Abstract
Human attentional control is unrivaled. We recently proposed that adults depend on distinct frontoparietal and cinguloopercular networks for adaptive online task control versus more stable set control, respectively. During development, both experience-dependent evoked activity and spontaneous waves of synchronized cortical activity are thought to support the formation and maintenance of neural networks. Such mechanisms may encourage tighter “integration” of some regions into networks over time while “segregating” other sets of regions into separate networks. Here we use resting state functional connectivity MRI, which measures correlations in spontaneous blood oxygenation level-dependent signal fluctuations between brain regions to compare previously identified control networks between children and adults. We find that development of the proposed adult control networks involves both segregation (i.e., decreased short-range connections) and integration (i.e., increased long-range connections) of the brain regions that comprise them. Delay/disruption in the developmental processes of segregation and integration may play a role in disorders of control, such as autism, attention deficit hyperactivity disorder, and Tourette's syndrome.
Keywords: attention, connectivity, functional MRI, spontaneous activity development
The human capacity to maintain task goals, selectively attend to relevant information, and avoid distraction is unrivaled. These attentional control abilities are thought to be accomplished through the adoption of task sets (or “rules”) that flexibly configure moment-to-moment information processing in response to task demands.
Previous models of control have come in several forms, including theories based on centralized frontal control (1–3), hierarchically organized processing (4), and those with multiple functional networks (5–7). Dissociating among these models remains a particularly difficult challenge. However, to paraphrase Johnson and Pennington (8), full comprehension of how the mature system works can be powerfully illuminated by “understanding how it is constructed in development” and “how development can go awry.”
The following report uses resting state functional connectivity MRI (rs-fcMRI) to examine the development of two putative, previously identified control networks (2, 6). We hope to gain deeper insight into their adult functions by revealing how these networks are constructed.
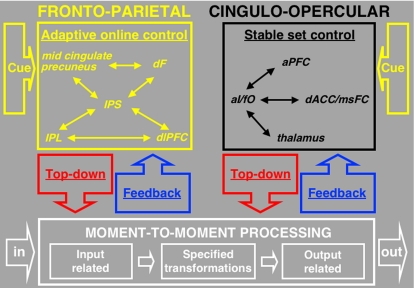
Using rs-fcMRI and graph theory analyses, Dosenbach et al. (6) recently proposed that adult task control may be implemented by distinct frontoparietal and cinguloopercular networks (Fig. 1). The proposed cinguloopercular network consists of the dorsal anterior cingulate/medial superior frontal cortex (dACC/msFC), bilateral anterior insula/frontal operculum (aI/fO), anterior prefrontal cortex (aPFC), and thalamus (6). The frontoparietal network, which appears to include elements of the dorsal attention network (9), consists of dorsolateral prefrontal cortex (dlPFC), intraparietal sulcus (IPS), inferior parietal lobule (IPL), precuneus, dorsal frontal (dF), and midcingulate (6) [see supporting information (SI) Table 1 for a list of abbreviations]. Although the two control networks showed strong intranetwork connectivity, internetwork connections, as measured with rs-fcMRI, were minimal. These results were consistent with earlier cross-studies analyses that combined fMRI activation data from 10 different tasks. These analyses showed that regions in the cinguloopercular and frontoparietal networks had varying combinations of three different task-control signals: set initiation (start cue), set maintenance (sustained across trials), and error/adjustment (error related) (2, 6).
Fig. 1.
Proposed dual networks for adult human task control. The dual-networks model of control in adults was motivated by results from cross-studies analyses of mixed blocked/event-related fMRI and rs-fcMRI data (2, 6). Both control networks are proposed to interpret cues, implement top-down control, and process bottom-up feedback, but use different mechanisms and over different temporal scales. The frontoparietal network (yellow) is proposed to act for rapid online control on a trial-by-trial basis (i.e., adaptive control) and the cinguloopercular (black) for more sustained task sets (i.e., stable control).
