causes an up-regulation of protein and mRNA levels of GRβ; 3) bombesin augments the expression of SRp30 protein; and 4) bombesin-induced alternative splicing of GR can be reversed by suppressing the SRp30c subspecies.
Figure 7. Schematic illustrating the role of bombesin in mediating GC resistance in PC cells.
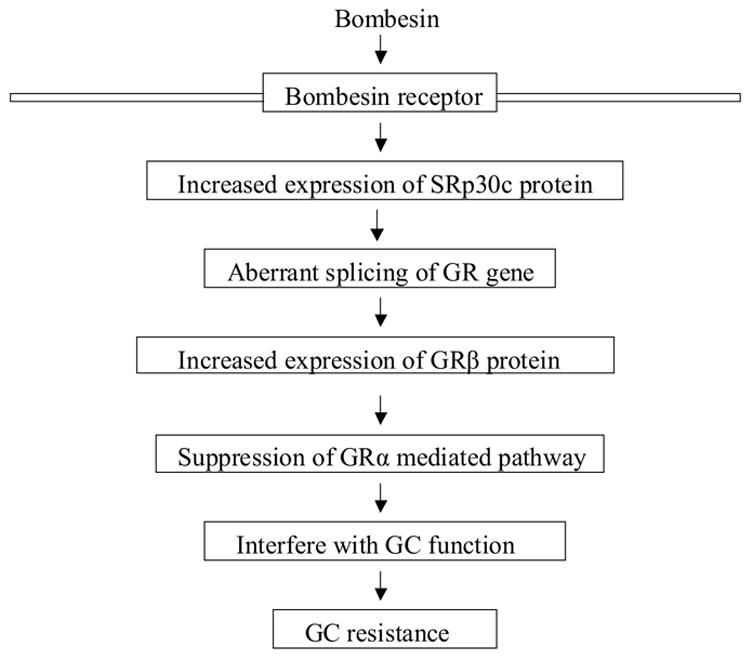
During androgen withdrawal, up-regulation of SRp30c protein by bombesin leads to aberrant splicing of GR gene and the increased level of GRβ protein, which in turn confers resistance to GC in PC cells.
Our data confirm and extend the finding that neuropeptides can antagonize the action of glucocorticoids on PC cells. Hormones or cytokines either employed in prostate cancer endocrine therapy or elevated in prostate cancer have been reported to function as GR antagonists. Krishnan, et al. demonstrated that estrogen, E2 had an anti-GC action in human breast cancer cells [35]. Autocrine or paracrine secretion of lymphokines with subsequent activation of survival pathways has been implicated in GC resistance in numerous systems [36]. Recently, the proinflammatory cytokine, IL-1α, was shown to antagonize DEX action in mouse L929 cells [37]. Our results suggest that bombesin modulates GR-mediated transactivation by altering expression of GRs. GRs are reported to be present in several human prostate cancer cell lines. GC activity is lacking in GR-negative LNCaP cells, but clearly present in GR-positive PC-3 and DU-145 cells [38].
Several studies report GRβ protein is capable of exhibiting a dominant negative function [32, 39]. The preferential increase in the beta isoform of the human GR has also been seen in several disease states resistant to GC therapy. Recently, increased GRβ levels have been reported in human T cells localized to airways, peripheral blood mononuclear cells, and in tuberculin-induced inflammatory lesions in GC-insensitive asthmatics [40]. In another report, higher levels of GRβ were found in 10 of 12 patients with GC-resistant colitis [41]. The cytokines TNF-α and IL-1 were reported to interfere with GC-induced apoptosis by changing the GRα and GRβ ratio [42, 43]. Additionally, isolated peripheral blood mononuclear cells, when stimulated with various superantigens, became insensitive to GC, and this insensitivity is believed to be the result of increased GRβ [44]. All these studies underscore the strong correlation between the expression of GRβ and generalized resistance to GC therapy in human disease states. Our studies are consistent with those previous observations and also reveal a molecular mechanism by which GC resistance may be achieved. The mechanisms could potentially impact multiple GC target genes and interact with downstream effectors of bombesin as well.
