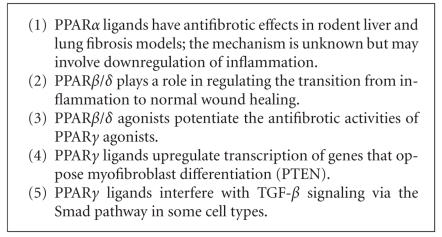
Abstract
Pulmonary fibrosis is a group of disorders characterized by accumulation of scar tissue in the lung interstitium, resulting in loss of alveolar function, destruction of normal lung architecture, and respiratory distress. Some types of fibrosis respond to corticosteroids, but for many there are no effective treatments. Prognosis varies but can be poor. For example, patients with idiopathic pulmonary fibrosis (IPF) have a median survival of only 2.9 years. Prognosis may be better in patients with some other types of pulmonary fibrosis, and there is variability in survival even among individuals with biopsy-proven IPF. Evidence is accumulating that the peroxisome proliferator-activated receptors (PPARs) play important roles in regulating processes related to fibrogenesis, including cellular differentiation, inflammation, and wound healing. PPARα agonists, including the hypolidipemic fibrate drugs, inhibit the production of collagen by hepatic stellate cells and inhibit liver, kidney, and cardiac fibrosis in animal models. In the mouse model of lung fibrosis induced by bleomycin, a PPARα agonist significantly inhibited the fibrotic response, while PPARα knockout mice developed more serious fibrosis. PPARβ/δ appears to play a critical role in regulating the transition from inflammation to wound healing. PPARβ/δ agonists inhibit lung fibroblast proliferation and enhance the antifibrotic properties of PPARγ agonists. PPARγ ligands oppose the profibrotic effect of TGF-β, which induces differentiation of fibroblasts to myofibroblasts, a critical effector cell in fibrosis. PPARγ ligands, including the thiazolidinedione class of antidiabetic drugs, effectively inhibit lung fibrosis in vitro and in animal models. The clinical availability of potent and selective PPARα and PPARγ agonists should facilitate rapid development of successful treatment strategies based on current and ongoing research.
1. INTRODUCTION
Pulmonary fibrosis is a potentially fatal disease characterized by accumulation of scar tissue in the lung interstitium, resulting in loss of alveolar function, destruction of normal lung architecture, and respiratory distress [1–3]. Known causes include inhalation of dusts and other particulates such as silica and asbestos, chemo- and radiation therapy, autoimmunity, hypersensitivity pneumonitis, and sarcoidosis [4, 5]. The idiopathic interstitial pneumonias, as the name suggests, are a group of fibrotic diseases of unknown etiology, the commonest of which is the usual intersitial pneumonitis (UIP), also called idiopathic pulmonary fibrosis (IPF) [6–8]. Some types of fibrosis respond to corticosteroids but many are refractory [9–11]. Prognosis is varied, but can be poor. UIP is considered to be the most severe of the idiopathic interstitial pneumonias. However, there is significant variability in the natural history of this disease. For example, the mean survival time after a diagnosis of UIP is less than three years [12], but there are patients who can survive for much longer periods of time with much slower (or rarely no) progression of their lung disease [13]. In contrast, other patients can develop acute exacerbations of their pulmonary fibrosis with the rapid onset of dyspnea, new radiographic abnormalities, respiratory failure, and death in 20%–86% of patients. Histological examination of their lungs reveals diffuse alveolar damage superimposed on a background of UIP [12]. The etiology of these exacerbations is unclear, but factors including infection have been implicated.
At the cellular level, pulmonary fibrosis is characterized by proliferation and accumulation of fibroblasts and scar-forming myofibroblasts in the lung interstitium with increased synthesis and deposition of extracellular matrix proteins including collagen and fibronectin [9, 14]. Although fibroblasts were previously regarded as simple structural cells, they are now recognized as having important sentinel and regulatory functions and are a rich source of regulatory cytokines and chemokines [15]. Fibroblasts differentiate to myofibroblasts after appropriate stimuli, including transforming growth factor (TGF)-β1 [9, 14, 16]. Myofibroblasts have some of the characteristics of smooth muscle cells, including contractility and expression of α-smooth muscle actin (α-SMA) [14, 17, 18]. The differentiation of fibroblasts to myofibroblasts, along with increased cellular proliferation and matrix deposition, leads to the development of fibroblastic foci similar in appearance to the early stages of normal wound healing. Fibrosis is usually progressive, leading to destruction of the normal lung architecture [2, 14, 17, 18]. Other organs can develop fibrosis, including the skin, liver, kidney, and pancreas, and the cellular events and signals are likely to be similar.