For example, the dACC/msFC and bilateral aI/fO regions of the cinguloopercular network showed all three task-control signals. The aPFC showed a similar profile but in fewer tasks. The presence of multiple control signals, in particular set-maintenance activity, in the cinguloopercular network suggested that this network may implement stable task control across the trials of a task (see Fig. 1) (6).
By comparison, the cross-studies analyses showed that the frontoparietal network was characterized by the presence of start-cue (set initiation) signals in the dF, IPS, precuneus, and midcingulate regions, as well as error-related signals in the IPL and dlPFC regions. Importantly, only 1 of 11 regions in this network (i.e., left IPL) showed sustained set-maintenance activity. The presence of start-cue and error-related signals, but lack of robust sustained activity, suggested that the frontoparietal network supports control initiation and adaptive, trial-related control adjustments in response to feedback (6).
Consistent with complex adaptive systems models (10), these observations suggested that the two control networks may function in parallel, each exerting top-down control with different properties and over different temporal scales (6, 10).
rs-fcMRI, the method used to define the two control networks, measures correlated low-frequency (usually <0.1 Hz) blood oxygenation level-dependent (BOLD) signal fluctuations between brain regions occurring at rest (11–13). These low-frequency BOLD fluctuations are thought to relate to “spontaneous” neural activity (11, 14). By cross-correlating the BOLD signal time series between different regions, one can determine which regions are “functionally connected.”
Early studies of brain networks defined by temporal correlations in spontaneous neural activity date back several years; however, recent theoretical and experimental findings have brought such network approaches into broader view (6, 12, 15–19). One reasonable hypothesis regarding the nature of correlated spontaneous BOLD fluctuations is that they may, at least in part, reflect a long-standing history of coactivation (20). In this sense, coactivation of brain regions across many tasks can lead to the Hebbian strengthening of the functional connections between them (21).
In addition, some evidence suggests that, along with experience-dependent evoked activity, spontaneous waves of synchronized activity during pre- and postnatal development support the integration (i.e., strengthening interregional relationships) and segregation (i.e., weakening interregional relationships) of neural networks (15–18, 20, 22). Such mechanisms may be important for gating information flow (15, 17), building internal representations (15, 17, 20), and developing and maintaining mature network relationships (17).
More than 10 years ago, Tononi and colleagues (23) emphasized “the functional segregation of different brain regions and their integration in perception and behavior” as a fundamental principle of brain organization in higher vertebrates. In this scheme, functional segregation is measured by statistical independence of components of a system, whereas integration is measured by deviation from statistical independence. Plausibly, temporally correlated spontaneous activity (i.e., rs-fcMRI) could provide a metric for determining the presence or absence of statistical independence. As such, the dual-networks control structure observed in adults (6) may be observed by examining synchronous activity over development.
A substantial literature from developmental psychology and cognitive science documents age-related improvements in several control processes, including inhibitory control, set switching, and set maintenance (24–26). In particular, working memory, or the ability to perform complex manipulations on stored items, continues to mature through childhood and into adolescence (26, 27). These behavioral developments are accompanied by activity related to working-memory tasks in the dlPFC and other associated regions that, although present in childhood, continue to increase with age (27, 28). These data have led some to suggest that “… the basic working-memory circuitry is in place by middle childhood, but also that working-memory circuitry is strengthened during middle childhood” (28). We therefore hypothesized that the functional connections within the frontoparietal network, which includes the dlPFC and posterior parietal regions, may already be present in childhood, but the relative connection strengths within the network will increase with age (28).
By age 6 (25, 26), children can perform many of the tasks designed to study control (24, 25, 29–31). Yet when compared with adults, children's accuracy is often decreased because of errors of perseveration (inability to switch set) and errors of distraction (inability to maintain set) (24, 25). fMRI studies suggest that improvement of set and rule maintenance over time, as opposed to active working memory, is at least in part related to activity changes in regions of the cinguloopercular network, such as the aI/fO (also referred to as ventrolateral prefrontal cortex), aPFC, and dACC/msFC (28, 29). Taken together, these data imply that the functional connections in the cinguloopercular network, as with those in the frontoparietal network, may undergo significant remodeling with age.
