Abstract
Objective
To assess microstructural changes in the retina that may explain incomplete visual recovery after anatomically successful repair of rhegmatogenous retinal detachments (RD) using ultrahigh-resolution optical coherence tomography (UHR OCT).
Design
Retrospective observational case series.
Participants
Seventeen patients with decreased visual acuity after RD repair. Twelve patients had macula-involving and 5 had macula-sparing RDs.
Methods
The UHR OCT prototype capable of ~3 μm axial resolution was developed for clinical use. The UHR OCT images through the center of the fovea in 17 patients with visual complaints after RD surgery were obtained. Patients were either postoperative patients from the New England Eye Center or tertiary referrals. Baseline visual acuity, preoperative lens status, location of retinal detachment, macular involvement, and postoperative visual acuity were recorded.
Main Outcome Measures
The UHR OCT images after RD repair.
Results
The UHR OCT images were obtained 1 to 84 months (median, 5 months) postoperatively. The mean preoperative logarithm of the minimum angle of resolution (logMAR) visual acuity was 1.37 (Snellen equivalent, 20/390). The mean postoperative logMAR visual acuity was 0.48 (Snellen equivalent, 20/60). Anatomical abnormalities that were detected included distortion of the photoreceptor inner/outer segments (IS/OS) junction in 14 of 17 patients (82%), epiretinal membranes in 10 of 17 patients (59%), residual subretinal fluid in 3 of 17 patients (18%), and cystoid macular edema in 2 of 17 patients (12%). Of the 5 patients with preoperative macula-on detachments, 4 had distortion of the outer retina after RD repair.
Conclusions
The higher resolution of UHR OCT facilitates imaging of the IS/OS junction. Therefore, UHR OCT is able to confirm prior histopathologic findings that damage to photoreceptor outer segments may occur as a consequence of retinal detachment. This may explain poor postoperative visual acuity in eyes with anatomically successful repair.
Postoperative visual complaints may occur after anatomically successful repair of rhegmatogenous retinal detachments (RD). Reduced postoperative visual acuity may result from epiretinal membranes (ERMs), pigment migration, cystoid macular edema (CME), macular hole, retinal folds, myopic shift, or cataract. Even in the absence of these clinically detectable complications, decreased final postoperative visual acuities can occur.1
Advances in retinal imaging have helped to demonstrate possible causes of delayed or incomplete visual recovery after anatomically successful surgical repair. The preoperative anatomy of RDs has been analyzed using optical coherence tomography (OCT) in an effort to predict visual outcome.2,3 The OCT measurements after surgical repair of RDs have shown that residual pockets of subretinal fluid may be present despite being undetectable by both biomicroscopy and fluorescein angiography.1 Postoperative subfoveal fluid can be demonstrated even in RDs in which the macula was uninvolved preoperatively.4 A recent study also showed that the outer nuclear layer of the neural retina thickens on OCT after retinal detachment, possibly indicating intraretinal fluid.5
Histopathology has shown that prolonged detachment of the retina can cause atrophy and death of the photoreceptors.6–9 As photoreceptor damage is not detectable biomicroscopically, this could explain unexpected visual loss in some patients after retinal detachment. Similarly, OCT has demonstrated photoreceptor loss in cases of central serous chorioretinopathy. Piccolino et al10 recently found that standard-resolution OCT could measure photoreceptor damage in central serous chorioretinopathy patients, and that this correlated with visual acuity. Decreased visual acuity has also been shown to correlate with photoreceptor loss on OCT after macular hole repair.11,12 However, photoreceptor loss has not been demonstrated with OCT in patients after rhegmatogenous retinal detachment.
Our group has recently developed a new ultrahigh-resolution optical coherence tomography (UHR OCT) system that significantly improves axial image resolution.13,14 The axial resolution of conventional OCT is limited to ~10 μm,15,16 whereas the UHR OCT has an axial resolution of ~3 μm. This improved resolution enables more detailed in vivo assessment of retinal architecture, particularly at the level of the photoreceptors and the retinal pigment epithelium.13,14,17 In the present study, we used UHR OCT to determine the presence of structural changes in the retina that may explain incomplete visual recovery in patients after retinal detachment.