It has been hypothesized that fibrosis is a consequence of abnormal regulation of wound repair [2, 19, 20]. An injury leads to acute inflammation, followed by an initial repair phase in which fibroblasts and myofibroblasts at the injury site replace damaged tissue with scar tissue. Normally, this phase of wound repair is self-limiting, with myofibroblasts eventually undergoing apoptosis, and the scar tissue may be remodeled and reconstructed as relatively normal functional tissue. In fibrosis, the fibroblasts and myofibroblasts do not undergo apoptosis, but continue to proliferate, resulting in progressive scarring. The cellular signals involved in the maintenance of the profibrotic phenotype are unknown, although it is likely that TGF-β is a critical factor [21–24].
2. PPARs AND LUNG DISEASE
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor family, that function to regulate a wide range of physiological activities [25]. Three different isoforms of PPARs have been identified: PPARα (NR1C1), PPARβ/δ (NUC1; NR1C2), and PPARγ (NR1C3), encoded by three separate genes. The PPARs and their obligate coreceptors, the retinoid X receptors (RXRs), bind a variety of ligands. The ligand-activated heterodimeric complexes then induce expression of target genes carrying peroxisome proliferators response elements (PPREs) in their promoters. PPARα was first identified as the mediator of the response to peroxisome proliferators in rodents [26]. Over the past decade, PPARs have been implicated as important regulators of various physiological processes, such as lipid and lipoprotein metabolism, glucose homeostasis, cellular proliferation, differentiation, and apoptosis. PPARα is found in high levels in liver, kidney, heart, and muscle, whereas PPARβ/δ is ubiquitously expressed [26, 27]. PPARγ is found in two main isoforms, PPARγ1 and PPARγ2, derived from different pre-mRNA splice variants that use different transcription start sites. PPARγ is widely expressed, and has been found in blood cells, such as macrophages [28], T and B lymphocytes [29, 30] and platelets [31], as well as in tissues including adipose, colon, spleen, retina, skeletal muscle, liver, bone marrow, and lung [27]. Within the lung, PPARγ is expressed by the epithelium, smooth muscle cells, fibroblasts, endothelium, macrophages, eosinophils, and dendritic cells [32].
The role of the PPARs in lung disease is not yet clear. Both PPARα and PPARγ have been localized in lung tissue, including bronchial epithelial cells, alveolar walls, and alveolar macrophages [27, 32, 33]. A comparison of nonsmokers, smokers with chronic obstructive pulmonary disease (COPD), and smokers without COPD found no statistically significant difference in the number of PPARγ-positive macrophages, but found an increased number of PPARα-positive alveolar macrophages in smokers with COPD [34]. Sarcoidosis and pulmonary alveolar proteinosis are two other disorders in which alveolar macrophages are deficient in PPARγ [35]. A causal relationship has not been determined, however, treatment of pulmonary alveolar proteinosis with granulocyte-macrophage colony-stimulating factor (GM-CSF) restores alveolar macrophage PPARγ levels [36].
There is evidence that the PPARs, particularly PPARα and PPARγ, play a role in regulating inflammation. For example, fatty-acid-derived inflammatory mediators, including prostaglandins and leukotrienes, are ligands for PPARα and γ [37]. Although the pathogenesis of fibrosis appears to be distinct from inflammation, and many forms of fibrosis are refractory to anti-inflammatory therapies such as corticosteroids, recent work has supported the hypothesis that fibrosis is a consequence of a dysregulated wound healing process with an initial injury and inflammatory response. Certainly, many important inflammatory signals and mediators, particularly TGF-β, TNF-α, and IL-1β, and prostaglandins, play key roles in fibrosis [21–24]. This review will discuss recent reports examining the link between PPARs and fibrosis, and the possibility of using PPAR ligands as antifibrotic therapies. Because the study of PPARs in lung fibrosis is relatively new, we will also review selected results from fibrotic disease models in other organs.