Using rs-fcMRI, we compared the network structure of putative control systems between adults and typically developing children. We sought to determine whether changes in the architecture of the brain's control networks over development would show evidence of functional segregation and integration. At younger ages, will the brain's control regions show more widespread statistical dependence that segregates into distinct control networks with age? Conversely, will the intraconnectivity of regions in their respective adult control networks increase across development leading to greater within-network integration?
Results
Thirty-nine putative task-control regions identified in previous cross-studies analyses (2) were analyzed for pairwise temporal BOLD correlations in three different groups: children (7–9 years; mean 8.6), adolescents (10–15 years; mean 11.9), and adults (20–31 years; mean 24.1). Pairwise correlations were represented as graphs for each group. Region pairs were treated as binary and were either connected or not connected. Previously, we evaluated the effects of a range of thresholds (r ≥ 0.2, 0.175, 0.15, 0.125, and 0.1) in adults to ensure that our results were not an artifact of a specific threshold (6). These thresholds were based on a natural division observed in the interregional r-value distribution of the adult population. The child sample used here had an r-value distribution similar to that of the adult sample (SI Fig. 5).
Thus, we compared children and adults with similar thresholds by using two approaches. First, the 75 strongest connections (r values) in each group (children, adolescents, and adults) were chosen. This approach generated graphs for each group with the exact same number of regions (nodes) and connections (vertices), ensuring that any differences among graphs would be because of specific changes in certain connections, not a greater overall level of connectivity in any group. This number of connections is similar to the number obtained at the r ≥ 0.15 threshold used for adults by Dosenbach et al. (6). Second, to carry out direct between-group comparisons, connections that had an r ≥ 0.1 in either children, adults, or both were analyzed. Using this criterion, children and adults had a similar number of total connections (children, 140; adults, 139). As implied elsewhere, pairwise correlations with r < 0.1, even if statistically significant, may not be biologically significant (32).
Child Graph Structure Deviates Significantly from Adult Architecture.
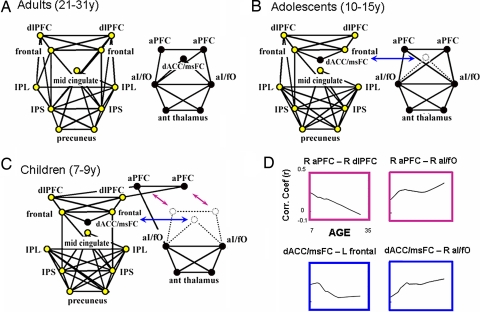
As previously described, the two control networks separated into two distinct components in adults (Fig. 2A and SI Table 2). The child control architecture showed three noteworthy differences from the adult layout (Fig. 2C). First, although regions in the frontoparietal network were connected in children, additional connections between the aPFC and dlPFC regions were identified that bridged the frontoparietal and cinguloopercular networks (Fig. 2C). A second important finding was the close relationship between the dACC/msFC and frontoparietal networks. The dACC/msFC region was not only disconnected from the cinguloopercular network in children, but also incorporated into the frontoparietal network through connections with the dorsal frontal cortex (Fig. 2C). Third, although the frontal and parietal cortices were connected in children through dorsal frontal to IPS connections (Fig. 2C), over time, additional links developed among the dlPFC, IPS, and IPL regions (Fig. 2A). These differences were found in both halves of a replication analysis described in SI Fig. 6.
Fig. 2.