Materials and Methods
Our group developed a prototype UHR OCT system capable of performing studies in the ophthalmology clinic.14 For this UHR OCT system, a specially designed femtosecond, titanium:sapphire laser was used as the light source for UHR OCT imaging.13 The femtosecond laser generates ~125 nm of bandwidth at ~815 nm center wavelength, and the UHR OCT system is capable of axial image resolution of ~3 μm. The UHR OCT image uses scans with a 1.5 mm axial depth and 6 mm in the transverse direction. The UHR OCT images have ~3 μm axial and 15 to 20 μm transverse resolution in tissue, and consists of 3000 axial and 600 transverse pixels (total = 1 800 000 pixels). The prototype UHR OCT clinical ophthalmic system has been described in detail in a previous study comparing standard imaging with UHR OCT imaging of macular holes.14
The OCT imaging is performed within well-established safe retinal exposure limits set by the American National Standards Institute. The standard for the American National Standards Institute for safe retinal exposure accounts for wavelength, duration, and multiple exposures of the same spot on the retina.18 The UHR OCT system uses the same incident optical power as used in StratusOCT; the regulation of incident optical power in the UHR OCT prototype has been previously described.14
The standard StratusOCT imaging protocol was followed on both OCT systems to enable a direct comparison of the resulting images. Six radial macular scans of 6 mm length each were acquired at angles separated by 30° intervals. After UHR OCT imaging was completed, all images were corrected for axial motion using standard re-registration algorithms. These algorithms have been used in all of the previous prototype and commercial OCT systems.19
Imaging was performed using our UHR OCT prototype at the New England Eye Center, Tufts–New England Medical Center. The study was approved by the institutional review board committees of both Tufts–New England Medical Center and Massachusetts Institute of Technology. Written informed consent was obtained from all the subjects in this study before UHR OCT imaging was performed. An experienced operator performed all UHR OCT examinations.
All tertiary referrals or postoperative patients from the New England Eye Center after anatomically successful repair of a rhegmatogenous RD were asked to participate in the study. A total of 33 eyes of 30 patients were imaged with the UHR OCT at the New England Eye Center. A complete history and ophthalmic examination was performed on all patients. Information about the location of the retinal detachment, involvement of the macula, initial visual acuity, time until repair, and type of surgery was recorded. Visual acuity was recorded using standard Snellen eye charts and use of the pinhole. Visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR) for data analysis.
Three patients had normal visual acuity and no visual complaints after surgery, and thus were not included in the analysis. The goal was to analyze images of patients with a persistent decrease in visual acuity after anatomically successful RD repair. Of note, however, all 3 patients without visual complaints had normal UHR OCT scans (Fig 1).
Figure 1.
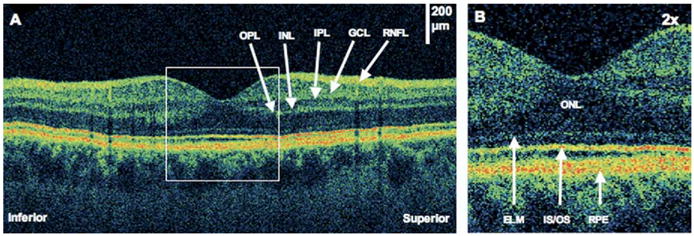
Normal macula after pneumatic retinopexy. The patient is a 28-year-old man with a macula-on retinal detachment (RD) treated with pneumatic retinopexy. Images were taken 6 months after RD repair. Visual acuity was 20/20 in the right eye. A, An ultrahigh-resolution optical coherence tomography (UHR OCT) image shows a normal macular image. The inner retinal layers are labeled: outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), and retinal nerve fiber layer (RNFL). B, The UHR OCT image (×2 magnification). The outer retinal layers are labeled: outer nuclear layer (ONL), external limiting membrane (ELM), photoreceptor inner/outer segments junction (IS/OS), and retinal pigment epithelium (RPE).
Six eyes were excluded due to inability to obtain either preoperative or intraoperative records. Two eyes were excluded due to poor scan quality; one had dense cataract formation and the other had retained perfluorocarbon liquid. Another eye was eliminated due to a history of endophthalmitis, which had led to RD. The authors believed that the endophthalmitis would complicate analysis of the images. Similarly, 1 eye was not included because of a retinal vascular abnormality. Finally, 1 eye was imaged only 1 day after pneumatic retinopexy and therefore was excluded due to inadequate postoperative follow-up. This left 17 eyes of 17 patients with visual complaints after retinal detachment imaged with the UHR OCT. More specifically, these 17 patients were dissatisfied with their visual acuity outcomes after surgery.