3. PPARα
PPARα was originally cloned as the molecular target for the hypolipidemic fibrate drugs, although arachidonic acid metabolites (eicosanoids, prostaglandins, and leukotrienes) are also important ligands [38]. PPARα plays a key role in lipid metabolism and is highly expressed in tissues involved in lipid and cholesterol metabolism, including the liver, kidney, and macrophages. PPARα ligands have important anti-inflammatory properties, although some studies have reported proinflammatory effects as well [37, 39]. Little is known about PPARα in lung disease, although other fibrosis models implicate PPARα in regulating fibrosis.
In the liver, the PPARα agonists fenofibrate and WY14643 dramatically reduced fibrosis in the thioacetamide model of cirrhosis [40]. N-3 polyunsaturated fatty acid, another PPARα ligand, reduced hepatic and serum TNF-α levels and reduced the degree of liver injury in a rat model of nonalcoholic steatohepatitis [41]. The synthetic PPARα agonist WY14643 reduced the severity of steatohepatitis in C57BL/6 mice fed a methionine- and choline-deficient diet, with reductions in hepatic mRNA levels of collagen alpha 1, tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2, and matrix metalloproteinase (MMP)-13 [42].
Fenofibrate also attenuated cardiac and vascular fibrosis in pressure-overloaded rat hearts, with reductions in collagen I and III mRNA [43], and inhibited fibrotic left ventricular remodeling in mineralcorticoid-dependent hypertension [44]. The PPARα agonist gemfibrozil attenuated glomerulosclerosis and collagen deposition in diabetic ApoE-knockout mice [45].
Recent reports have found significantly reduced PPARα mRNA levels in lymphocytes from cystic fibrosis patients [46], while PPARα knockout mice develop more severe carageenan-induced pleural inflammation [47], suggesting a connection between diminished PPARα-dependent gene activation and disease pathology.
The role of PPARα in lung fibrosis was investigated in mice using the bleomycin model of lung injury and fibrosis. Intratracheal instillation of the antineoplastic agent bleomycin causes acute lung inflammation that develops into severe fibrosis, with proliferation of α-SMA-positive myofibroblasts, increased collagen deposition, and loss of normal alveolar architecture [48, 49]. PPARα-knockout mice treated with bleomycin developed more severe inflammation and fibrosis than wild-type mice, with increased immunohistochemical detection of TNF-α and IL-1β, increased apoptosis of interstitial cells, and decreased survival [50]. Treatment of wild-type mice with the PPARα agonist WY-14643 enhanced survival and reduced the severity of fibrosis, as well as reducing the detection of TNF-α and apoptosis by immunohistochemistry. The authors concluded that endogenous PPARα ligands play an important role in limiting the fibrotic response in wild-type mice, and that treatment with PPARα ligands has potential as an antifibrotic therapy.
As yet, there have been no molecular mechanisms proposed to explain these results. Since bleomycin treatment results in an acute inflammatory response that later resolves into fibrosis, it is possible that PPARα agonists act to inhibit fibrosis by moderating the initial inflammatory response. This could be addressed by using a fibrogenic insult that provokes minimal inflammation, such as adenovirus-mediated overexpression of TGF-β [24].
Interestingly, there is some evidence that the effects of PPARα agonists are not entirely dependent on PPARα-dependent transcription [51]. Since the above study did not report treating PPARα-knockout mice with WY-14643, the issue of the PPARα dependence or independence of the effect was not addressed. It should also be noted that WY-14643 is also a weak PPARγ agonist [52], and PPARγ agonists may have antifibrotic activity as well (discussed below). One way to investigate the PPARα dependence or independence of PPARα agonists would be to study their effects in PPARα-knockout fibroblasts in vitro and PPARα-knockout mice in vivo. Studies using additional in vivo models of fibrosis (such as thoracic radiation or inhalation of crystalline silica) should also prove informative.
4. PPARβ/δ
Although little is known about PPARβ/δ in the lung, PPARβ/δ does play a critical role in wound healing in the skin. PPARβ/δ expression is upregulated following skin injury. Further, PPARβ/δ-knockout mice exhibit defective in vivo wound healing, and keratinocytes from PPARβ/δ-knockout mice show decreased adhesion and migration in vitro [53]. It has been suggested that PPARβ/δ is a critical regulator of the transition from the initial inflammatory response to the later wound healing program [54].