To investigate the aberrant splicing of GRβ, we studied the expression of splicing factors SR proteins. Specifically, the SRp30c subspecies leads to increased alternative splicing of GRβ mRNA and expression in the presence of bombesin. SRp30c is a repressor of 3′ splice site utilization [19] and has been found to stimulate alternative splice site selection in CD45 pre-mRNA in leukocytes [45]. A bioinformatics search of the region covering the sequences responsible for splicing of GR gene revealed the presence of several exonic splicing enhancers (ESEs) for SRp30c interaction (data not shown), suggesting that the augmented expression of SRp30c by bombesin might lead to the repression of the splicing of exon 9α (or enhancement of the splicing of exon 9β) to generate a higher level of GRβ.
Our studies demonstrated that SRp30c played the primary function in regulation of GR splicing both in vitro and in vivo. Nevertheless, the antagonizing effects of SRp30c siRNA on cell growth was relatively weak when compared to that in vitro. Other splicing factors, such as hnRNPs which antagonize the effects of SR proteins, may play a role in bombesin-mediated GR splicing in vivo. Recently it has been reported that transcription may couple with the splicing process. All these warrant our further studies when considering the mechanisms underlying bombesin-induced GC resistance.
This study is first to demonstrate neuropeptide antagonism of GC function by altering GR splicing. Anti-survival factor therapy aims at neutralizing the protective effect conferred by the survival factors derived from the local microenvironment. Since GRβ is intimately involved in GC insensitivity, and GRβ is dependent on the presence of SRp30c, the pathway involving SRp30c protein may be an attractive therapeutic target for restoring GC sensitivity to hormone refractory PC and may account for patient differences in GC responsiveness. Moreover, the ability of neuropeptides to induce GC resistance may play an important role in other clinical settings such as bone turnover and the neuroendocrine response to stress.
Acknowledgments
This work was supported by NIH grants RO1 DK060908-02, RO1 CA80240, the Robert H. McCooey Memorial Cancer Research Fund, Ronald and Susan Lynch Professorship in Urologic Oncology (to J.G.), and Brady Urology Foundation of the Department of Urology. We acknowledge Drs. Rong Zheng, Sandra Houser and Akio Horiguchi for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishimura K, Nonomura N, Satoh E, Harada Y, Nakayama M, Tokizane T, Fukui T, Ono Y, Inoue H, Shin M, Tsujimoto Y, Aozasa K, Okuyama A. Potential mechanism for the effects of Dexamthasone on growth of androgen-independent prostate cancer. J Natl Can Inst. 2001;93:1739–1746. doi: 10.1093/jnci/93.22.1739. [DOI] [PubMed] [Google Scholar]
- 2.Fakih M, Cnadance S, Johnson L, Trump D. GC and treatment of prostate cancer. Urology. 2002;60:553–561. doi: 10.1016/s0090-4295(02)01741-7. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Su A, Chen J, Yvonne A, Lefebvre A, Hache R. Attenuation of glucocorticoid signaling through target degradation of p300 via the 26S proteasome pathway. Mol Endocrin. 2002;16:2819–2827. doi: 10.1210/me.2002-0154. [DOI] [PubMed] [Google Scholar]
- 4.Storlie JA, Buckner JC, Wiseman GA, Burch PA, Hartmann LC, Richardson RL. Prostate specific antigen levels and clinical response to low dexamethasone for hormone-refractory metastatic prostate carcinoma. Cancer. 1995;76:96–100. doi: 10.1002/1097-0142(19950701)76:1<96::aid-cncr2820760114>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Sartor O, Weinberger M, Moore A, Li A, Figg WD. Effect of prednisone on prostate-specific antigen in patients with hormone-refractory prostate cancer. Urology. 1998;52:252–256. doi: 10.1016/s0090-4295(98)00149-6. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama T, Terunuma M. Hormone/antihormone withdrawal and dexamethasone for hormone-refractory prostate cancer. Int J Urol. 1998;5:44–47. doi: 10.1111/j.1442-2042.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 7.Saika T, Kusaka N, Tsushima T, Yamato T, Ohashi T, Suyama B, Arata R, Nasu Y, Kumon H, Okayama Treatment of androgen-independent prostate cancer with dexamethasone: a prospective study in stage D2 patients. Intl J Urol. 2001;8:290–294. doi: 10.1046/j.1442-2042.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 8.Tannock IF, Wit RD, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenhal MA, Eisenberger MA. Docetaxel plus prednisone or mitoantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 9.Cidlowski JA, King KL, Evans-Storms RB, Montague JW, Bortner CD, Hughes FM., Jr The biochemistry and molecular biology of glucocorticoid-induced apoptosis in the immune system. Recent Prog Hom Res. 1996;51:457–490. [PubMed] [Google Scholar]