Results
The mean age of the 17 patients was 54±9 years (range, 38 – 69 years). Ten patients had RDs involving the right eye and 7 had RDs in the left eye. Twelve patients had macula-involving RDs preoperatively and 5 patients had RDs not involving the macula. It was not possible to accurately estimate the duration of macular detachment prior to surgery.
Surgical procedures included pneumatic retinopexy (1 eye), primary scleral buckle (2 eyes), primary vitrectomy (6 eyes), and vitrectomy with scleral buckle (8 eyes). Two of the 17 patients required >1 procedure to achieve retinal reattachment. One patient failed a pneumatic retinopexy and thus was treated with a vitrectomy and scleral buckle. The other patient required 2 pneumatic retinopexies, a scleral buckle segment, and a vitrectomy to achieve anatomic success. His final Snellen visual acuity was 20/25. A third patient had a membrane peel for an ERM after his RD repair.
Images were taken between 1 to 84 months postoperatively (median, 5 months). Eleven patients had imaging between 1 and 12 months after surgery, and the remaining 6 patients were imaged more than a year after surgery.
Best-corrected visual acuity was estimated using the pinhole. The mean preoperative logMAR visual acuity was 1.37 (Snellen equivalent, 20/390). The mean postoperative logMAR visual acuity was 0.48 (Snellen equivalent, 20/60). Postoperative Snellen visual acuity ranged from 20/25 to 20/400.
The UHR OCT images illustrated a range of retinal abnormalities associated with incomplete visual recovery. Findings included distortion of the photoreceptor inner/outer segments (IS/OS) junction (Fig 2), CME (Fig 3), pockets of subretinal fluid (Figs 4, 5), and ERMs (Fig 6). Distortion of the IS/OS junction was defined as a disruption in the highly-reflective IS/OS band. Distortion of the IS/OS junction was seen in 14 of 17 patients (82%), CME was present in 2 of 17 patients (12%), subretinal fluid was detected in 3 of 17 patients (18%), and ERM was demonstrated in 10 of 17 patients (59%). One patient also had a small lamellar hole (Fig 7). Of the 5 patients with preoperative macula-on detachments, 4 had distortion of the IS/OS junction after RD repair, 2 had ERMs, 1 had CME, and 1 had subretinal fluid.
Figure 2.
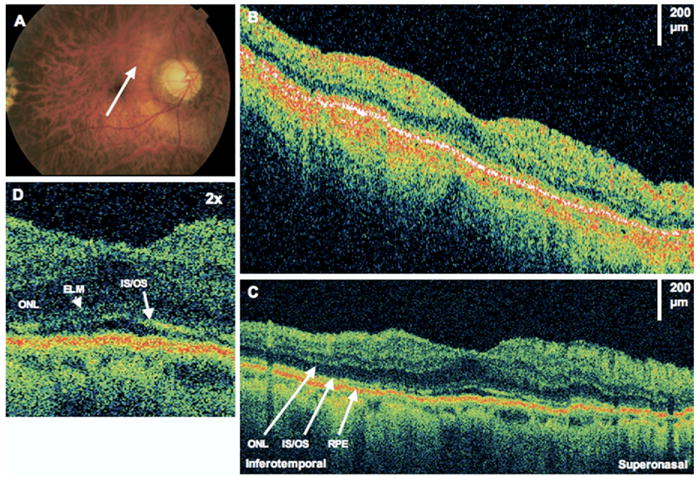
Photoreceptor distortion. The patient is a 50-year-old man with a macula-off retinal detachment (RD) treated with a scleral buckle, vitrectomy, and sulfur hexafluoride 20% gas bubble injection. Images were taken 18 months after RD repair. Visual acuity was 20/40 in the right eye. A, Fundus photograph showing the direction of optical coherence tomography (OCT) scans. B, StratusOCT image demonstrates an apparently normal retina. C, The corresponding ultrahigh-resolution optical coherence tomography (UHR OCT) image shows attenuation of the photoreceptor inner/outer segments (IS/OS) signal. Outer retinal layers are labeled. ONL = outer nuclear layer; RPE = retinal pigment epithelium. D, The UHR OCT image (×2 magnification), demonstrating attenuation of the IS/OS signal. ELM = external limiting membrane.