An intriguing recent study suggested that PPARβ/δ may be a target of prostacyclin mimetics used in treating pulmonary hypertension. Treprostinil sodium activated a PPARβ/δ reporter gene and inhibited proliferation of lung fibroblasts in vitro. The effect was not seen in lung fibroblasts from PPARβ/δ-knockout mice, demonstrating that the effect was dependent on PPARβ/δ and not on the prostacyclin receptor [55]. Finally, PPARβ/δ agonists enhance the efficacy of PPARγ agonists in mediating adipocyte differentiation in vitro [56], suggesting that PPARβ/δ agonists may also potentiate the antifibrotic effects of PPARγ agonists discussed below.
5. PPARγ
PPARγ is expressed in many types of lung cells including fibroblasts, ciliated airway epithelial cells and alveolar type II pneumocytes, alveolar macrophages, T lymphocytes, and airway smooth muscle cells [57]. Endogenous ligands of PPARγ include 15-deoxy −Δ12,14-prostaglandin J2 (15d-PGJ2) [58, 59], lysophosphatidic acid [60], and nitrolinoleic acid [61]. PPARγ can also be activated by synthetic ligands including the thiazolidinedione (TZD) class of clinically used insulin-sensitizing drugs [62] including rosiglitizone and pioglitizone, as well as oleanic acid derivatives known as triterpenoids [63].
The anti-inflammatory properties of PPARγ ligands have been well described [37, 64]. In the lung, PPARγ ligands inhibit LPS-induced neutrophilia [65, 66] and allergic airway inflammation and hyperresponsiveness in a mouse model of asthma [67, 68]. PPARγ ligands also inhibit the release of proinflammatory mediators from airway epithelial cells and alveolar macrophages [69, 70]. In addition, PPARγ plays an important role in regulating cellular differentiation, as PPARγ ligands promote differentiation of preadipocyte fibroblasts to adipocytes [58, 59, 71].
A number of studies have investigated PPARγ ligands as potential antifibrotic agents in vivo. Pioglitazone reduced carbon-tetrachloride-induced hepatic fibrosis in rats, with decreases in hydroxyl proline content, procollagen I mRNA, and α-SMA-positive hepatic stellate cells [72]. A similar effect was observed when fibrosis was induced by a choline-deficient diet [73, 74]. Rosiglitazone inhibits cardiac fibrosis in rats [44] and kidney fibrosis in diabetic mice and rats [45]. Intriguingly, improvements in renal function have been noted in patients with type II diabetes who are treated with TZDs [75, 76].
Only a limited amount of data is available on the effects of PPARγ agonists on lung fibrosis in vivo. Ciglitazone administered by nebulization in a mouse model of asthma not only reduced lung inflammation and eosinophilia, but also reduced basement membrane thickening and collagen deposition associated with airway remodeling, as well as synthesis of the profibrotic cytokine TGF-β [68]. This effect was abolished by concomitant use of GW9662, an irreversible PPARγ antagonist. Rosiglitazone and 15d-PGJ2 significantly reduced mortality, inflammation, cellular infiltrates, and histological fibrosis following intratracheal administration of bleomycin [77]. Studies of the in vivo effects of PPARγ agonists have been hampered by the fact that unlike PPARα, homozygous germline deletion of the PPARγ gene results in embryonic lethality [78]. A conditional knockout mouse, in which exon 2 of the PPARγ gene has been flanked by loxP sites, has been developed [78], and strategies to inducibly knock out PPARγ expression in the adult mouse lung prior to fibrotic insult are being explored in a number of laboratories.
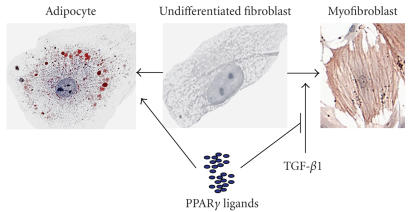
The antifibrotic effects of PPARγ ligands have been studied in vitro, leading to new insights into their mechanism of action. As previously discussed, TGF-β drives differentiation of lung fibroblasts to myofibroblasts, a key effector cell in fibrosis [16, 23, 24]. In contrast, PPARγ ligands differentiate fibroblasts to fat-storing adipocytes [58, 59]. This suggests that PPARγ ligands may oppose the fibrogenic effects of TGF-β (Figure 1). We investigated the ability of PPARγ ligands to counter the profibrotic effects of TGF-β on primary human lung fibroblasts. Rosiglitazone and 15d-PGJ2 efficiently inhibited TGF-β-driven differentiation of human lung fibroblasts to myofibroblasts, with reductions in the expression of α-SMA (a myofibroblast marker) and production of collagen [79].