Graphs formed from putative task-control regions in children, adolescents, and adults. ROI locations are drawn to correspond to topographic brain locations. Right-sided ROIs are displayed on the right and anterior ROIs at the top of each graph. (A) rs-fcMRI revealed two separate control networks in adults as previously described (6). (B) The top 75 connections in adolescents revealed a similar two-component system as seen in adults; however, the dACC/msFC region was incorporated into the frontoparietal network. (C) The top 75 connections in children revealed a significant deviation from the adult architecture. The two networks were connected by a bridge connection (aPFC–dlPFC). The dACC/msFC region was incorporated into the frontoparietal network. Children lacked connections from the dlPFC to IPS and IPL. (D) Fit LOWESS curves of connection strength (r) versus age. As connection strength between the dACC/msFC region and the dF cortex decreased with age, correlation strength increased between the dACC/msFC and aI/fO regions. The aPFC region also decreased its connection strength with the dlPFC region with age but was already strongly connected to the aI/fO region in children. The strength of the aI/fO–aPFC connection was maintained into adulthood.
Adolescent Graph Structure Appears Intermediate to That of Children and Adults.
Data from a third group consisting of adolescent-age subjects (10–15 years) were analyzed to determine whether the graph structure would appear intermediate to the graphs shown for younger children and adults. Indeed, the network graph for the adolescent group was qualitatively “intermediate” to the adult and child structures. In adolescents, as in adults, the frontoparietal and cinguloopercular networks were segregated; however, as in children, the adolescent dACC/msFC region still remained incorporated into the frontoparietal network.
LOWESS Curves Describe Developmental Trajectory of Interregional Connection Strength.
To create a visual representation of the development of specific functional connections, data of connection strength (r) versus age were fit with LOWESS curves (locally weighted sum of squares), a local regression-smoothing procedure (33). This method makes no assumptions about the form of the bivariate relationship. Rather, it allows the form to be discovered by using the actual data. This approach is useful for identifying data patterns that may otherwise be overlooked by using curve-fitting procedures that assume specific shapes.
The LOWESS curves provide a complementary view of the development of interregional connections. As correlation strength between the dACC/msFC and dF cortices decreased with age, correlation strength increased between the dACC/msFC and aI/fO regions (Fig. 2D, see also SI Fig. 7). The aPFC region also decreased its connection strength with the dlPFC over time (Fig. 2D). The aPFC region was already strongly connected to the aI/fO in children, and the strength of this connection was maintained into adulthood (Fig. 2D). The LOWESS curves for all regions (along with original data points) are shown in SI Fig. 8.
Direct Comparisons Between Children and Adults.
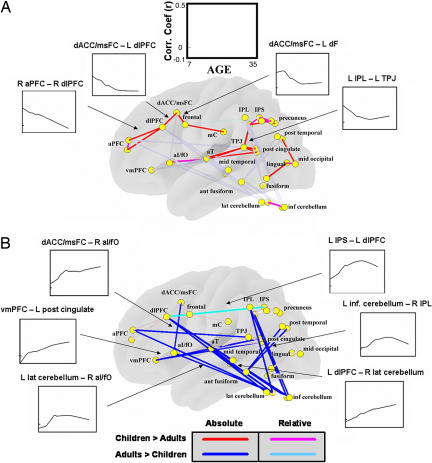
Direct comparisons of all possible connections between adults and children (see Methods) were performed to test the statistical reliability of the between-group differences observed with graph theory methods (P ≤ 0.05, multiple comparison corrected; see Methods). As mentioned previously, these results for both the children and adult groups were verified with a replication analysis (see SI Fig. 6 and SI Text). The disengagement of the dACC/msFC region from the frontoparietal network with age was reliable in that the dACC/msFC–dorsal frontal cortex connection was significantly stronger in children than adults (Fig. 3). Likewise, connections between the dACC/msFC region and other parts of the cinguloopercular network (i.e., aI/fO) gained with age were significantly stronger in adults than children (Fig. 3). The direct comparisons also validated the developmental segregation of the aPFC from the dlPFC (Fig. 3).
Fig. 3.