Figure 3.
Cystoid macular edema (CME). The patient is a 59-year-old man who had a macula-on retinal detachment treated with a scleral buckle. Images were taken 1 month after surgery, at which time his visual acuity was 20/80. Optical coherence tomography (OCT) images were taken from the right eye at 60°, temporal to nasal. A, StratusOCT image demonstrates CME. B, The corresponding ultrahigh-resolution OCT image demonstrates attenuation of signal of the inner/outer segments (IS/OS) junction below the area of the CME. The outer nuclear layer (ONL) also seems thickened. ELM = external limiting membrane; RPE = retinal pigment epithelium.
Figure 4.
Residual subretinal fluid. The patient is a 38-year-old woman who had a macula-off retinal detachment (RD) and underwent a primary scleral buckle with sulfur hexafluoride 20% gas injection. Images were taken 4 months after RD repair. Visual acuity was 20/40. A, Fundus photograph showing the direction of optical coherence tomography (OCT) scans. B, StratusOCT image showing several small pockets of subretinal fluid (horizontal and vertical images show similar findings). C, Corresponding ultrahigh-resolution OCT image shows the photoreceptor inner/outer segments (IS/OS) junction is lifted off the retinal pigment epithelium (RPE). The external limiting membrane (ELM) also seems intact above areas of subretinal fluid.
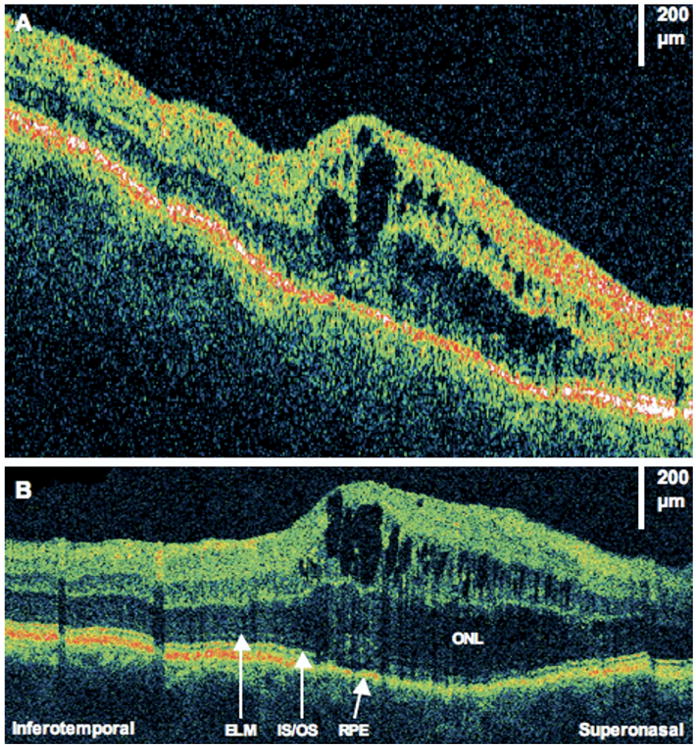
Figure 5.