Figure 1.
PPARγ ligands promote fibroblast differentiation to adipocytes and inhibit differentiation to myofibroblasts. Primary human fibroblasts (center panel) can be differentiated to adipocyte-like cells (left panel) by treatment with 1 μM 15d-PGJ2 for 8 days. Lipid droplets were visualized with oil red O staining. Alternatively, incubation with 10 ng/mL TGF-β for 3 days will differentiate fibroblasts to myofibroblasts (right panel). α-SMA was detected by immunocytochemistry. Note the long bundles of contractile fibers.
Similar results have been observed in other cell types. Differentiation of hepatic stellate cells to a myofibroblast phenotype is a key step in liver fibrosis [80–82]. PPARγ agonists suppress proliferation of hepatic stellate cells and chemotaxis in response to platelet-derived growth factor (PDGF) [83], and induce hepatocyte growth factor (HGF), an anti-fibrotic cytokine [84]. PPARγ ligands also block PDGF-dependent proliferation, prolyl4-hydroxylase (α) mRNA, and the expression of collagen and α-SMA by pancreatic stellate cells [85]. Renal cortical fibroblasts treated with glucose induce myofibroblastic markers. Treatment of these cells with pioglitizone decreased collagen IV production, incorporation of proline, fibronectin production, and MMP-9 activity as well as reduced secretion of TIMP-1 and -2 [86, 87].
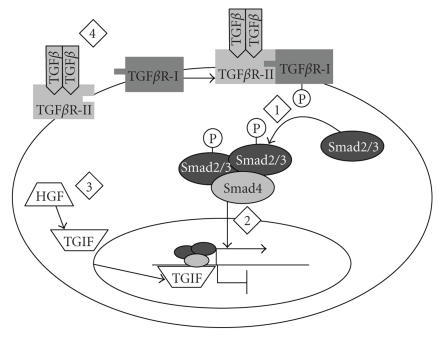
The molecular mechanisms by which PPARγ ligands inhibit myofibroblast differentiation and effector function are under investigation. Because TGF-β appears to be a key profibrotic cytokine in lung fibrosis [2, 21], several groups have investigated the ability of PPARγ ligands to interfere with TGF-β signaling. TGF-β signaling is mediated by the Smad family of transcription factors [21]. Binding of TGF-β to type 2 TGF-β receptor recruits type 1 TGF-β receptors (TGF-βR-I), forming a heterotetrameric structure that phosphorylates Smad2 and Smad3. Smad2 and Smad3 form heteromeric complexes with Smad4, which translocate to the nucleus and activate transcription of target genes (Figure 2). In human hepatic stellate cells, TGF-β causes a time- and dose-dependent increase in Smad3 phosphorylation, followed by increased collagen production. Cotreatment with either a TGF-βR-I kinase inhibitor or the synthetic PPARγ agonist GW7845 resulted in dose-dependent inhibition of both collagen production and Smad3 phosphorylation [88]. In contrast, the natural PPARγ agonist 15d-PGJ2 did not inhibit nuclear translocation of Smad2/3 complexes in human renal mesangial cells treated with TGF-β. Instead, 15d-PGJ2 induced expression of the antifibrotic hepatocyte growth factor (HGF) via a peroxisome proliferator response element in the HGF promoter, and upregulated the Smad corepressor TG-interacting factor (TGIF), leading to inhibition of α-SMA and fibronectin expression [84]. Interestingly, the same study reported that 15d-PGJ2 did inhibit Smad2/3 nuclear translocation in rat kidney fibroblasts treated with TGF-β, while we have reported that 15d-PGJ2 does not inhibit TGF-β-stimulated phosphorylation of Smad2 in human lung fibroblasts [79]. It is possible that inhibition of myofibroblast differentiation by PPARγ agonists is mediated by different mechanisms in different cell types, or that natural and synthetic agonists act by different mechanisms.
Figure 2.