Increased long-range and decreased short-range connectivity with age. Direct comparisons of all possible connections between adults and children were performed to test the statistical reliability of between-group differences. Both left- and right-hemisphere regions are placed on a transparent brain to aid with visualization. Red and blue lines highlight significant between-group differences for connections with an r ≥ 0.1 in either children or adults (i.e., absolute difference). Light blue and pink lines highlight connections present in both children and adults (r ≥ 0.1) that differed significantly in connection strength between groups (relative difference; P ≤ 0.05). (A) The segregation of the dACC/msFC region from the frontoparietal network (Fig. 2) was statistically significant, as was the disconnection of the aPFC from the dlPFC region (P ≤ 0.05). Most of the connections that “grew down” with age constituted short-range connections. Connections that “grew up” with age are faded to highlight this observation. (B) Connections between the dACC/msFC region and the cinguloopercular network that grew stronger with age were statistically significant (P ≤ 0.05). The connections of left dlPFC to left IPS and left frontal to left IPS were already present in children but significantly increased in strength with age. Most of the connections that “grew up” with age constituted long-range connections. Connections that “grew down” with age are faded to highlight this observation. Selected LOWESS curves are presented in A and B.
Some of the functional connections between frontal and parietal regions, present in both groups, differed significantly in strength between children and adults. The connection between left dlPFC and left IPS (see Fig. 3) was already present in children but significantly increased in strength with age. The left dorsal frontal–left IPS connection also grew stronger (see SI Fig. 6). Other connections between frontal and parietal regions were present in both groups but not statistically different.
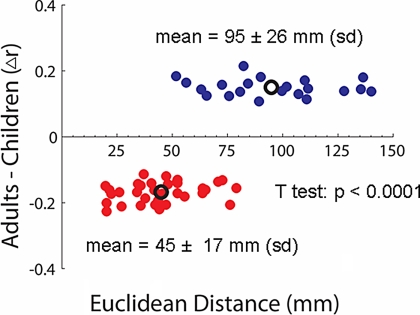
One of the most intriguing findings here was the overall increase in connection strength between regions that were spatially remote (i.e., long-range) and the concomitant decrease in connection strength for regions close in space (i.e., short- range) (Figs. 3 and 4). Some of the increases in long-range connectivity were particularly interesting. Regions in both control networks (e.g., dlPFC, IPL, aPFC, and aI/fO) developed stronger connections with the cerebellum (Fig. 3). Another notable long-range connection that developed with age was between the posterior cingulate and ventral medial prefrontal cortex (vmPFC) (Fig. 3), important elements of the brain's default system (also see SI Text) (17). This result is quantified in Fig. 4.
Fig. 4.
Euclidean distance as a function of Δr. The Euclidean distance (millimeters) of all connections that differed significantly between children and adults is plotted against the change in correlation strength (Δr) across development. Connections that increased in strength with age are displayed in blue, and those connections that decreased with age are displayed in red. The mean r and Euclidean distance for each group are also plotted (black circles). A t test (P < 0.0001) confirmed the qualitative finding (Fig. 3) that connections between spatially distant regions are more likely to “grow up” with age, whereas connections between closely adjacent regions tend to “grow down” with age.
Discussion
Recent evidence (6) suggests that adult control is implemented through two possibly parallel task-control networks. In this scheme, the frontoparietal network implements adaptive control on a trial-by-trial timescale, and the cinguloopercular network implements set-maintenance functions (6) at the task level.
Based on prior research (25–28, 34), we expected that both the frontoparietal and cinguloopercular networks would undergo remodeling with age. As predicted, the graph methods showed a frontoparietal network that continued to strengthen its frontal–parietal connections with age (Figs. 2 and 3). In addition, the cinguloopercular network was partially incomplete with many of the adult connections still missing (Fig. 2). A more nuanced characterization of the developmental dynamics of the two networks is presented in the following discussion.
Segregation/Integration Lead to Mature Dual-Network Control Architecture.
The control network structure of children significantly deviated from the adult organization in two fundamental ways (Fig. 2C). First, in children, the two networks were connected by the aPFC and dlPFC regions. Second, the dACC/msFC region was closely connected to the frontoparietal network. With age, there was segregation of both the aPFC and dACC/msFC regions from the frontoparietal network and integration of the dACC/msFC region into the cinguloopercular network (Fig. 2C).