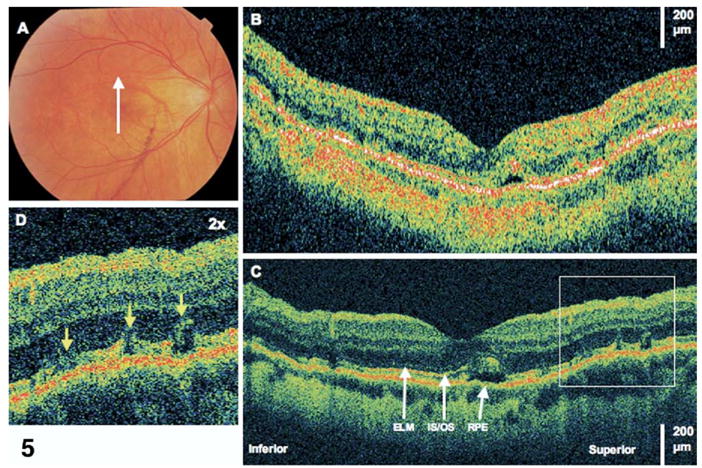
Resolving subretinal fluid. This is the same patient as in Figure 4. Images were taken 10 months after retinal detachment repair. Visual acuity remained 20/40. A, Fundus photograph showing direction of optical coherence tomography (OCT) images. B, StratusOCT demonstrates one small pocket of residual subretinal fluid. C, The corresponding ultrahigh-resolution OCT (UHR OCT) image demonstrates several disruptions of the photoreceptor inner/outer segments (IS/OS) junction where fluid is less apparent from 6 months prior. ELM = external limiting membrane; RPE = retinal pigment epithelium. D, The UHR OCT image (×2 magnification), demonstrating disruptions of the IS/OS junction.
Figure 6.
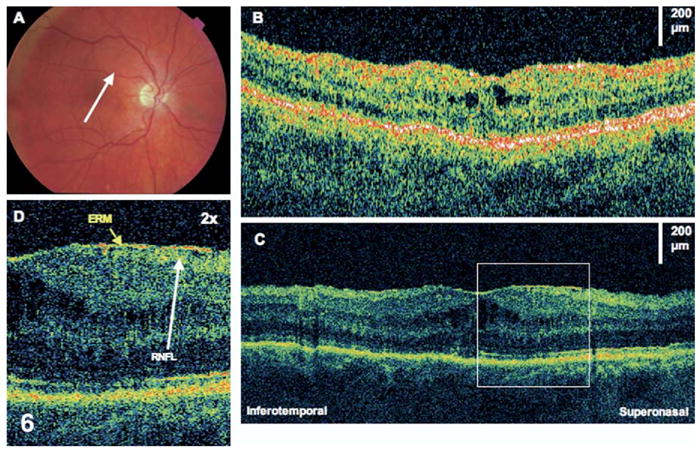
Epiretinal membrane (ERM). The patient is a 58-year-old man who had a macula-off retinal detachment in the right eye and underwent a scleral buckle and pars plana vitrectomy procedure. Images were taken 7 months later, at which time visual acuity was 20/100. A, Fundus photograph showing direction of optical coherence tomography (OCT) images. B, StratusOCT demonstrates a small amount of cystoid macular edema. C, The corresponding ultrahigh-resolution OCT image additionally demonstrates a thin ERM. D, The ERM (×2 magnification). The retinal nerve fiber layer (RNFL) seems wrinkled underneath the membrane.
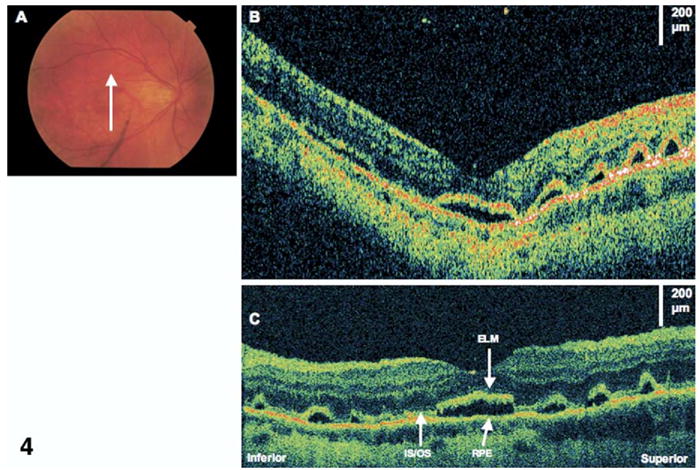
Figure 7.