The TGF-β signaling pathway. Binding of TGF-β to TGF-β receptor II recruits TGF-β receptor I (TGF-βR-I). The kinase domain of TGF-βR-I phosphorylates Smad2 and 3, which form a heteromeric complex with Smad4 that translocates into the nucleus where it activates transcription of target genes. Numbers indicate points in the pathway where PPARγ ligands have been demonstrated to interfere with TGF-β signaling. (1) GW7845, a PPARγ ligand, inhibited Smad3 phosphorylation in human hepatic stellate cells [88]. (2) 15d-PGJ2 inhibited nuclear translocation of Smad2/3 in rat kidney fibroblasts [84]. (3) In human renal mesangial cells, 15d-PGJ2 induced hepatocyte growth factor (HGF), which upregulates the Smad corepressor TG-interacting factor (TGIF) [84]. (4) In mouse L929 fibroblasts, 15d-PGJ2 or retinoic acid upregulated the phosphatase and tensin homologue deleted on chromosome 10 (PTEN), leading to repression of TGF-β1 transcription [89].
Another candidate mechanism for inhibition of profibrotic effector functions of fibroblasts involves upregulation of the tumor-suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN). The PTEN promoter contains a PPRE, and PPARγ ligands upregulate PTEN expression [90]. In vitro studies have shown that PTEN inhibits fibroblast-myofibroblast differentiation and expression of α-SMA and collagen in human and mouse lung fibroblasts [91], while loss of PTEN activity contributes to the migratory/invasive phenotype of lung fibroblasts isolated from IPF patients [92]. It has also been reported that PTEN levels are decreased in the lung tissue of IPF patients, and that PTEN knockout mice are more susceptible to bleomycin-induced fibrosis [91]. Interestingly, both 15d-PGJ2 and the RXR ligand 9-cis-retinoic acid inhibited transcription of the TGF-β1 gene via PTEN upregulation in mouse L929 fibroblasts [89], providing an additional mechanism by which PPARγ ligands might interfere directly with the profibrotic effects of TGF-β.
One important consideration is that the effects of PPARγ ligands may not all be dependent on PPARγ-dependent transcriptional activation. PPARγ-dependent transcriptional repression has been described in adipogensis, but not in myofibroblast differentiation [93, 94]. Additionally, recent reports have suggested that some of the biological effects of 15d-PGJ2 are moderated by a PPARγ-independent mechanism involving modification of protein thiols by an electrophilic carbon on the imidazole ring of 15d-PGJ2 [95, 96]. For example, the ability of troglitazone or 15d-PGJ2 to inhibit proliferation of hepatic stellate cells was shown to be PPARγ-independent [97], while 15d-PGJ2 inhibts the proliferation of human breast carcinoma cell lines by covalent modification of the estrogen receptor DNA-binding domain [98]. We examined the PPARγ dependence of the antifibrotic effects of PPARγ ligands on human lung fibroblasts. Neither the irreversible PPARγ antagonist GW9662 nor a dominant-negative PPARγ mutant significantly blocked the ability of 15d-PGJ2 to inhibit TGF-β-induced α-SMA expression, suggesting that this effect of 15d-PGJ2 was largely PPARγ-independent [79]. However, the antifibrotic effects of rosiglitizone were rescued significantly by the dominant-negative PPARγ, suggesting that while rosiglitizone was less effective at inhibiting myofibroblast differentiation, the effect was mostly dependent on PPARγ [79].
6. RETINOID X RECEPTOR
The PPARs must form heterodimers with the retinoid X receptor (RXR) in order to initiate gene transcription [99]. Therefore, it has been proposed that the anti-inflammatory and antifibrotic functions of PPARs may be addressed or enhanced by RXR ligands, predominantly the retinoic acids [100, 101]. In the rat liver, endogenous and synthetic retinoic acids (RA) reduced proliferation of HSCs and production of collagen I. In addition, all-trans RAs inhibited the synthesis of collagen I/II and fibronectin but did not affect HSC proliferation [102]. Levels of RXR-α and RXR-β were decreased in the HSC of rats with cholestatic liver fibrosis [103]. In addition, there were decreases in all-trans RA and 9-cis-RA levels and RA binding to the retinoid receptor response element (RARE) in fibrotic liver tissue. Similar findings have been demonstrated in glomerular mesangial cells where 9-cis-RA induced the antifibrotic growth factor HGF and inhibited TGF-β-stimulated induction of α-SMA and fibronectin [104]. Synergistic effects between RXR ligands and PPAR ligands have not yet been reported in lung fibroblasts in vitro or in animal models of lung fibrosis, though this is under investigation.
7. CONCLUSION
Although the role of the PPARs in fibrosing diseases has been less well studied than their role in regulating inflammation, a number of key results have emerged. PPARγ agonists inhibit the differentiation of lung fibroblasts to myofibroblasts in vitro, and also inhibit airway remodeling and fibrosis in animal models [77, 79]. PPARα agonists also attenuated fibrosis in the mouse bleomycin model, while PPARα knockout mice developed more severe disease [50].