- 10.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 11.Jondal M, Pazirandeh A, Okret S. A role for glucocorticoids in the thymus? Trends Immunol. 2001;22:185–186. doi: 10.1016/s1471-4906(01)01871-3. [DOI] [PubMed] [Google Scholar]
- 12.Schaaf MJM, Cidlowski JA. Molecular mechanism of glucocorticoid action and resistance. J Steroid Biochem & Mol Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 13.Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, Picado C. Expression of glucocorticoid receptor alpha- and beta- isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002;283:C1324–1331. doi: 10.1152/ajpcell.00363.2001. [DOI] [PubMed] [Google Scholar]
- 14.Yudt MR, Cidlowski JA. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol Endocrinol. 2002;16:1719–1726. doi: 10.1210/me.2002-0106. [DOI] [PubMed] [Google Scholar]
- 15.Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30:13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- 16.Stamm S, Ben-Ari S, Rafalska I, Tang Y, Zhang Z, Toiber D, Tanaraja TA, Soreq H. Function of alternative splicing. Gene. 2005;344:1–20. doi: 10.1016/j.gene.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanford JR, Longman D, Caceres JF. Multiple roles of the SR protein family in splicing regulation. Progress in Molecular Subcell Biology. 2003;31:33–58. doi: 10.1007/978-3-662-09728-1_2. [DOI] [PubMed] [Google Scholar]
- 19.Simard MJ, Chabot B. SRp30c is a repressor of 3′ splice site utilization. Mol Cell Biol. 2002;22:4001–4010. doi: 10.1128/MCB.22.12.4001-4010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Q, Leung DY, Kisich KO. Serine-arginine-rich protein p30 directs alternative splicing of glucocorticoid receptor pre-mRNA to glucocorticoid receptor beta in neutrophils. J Biol Chem. 2003;278:27112–27118. doi: 10.1074/jbc.M300824200. [DOI] [PubMed] [Google Scholar]
- 21.Aprikian AG, Tremblay L, Han K, Chevalier S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Intl J Cancer. 1997;72:498–504. doi: 10.1002/(sici)1097-0215(19970729)72:3<498::aid-ijc19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signaling pathways. Trends in Pharmacological Sciences. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- 23.Sumitomo M, Shen R, Goldberg JS, Dai J, Navarro D, Nanus DM. Neutral endopeptidase promotes phorbol ester-induced apoptosis in prostate cancer cells by inhibiting neuropeptide-induced protein kinase δ degradation. Cancer Res. 2000;60:6590–6596. [PubMed] [Google Scholar]
- 24.Sumitomo M, Shen R, Geng Y, Walburg M, Dai J, Geng Y, Navarro D, Boileau G, Papandreou CN, Giancotti FG, Knudsen B, Nanus DM. Neutral endopeptidase (CD10, CALLA) inhibits prostate cancer cell migration by blocking neuropeptide-mediated focal adhesion kinase (FAK) signaling. J Clin Invest. 2000;106:1399–1407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumitomo M, Milowsky MI, Shen R, Navarro D, Dai J, Asano T, Hayakawa M, Nanus DM. Neutral endopeptidase inhibits neuropeptide-mediated transactivation of the insulin-like growth factor receptor-Akt cell survival pathway. Cancer Res. 2001;61:3294–3298. [PubMed] [Google Scholar]
- 26.Dai J, Shen R, Sumitomo M, Stahl R, Navarro D, Gershengorn MC, Nanus DM. Synergistic activation of androgen receptor by bombesin and low-dose androgen. Clin Cancer Res. 2002;8:2399–2405. [PubMed] [Google Scholar]
- 27.Gong J, Zhu J, Goodman O, Pestell RG, Schlegel PN, Nanus DM, Shen R. Activation of p300 histone cetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene. 2006;25:2011–2221. doi: 10.1038/sj.onc.1209231. [DOI] [PubMed] [Google Scholar]