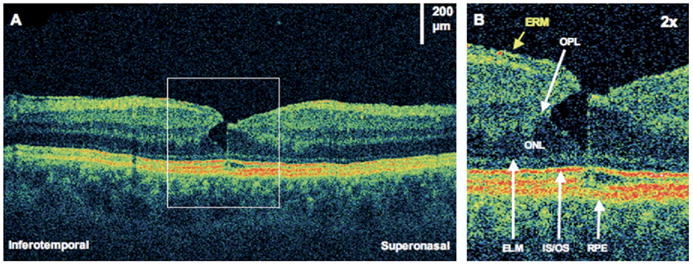
Lamellar macular hole. The patient is a 59-year-old man who had a giant retinal tear and subsequent scleral buckle and pars plana vitrectomy. Images were taken 7 years later, at which time visual acuity was 20/25. A, Ultrahigh-resolution optical coherence tomography demonstrating an epiretinal membrane (ERM) and a small lamellar hole. B, The lamellar hole (×2 magnification), showing a sharp break through the inner layers of the retina. There seems to be separation between the outer plexiform layer (OPL) and the outer nuclear layer (ONL). There is also a very small disruption of the photoreceptor inner/outer segments (IS/OS) junction at the foveola. ELM = external limiting membrane; RPE = retinal pigment epithelium.
Discussion
The histopathologic effects of RD surgery have been examined by Barr7 and Wilson and Green.9 The most common factors complicating RD surgery were the formation of ERMs and marked photoreceptor atrophy with disorganization of the lamellar architecture of the retina. This may explain the lack of visual improvement after ERM peeling in patients with previous RD repair.
Experimental RDs in cats have shown that alterations in the outer nuclear layer can occur after only 1 hour of RD, and continued progressive loss of photoreceptors occurs in retinas detached 13 to 30 days.7 In an eye with an RD, the outer retina is deprived of its nutrient supply from the underlying choroid, and thus, photoreceptor atrophy ensues. After reattachment of the cat retina, atrophy of the photoreceptors was present in 42-day detachments, but there was only limited atrophy in retinas detached only 3 to 7 days.6 Photoreceptor atrophy may be responsible for the reduced visual acuity seen in patients after anatomically successful RD repair.
The UHR OCT enables unprecedented visualization of in vivo retinal morphology. Enhanced visualization of inner and outer segments of the photoreceptors in cases of macular holes has been demonstrated.11 The current study outlines a series of postoperative RDs imaged with UHR OCT. As previously discussed, all of our patients had anatomically successful repair of RDs but had persistent visual complaints. Postoperative Snellen visual acuity ranged from 20/25 to 20/400. Many of these patients suffered photoreceptor damage visible on UHR OCT. These changes are represented by alterations in the reflectivity of the inner and outer segments junction. It is possible that these subtle changes in reflectivity may represent damage to the photoreceptors. This may explain their incomplete visual recovery. In addition, CME, ERM, and a lamellar hole were noted, which could also explain decreased visual acuity in some patients. Remodeling at the cellular level also occurs in detached retina. Visual recovery may depend on the success of neural remodeling. These changes at the cellular level are not evident on UHR OCT.
Interestingly, patients with retinal detachments not involving the macula had similar abnormalities on UHR OCT, indicating that an attached macula does not necessarily mean that macular photoreceptors are uninvolved. Yetik et al5 examined OCT images of attached retinal sites of eyes with RDs and compared them with corresponding retinal sites of fellow eyes.5 Their analysis showed that healthy-appearing areas of attached retina may not be structurally normal in cases of rhegmatogenous RDs.
It is important to note that in our study, preoperative macular status was determined clinically and not by OCT. Therefore, it is possible that subclinical subretinal fluid may have been present in the patients with clinically macula-on detachments. Alternatively, subretinal fluid might be displaced by intraocular gas into the macular region, resulting in a period of macular detachment and consequent retinal changes.
Limitations of this study include small patient number and the need for correction for axial patient motion with computer algorithms. Future studies with larger numbers of patients may be helpful to determine whether a correlation exists between length of time between retinal detachment and repair with degree of photoreceptor damage, as well as a correlation between photoreceptor loss and final visual acuity. The correction for axial motion eliminates the ability to image true retinal topography, which may introduce errors in image analysis. In the future, high-speed OCT will help to eliminate motion artifacts and the need for motion correction, enabling enhanced visualization of the true retinal topography.20,21 In addition, some images were performed as early as 1 month postoperatively. Visual recovery can take several months, and thus, these patients may continue to improve.