These developmental dynamics may represent a learning mechanism whereby precursors to adult task sets are originally derived from more available signals generated by regions of the more rapidly adaptive control network (i.e., frontoparietal). In this sense, the performance of tasks with novel components would rely more heavily on rapidly adaptive control generated by the frontoparietal network. With greater age, and therefore greater experience, stored task sets may be retrieved and stably maintained throughout the task epoch by the cinguloopercular network.
Consistent with this idea, Weisman et al. (35) showed that, for a Stroop-like task, which contains novel components even for adults, control activity in the dlPFC and parietal cortex decreases with learning, whereas control-related activity in the cingulate increases with learning. Other investigators have made similar observations (36). In adults, difficult novel paradigms such as Stroop or dual-task situations may drive a resynchronization between the frontoparietal and cinguloopercular networks, as is seen in childhood, which may disappear again with practice. Future work in adults using effective connectivity, which measures the direct influence of one region on another using a predefined model, and/or fcMRI during completely novel tasks, may unveil such novelty-induced changes in task-control strategies.
Long-Range Functional Connections Increase and Short-Range Connections Decrease with Age.
One of the most compelling findings was the strong tendency toward reduction in short-range connections with age and the concomitant addition of long-range connections (Figs. 3 and 4). This observation fits well with suggestions that perceptual and cognitive development involves the simultaneous segregation and integration of information-processing streams (28, 37–40).
By ≈9 months of age, long-range anatomical connectivity is adult-like (41), indicating that the developmental changes presented here cannot be accounted for by neuroanatomical changes alone. Increasing long-range functional connectivity with age may reflect increased myelination, which continues into young adulthood (42). Increased efficiency of signal propagation after the addition of the myelin sheath may be important for efficient information transfer and, hence, the functional integration between distant regions (28, 38). In principle, such processes over time could allow for stronger spontaneous correlations seen with rs-fcMRI. Conversely, the protracted period of selective pruning of synapses between specific brain regions, which occurs from early infancy and well into the second decade of life (43, 44), may contribute to the significant reduction in short-range functional connectivity (also see SI Text and SI Fig. 7).
Although developmental phenomena such as myelination and synaptic pruning may influence our current results, they likely do not account for all of the observed rs-fcMRI differences between children and adults (28). Other processes, such as coordinated evoked and spontaneous activity, may directly contribute to the creation of the adult functional architecture. For example, in a recent publication, Honey et al. (18) simulated spontaneous neuronal firing (millisecond timescale) for a network of nodes only constrained by the known anatomical connections of macaque neocortex. Complex spatial and temporal patterns of synchronous activity developed over time in the absence of external input and without changes in synaptic strengths (18). These findings suggest that a process of “integration through synchronization” may partially underlie the development of the control networks described here (16).
Cerebellar Connections to Control Networks Grow Stronger with Age.
Recent neuropsychological findings suggest that children are worse at monitoring performance feedback signals than adults (24). Other work has indicated that the cerebellum is important for generating error codes (45). The relatively late appearance of long-range functional connections between the cerebellum and the brain's control networks may contribute to children's inferior ability to monitor performance feedback (30).
Conclusions
The brain is a complex network whose structure simultaneously satisfies two major challenges of neural information processing: the segregation of specialized information and functional integration (23). The study of temporally correlated neural activity revealed that segregation (i.e., decreased short-range connections) and integration (i.e., increased long-range connections) can be observed across development. These global developmental processes support the maturation of a dual-network control system (6).