Our understanding of the role of PPARs in lung fibrosis is hindered by the relative lack of experiments directly involving the lung or lung cells. However, progress has also been made toward determining the role of the PPARs in fibrosing diseases of the liver, kidney, and pancreas. Hepatic stellate cells and pancreatic stellate cells differentiate to myofibroblast-like cells under the same stimulus as lung fibroblasts, and this differentiation is inhibited by both natural and synthetic PPARγ ligands [83–85]. The TZD class of PPARγ agonists is effective in reducing liver, cardiac, and kidney fibrosis in rats and mice [44, 45, 72]. PPARα agonists, including the fibrate drugs, have also shown promise in attenuating liver, kidney, and cardiac fibrosis [40, 43, 45].
The mechanisms by which PPAR ligands alter fibrosis are not well understood, but appear to involve multiple regulatory pathways (see Figure 3). Natural and synthetic PPARγ agonists inhibit TGF-β-driven myofibroblast differentiation and activation in hepatic stellate cells, kidney fibroblasts, and lung fibroblasts. In human hepatic stellate cells, the PPARγ agonist GW7845 inhibited Smad3 phosphorylation and nuclear translocation [88], while a similar result was seen with 15d-PGJ2 in rat kidney fibroblasts [84]. However, 15d-PGJ2 did not alter Smad2 phosphorylation in human lung fibroblasts [79] or human renal mesangial cells, but instead upregulated HGF and TGIF [84]. It is likely that the precise mechanism of action of PPARγ ligands varies depending on the cell type and agonist used. A further complication is that PPARγ agonists appear to have PPARγ-independent effects. Further studies using pharmaceutical inhibitors of PPARγ or PPARγ knockout cell lines may prove useful in further investigations.
Figure 3.
Key concepts in the regulation of fibrosis by PPARs.
A very intriguing recent report found that 15d-PGJ2 altered transcriptional activity of the estrogen receptor by covalent modification of cysteine residues in its zinc finger DNA-binding domain [98]. Since cysteine is a ready target of covalent modification by 15d-PGJ2 [95, 96] and many transcription factors use cysteine-rich zinc finger DNA-binding domains, this suggests that one possible mechanism by which PPARγ ligands can affect the regulation of cell differentiation independently of PPARγ itself is via modification of other transcription factors.
There are less data available on the mechanism of action of PPARα and β/δ agonists. Although PPARα agonists attenuate animal preclinical fibrosis models, studies of the direct effect of PPARα ligands on myofibroblast activation have not been reported. Treprostinil inhibition of lung fibroblast proliferation is PPARβ/δ-dependent [55], and PPARβ/δ also appears to play a role in keratinocyte maturation and function [53]. It has been hypothesized that fibrosis is a consequence of dysregulated wound healing and tissue remodeling following an initial injury [54]. This may provide the mechanistic link between PPARα and β/δ and fibrosis. Rather than directly acting on fibroblasts and myofibroblasts, PPARα may regulate inflammation, while PPARβ/δ regulates the transition from inflammation to wound healing [54, 105]. Thus, PPARα and β/δ agonists may ameliorate fibrosis by altering the initial inflammatory response and the transition to a fibrogenic milieu, respectively.
The relationship between the PPARs and fibrosis is likely to be complex. As discussed above, PPARα and PPARγ are involved in regulating both inflammation and fibrosis, and some ligands have affinity for more than one PPAR. In addition, because RXR is the obligate dimerization partner for all three PPARs, modulating RXR activity may have multiple overlapping or even conflicting effects. A number of useful tools exist to study these relationships, including highly specific synthetic agonists and antagonists, dominant negative expression constructs, and germline and conditional gene knockouts. Each of these approaches has potential advantages and drawbacks. In particular, genetic ablation of PPAR genes will eliminate their function from both inflammatory and repair processes, making it difficult to determine their role in each process independently. This problem can be addressed by using multiple complimentary approaches to examine PPAR function in both normal and abnormal wound repair and fibrosis.