In summary, UHR OCT was able to detect photoreceptor disruptions in the majority of patients with visual complaints after retinal detachment repair. This photoreceptor damage may persist long after surgery, and may help explain the lack of return to normal visual acuity. Other retinal abnormalities included epiretinal membranes, subretinal fluid, and CME. These abnormalities likely play a role in decreased visual acuity and visual distortion in some patients after RD repair.
Footnotes
Supported by royalties Drs Fujimoto and Schuman received from intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec. Drs Fujimoto and Schuman also received research support from Carl Zeiss Meditec, Dublin, California. Also supported in part by National Institutes of Health, Bethesda, Maryland (grant nos.: RO1-EY11289-16, R01-EY13178, P30-EY13078); National Science Foundation, Arlington, Virginia (grant no.: ECS-0119452); Air Force Office of Scientific Research, Arlington, Virginia (grant no.: F49620-98-1-0139); and Medical Free Electron Laser Program (grant no.: F49620-01-1-0186).
References
- 1.Wolfensberger TJ, Gonvers M. Optical coherence tomography in the evaluation of incomplete visual acuity recovery after macula-off retinal detachments. Graefes Arch Clin Exp Ophthalmol. 2002;240:85–9. doi: 10.1007/s00417-001-0410-6. [DOI] [PubMed] [Google Scholar]
- 2.Hagimura N, Suto K, Iida T, Kishi S. Optical coherence tomography of the neurosensory retina in rhegmatogenous retinal detachment. Am J Ophthalmol. 2000;129:186–90. doi: 10.1016/s0002-9394(99)00314-1. [DOI] [PubMed] [Google Scholar]
- 3.Lecleire-Collet A, Muraine M, Menard JF, Brasseur G. Predictive visual outcome after macula-off retinal detachment surgery using optical coherence tomography. Retina. 2005;25:44–53. doi: 10.1097/00006982-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Theodossiadis PG, Georgalas IG, Emfietzoglou J, et al. Optical coherence tomography findings in the macula after treatment of rhegmatogenous retinal detachments with spared macula preoperatively. Retina. 2003;23:69–75. doi: 10.1097/00006982-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Yetik H, Guzel H, Ozkan S. Structural features of attached retina in rhegmatogenous retinal detachments. Retina. 2004;24:63–8. doi: 10.1097/00006982-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–83. [PubMed] [Google Scholar]
- 7.Barr CC. The histopathology of successful retinal reattachment. Retina. 1990;10:189–94. [PubMed] [Google Scholar]
- 8.Erickson PA, Fisher SK, Anderson DH, et al. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24:927–42. [PubMed] [Google Scholar]
- 9.Wilson DJ, Green WR. Histopathologic study of the effect of retinal detachment surgery on 49 eyes obtained post mortem. Am J Ophthalmol. 1987;103:167–79. doi: 10.1016/s0002-9394(14)74222-9. [DOI] [PubMed] [Google Scholar]
- 10.Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99. doi: 10.1016/j.ajo.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Kitaya N, Hikichi T, Kagokawa H, et al. Irregularity of photoreceptor layer after successful macular hole surgery prevents visual acuity improvement. Am J Ophthalmol. 2004;138:308–10. doi: 10.1016/j.ajo.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Villate N, Lee JE, Venkatraman A, Smiddy WE. Photoreceptor layer features in eyes with closed macular holes: optical coherence tomography findings and correlation with visual outcomes. Am J Ophthalmol. 2005;139:280–9. doi: 10.1016/j.ajo.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121:695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 14.Ko TH, Fujimoto JG, Duker JS, et al. Comparison of ultra-high- and standard-resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology. 2004;111:2033–43. doi: 10.1016/j.ophtha.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 17.Drexler W, Morgner U, Ghanta RK, et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7:502–7. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American National Standard for the Safe Use of Lasers. ANSI Z136.1-1993. New York: American National Standards Institute; 1993. [Google Scholar]
- 19.Swanson EA, Izatt JA, Hee MR, et al. In-vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18:1864–6. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- 20.Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004;138:412–9. doi: 10.1016/j.ajo.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Unterhuber A, Povazay B, Hermann B, et al. Compact, low-cost Ti:Al2O3 laser for in vivo ultrahigh-resolution optical coherence tomography. Opt Lett. 2003;28:905–7. doi: 10.1364/ol.28.000905. [DOI] [PubMed] [Google Scholar]