The results presented here lend credence to the idea that the function of a system is not fully described by the activity of its individual components, but also depends on the nature of the dynamic links among them (15–18). Work in several laboratories has revealed that developmental disorders may not only be tied to dysfunction of a set of brain areas, but also to the way these regions are connected functionally and anatomically (16, 46). For example, an overabundance of short-range connections and insufficient long-range connections are now thought to contribute to autism spectrum disorders (46, 47). Other work has shown that the disruption of synchronous neural activity is related to cognitive deficits in schizophrenic patients (16). These results suggest that a delay or interruption of the developmental processes described in this article may underlie such conditions and other putative developmental disorders of control, including attention deficit hyperactivity disorder and Tourette's syndrome.
Methods
Data Acquisition.
fMRI data were acquired on a Siemens 1.5 Tesla MAGNETOM Vision system (Erlangen, Germany) and processed as previously described (48). See SI Table 3 and SI Text for details.
rs-fcMRI Preprocessing.
Preprocessing for fc analyses was carried out as previously described (12, 13). See SI Text for details.
Extraction of Regionwise Resting State Time Series.
Resting state (fixation) data from 210 subjects (66 ages 7–9; 53 ages 10–15; 91 ages 19–31; 74 ages 32+) (6) were included in the analyses. For each subject, at least 555 sec (9.25 min) of resting state BOLD data were collected. For each of the 39 regions of interest (ROIs), a resting state time series was extracted separately for each individual. ROIs were originally derived from cross-studies fMRI analyses of task-control signals in adults (2, 6). Hence, the roles of putative brain regions that contribute to task control in children and adolescents but not in adults should be addressed by the inclusion of such regions in future research.
For 10 adult subjects, resting fixation was continuous. For the remaining 200 subjects, by using a recently validated method (13), resting periods were extracted from different interleaved experimental designs that also contained task periods.
Computation of Mean Regionwise Correlation Matrix for Graph.
The resting state BOLD time series were correlated region by region for each subject across the full length of the resting time series, creating 210 square correlation matrices (39 × 39).
Because of the potential effects of head movement on rs-fcMRI data, even after movement correction,†† the child, adolescent, and adult groups were matched for movement to limit its effects. From a sample of 210 subjects, 139 movement-matched subjects (49 children ages 7–9 years, 43 adolescents ages 10–15 years, and 47 adults 21–31 years) were used for the graph visualization and subsequent direct comparisons. Within-run subject motion was <1 mm rms for all matched groups and not significantly different among groups (rms: children, 0.637 mm; adolescents, 0.636 mm; adults, 0.633 mm).
For graph analyses, the correlation coefficients (r) across matched subjects were combined by using the Schmidt–Hunter method for meta-analyses of r values (6).
Direct Comparisons Between Children and Adults.
We performed two-sample two-tailed t tests (assuming unequal variance; P ≤ 0.05) on all potential connections represented in the 39 × 39 correlation matrices (741 possible connections) between movement-matched children and adults. Fischer's Z transformation was applied to the correlation coefficients to improve normality for the random effects analyses. To account for multiple comparisons, the Benjamini and Hochberg False Discovery Rate (49) was applied. Connections that were significantly different between groups, but r < 0.1 in both groups, were not displayed.
Application of LOWESS Smoothing.
Data on connection strength versus age were fit with LOWESS curves by using the full complement of subjects (210 subjects) (33). LOWESS smoothing is a weighted least-squares fit. Each smoothed value is determined by the neighboring data points defined within a span, with the data point being smoothed carrying the most weight. Smooth lines were computed by using a tension of 50.
Supplementary Material
Acknowledgments
We thank Steven M. M. Nelson and Alecia C. Vogel for helpful discussions and Mark McAvoy and Abraham Z. Snyder for help with data analysis. This work was supported by National Institutes of Health Grants NS053425 (to B.L.S.), NS41255, and NS46424 (to S.E.P.); the John Merck Scholars Fund (B.L.S.); the Burroughs–Wellcome Fund (B.L.S.); the Dana Foundation (B.L.S.); the Ogle Family Fund (B.L.S.); a Washington University Chancellor's Graduate Fellowship (to D.A.F.); and a United Negro College Fund/Merck Graduate Science Research Dissertation Fellowship (to D.A.F.).