- 28.Levine L, Lucci JA, Pazdrak B, Cheng JZ, Guo YS, Townsend CM, Jr, Hellmich MR. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003;63:3495–3502. [PubMed] [Google Scholar]
- 29.Amorino GP, Parsons SJ. Neuroendocrine cells in prostate cancer. Critical Rev Eukaryot Gene Expr. 2004;14:287–300. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.40. [DOI] [PubMed] [Google Scholar]
- 30.Shen R, Sumitomo M, Dai J, Hardy DO, Navarro D, Usmani B, Papandreou CN, Hersh LB, Shipp MA, Freeman LP, Nanus DM. Identification and characterization of two androgen response regions in the human neutral endopeptidase gene. Mol Cell Endocrinol. 2000;22:131–142. doi: 10.1016/s0303-7207(00)00326-9. [DOI] [PubMed] [Google Scholar]
- 31.Frances F, Corella D, Sorli JV, Guillen M, Gonzalez JI, Portoles O. Validating a rapid method for detecting common polymorphisms in the APOA5 gene by melting curve analysis using Light. Typer Clin Chem. 2009;51:1279–1282. doi: 10.1373/clinchem.2005.049676. [DOI] [PubMed] [Google Scholar]
- 32.Tannocki I, Gospodarowicz M, Meakin W, Panzarella T, Steart L, Rider L. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7:590–597. doi: 10.1200/JCO.1989.7.5.590. [DOI] [PubMed] [Google Scholar]
- 33.Fruchter O, Kino T, Zoumakis E, Alesci S, De Martino M, Chrousos G, Hochberg Z. The human glucocorticoid receptor (GR) isoform {beta} differentially suppresses GR{alpha}-induced transactivation stimulated by synthetic glucocorticoids. J Clin Endocrin Metabolism. 2005;90:3505–3509. doi: 10.1210/jc.2004-1646. [DOI] [PubMed] [Google Scholar]
- 34.Sanford JR, Longman D, Caceres JF. Multiple roles of the SR protein family in splicing regulation. Progress in Mol Subcell Biology. 2003;31:33–58. doi: 10.1007/978-3-662-09728-1_2. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan AV, Awami S, Feldman D. Estradiol inhibits glucocorticoid receptor expression and induces glucocorticoid resistance in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol. 2001;77:29–37. doi: 10.1016/s0960-0760(01)00030-9. [DOI] [PubMed] [Google Scholar]
- 36.Aruna Krishnan V, Srilatha S, Feldman D. Estradiol inhibits glucocorticoid receptor expression and induces glucocorticoid resistance in MCF-7 human breast cancer cells. J Steroid Biochem & Mol Biol. 2000;77:29–37. doi: 10.1016/s0960-0760(01)00030-9. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies FE, Shima Y, Tai YT, Rosen S, Avraham S, Kharbanda S, Anderson KC. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 38.Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- 39.Fakih M, Candace S, Johnson M, Trump LDM. Glucocorticoids and treatment of prostate cancer: a preclinical and clinical review. Urology. 2002;60:553–561. doi: 10.1016/s0090-4295(02)01741-7. [DOI] [PubMed] [Google Scholar]
- 40.Webster JC, Robert H, Oakley H, Jewell MC, Cidlowski AJ. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative β isoform: A mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro MD, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos GP. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): tissue levels, mechanism of action, and potential physiologic role. Mol Med. 1996;2:597–607. [PMC free article] [PubMed] [Google Scholar]
- 42.Honda M, Orii F, Ayabe T, Imai S, Ashida T, Obara T, Kohgo Y. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000;118:859–866. doi: 10.1016/s0016-5085(00)70172-7. [DOI] [PubMed] [Google Scholar]
- 43.Kofler R, Schmidt S, Kofler A, Ausserlechner MJ. Resistance to glucocorticoid-induced apoptosis in lymphoblastic leukemia. J Endocrinol. 2003;178:19–27. doi: 10.1677/joe.0.1780019. [DOI] [PubMed] [Google Scholar]
- 44.Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000;105:782–787. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]
- 45.Wang HY, Xu X, Ding JH, Bermingham JR, Jr, Fu XD. SC35 plays a role in T cell development and alternative splicing of CD45. Mol Cell. 2001;7:331–334. doi: 10.1016/s1097-2765(01)00181-2. [DOI] [PubMed] [Google Scholar]
- 46.Charmandari E, Chrousos GP, Ichijo T, Bhattacharyya N, Vottero A, Souvatzoglou E, Kino T. The human glucocorticoid receptor (hGR) beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes. Mol Endocrin. 2005;19:52–64. doi: 10.1210/me.2004-0112. [DOI] [PubMed] [Google Scholar]