Abbreviations
- aI/fO
anterior insula/frontal operculum
- aPFC
anterior prefrontal cortex
- BOLD
blood oxygenation level-dependent
- dACC/msFC
dorsal anterior cingulate cortex/medial superior frontal cortex
- dlPFC
dorsolateral prefrontal cortex
- dF
dorsal frontal
- IPL
inferior parietal lobule
- IPS
intraparietal sulcus
- ROI
region of interest
- rs-fcMRI
resting state functional connectivity MRI.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705843104/DC1.
Cohen, A. L., Fair, D. A., Miezin, F. M., Dosenbach, N. U. F., Wenger, K. K., Fox, M. D., Snyder, A. Z., Vincent, J. L., Raichle, M. E., Schlaggar, B. L., Petersen, S. E. (2006) Soc. Neurosci. (abstr.).
References
- 1.Desimone R, Duncan J. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 2.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller EK, Cohen JD. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Koechlin E, Ody C, Kouneiher F. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 5.Posner MI, Petersen SE. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 6.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson MH, Pennington BF. In: Neurodevelopmental Disorders. Tager-Flusberg H, editor. Cambridge, MA: MIT Press; 1999. p. ix. [Google Scholar]
- 9.Corbetta M, Shulman GL. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 10.Holling CS, Gunderson LH. In: Panarchy: Understanding Transformations in Human and Natural Systems. Gunderson LH, Holling CS, editors. Washington, DC: Island Press; 2002. pp. 25–62. [Google Scholar]
- 11.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 12.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leopold DA, Murayama Y, Logothetis NK. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 15.Salinas E, Sejnowski TJ. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varela F, Lachaux J-P, Rodriguez E, Martinerie J. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 17.Raichle ME, Snyder AZ. NeuroImage. 2007 in press. [Google Scholar]
- 18.Honey C, Kotter R, Breakspear M, Sporns O. Proc Natl Acad Sci USA. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Nature. 2007;447:46–47. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 20.Miltner WH, Braun C, Arnold M, Witte H, Taub E. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- 21.Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 22.Johnson MH, Munakata Y. Trends Cogn Sci. 2005;9:152–158. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Tononi G, Sporns O, Edelman GM. Proc Natl Acad Sci USA. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crone EA, Ridderinkhof KR, Worm M, Somsen RJ, van der Molen MW. Dev Sci. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MC, Amso D, Anderson LC, Diamond A. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond A. In: Principles of Frontal Lobe Function. Stuss DT, Knight RT, editors. Vol. 466. London: Oxford Univ Press; 2002. 503 pp. [Google Scholar]
- 27.Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunge SA, Wright SB. Curr Opin Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 31.Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 33.Cleveland WS. Am Stat. 1981;35:54. [Google Scholar]
- 34.Sakai K, Rowe JB, Passingham RE. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 35.Weissman DH, Woldorff MG, Hazlett CJ, Mangun GR. Brain Res Cogn Brain Res. 2002;15:47–60. doi: 10.1016/s0926-6410(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 36.Fincham JM, Anderson JR. Proc Natl Acad Sci USA. 2006;103:12941–12946. doi: 10.1073/pnas.0605493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson MH. Nat Rev Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- 38.Luna B, Sweeney JA. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 39.Fair DA, Brown TT, Petersen SE, Schlaggar BL. Neurology. 2006;67:2246–2249. doi: 10.1212/01.wnl.0000249348.84045.0e. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan R. Clin Neurophysiol. 1999;110:1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 41.Conel JL. The Postnatal Development of the Human Cerebral Cortex. Cambridge, MA: Harvard Univ Press; 1939–1963. [Google Scholar]
- 42.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 43.Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Neurosci Lett. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 44.Chugani HT, Phelps ME, Mazziotta JC. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 45.Fiez JA. Neuron. 1996;16:13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- 46.Courchesne E, Pierce K. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 49.Benjamini Y, Hochberg Y. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.