It must be emphasized that important classes of PPARα (the fibrate drugs) and PPARγ (TZDs) agonists are currently available in the clinic. Although the frequency of lung fibrosis in the general population is not high, it may be possible to perform retrospective studies of long-term users of TZDs and fibrates to determine whether these drugs reduce the incidence or severity of lung fibrosis and other fibrosing diseases. More importantly, the clinical availability of these drugs means that significant results from animal studies of fibrosis models may be rapidly applied in the clinical setting. Recent advances in drug delivery by inhalation may allow delivery of antifibrotic PPAR agonists directly to the site of fibrosis (as has already been demonstrated with the use of ciglitazone in a mouse model of airway remodeling [68]), achieving higher effective doses at the target site with lower systemic side effects. As most forms of lung fibrosis are refractory to current treatment, the rapid translation of basic research to bedside practice holds great promise for a patient population suffering from a largely untreatable disease.
ACKNOWLEDGMENTS
This work was supported in part by HL-04492, HL-75432l, the James P. Wilmot Foundation, The National Institute of Environmental Health Sciences Center Grant ES-01247, National Institute of Environmental Health Sciences Training Grant ES-07026, and The Connor Fund. R. P. Phipps was supported by DE-011390, HL-078603, and HL-086367. T. H. Thatcher was supported in part by a postdoctoral fellowship from Philip Morris, USA.
References
- 1.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annual Review of Medicine. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 2.Sime PJ, O'Reilly KMA. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clinical Immunology. 2001;99(3):308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 3.Geiser T. Idiopathic pulmonary fibrosis—a disorder of alveolar wound repair? Swiss Medical Weekly. 2003;133(29-30):405–411. doi: 10.4414/smw.2003.09986. [DOI] [PubMed] [Google Scholar]
- 4.Fujimura N. Pathology and pathophysiology of pneumoconiosis. Current Opinion in Pulmonary Medicine. 2000;6(2):140–144. doi: 10.1097/00063198-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. American Journal of Respiratory and Critical Care Medicine. 1998;157(5, part 1):1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 6.Zisman DA, Keane MP, Belperio JA, Strieter RM, Lynch JP., III Pulmonary fibrosis. Methods in Molecular Medicine. 2005;117:3–44. doi: 10.1385/1-59259-940-0:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner KB, Samet JM, Coultas DB, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. American Journal of Epidemiology. 2000;152(4):307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 9.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. Journal of Pathology. 2003;201(3):343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vuyst P, Camus P. The past and present of pneumoconioses. Current Opinion in Pulmonary Medicine. 2000;6(2):151–156. doi: 10.1097/00063198-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Collard HR, Ryu JH, Douglas WW, et al. Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest. 2004;125(6):2169–2174. doi: 10.1378/chest.125.6.2169. [DOI] [PubMed] [Google Scholar]
- 12.Swigris JJ, Kuschner WG, Kelsey JL, Gould MK. Idiopathic pulmonary fibrosis: challenges and opportunities for the clinician and investigator. Chest. 2005;127(1):275–283. doi: 10.1378/chest.127.1.275. [DOI] [PubMed] [Google Scholar]
- 13.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proceedings of the American Thoracic Society. 2006;3(4):285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(supplement 6):286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 15.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. American Journal of Pathology. 1997;151(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-β1 promotes the morphological and functional differentiation of the myofibroblast. Experimental Cell Research. 2000;257(1):180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 17.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. Journal of Pathology. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 18.Phan SH, Zhang K, Zhang HY, Gharaee-Kermani M. The myofibroblast as an inflammatory cell in pulmonary fibrosis. Current Topics in Pathology. 1999;93:173–182. doi: 10.1007/978-3-642-58456-5_18. [DOI] [PubMed] [Google Scholar]
- 19.Selman M, King TE, Jr, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Annals of Internal Medicine. 2001;134(2):136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 20.Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respiratory Research. 2001;3:1. doi: 10.1186/rr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartram U, Speer CP. The role of transforming growth factor β in lung development and disease. Chest. 2004;125(2):754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 22.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Current Pharmaceutical Design. 2003;9(1):39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H-Y, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor β 1 . American Journal of Respiratory Cell and Molecular Biology. 1999;21(6):658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 24.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor- β 1 induces prolonged severe fibrosis in rat lung. Journal of Clinical Investigation. 1997;100(4):768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. Journal of Steroid Biochemistry and Molecular Biology. 2003;85(2–5):267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 26.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 27.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 28.Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator-activated receptor γ (PPARγ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends in Immunology. 2002;23(3):144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 30.Padilla J, Leung E, Phipps RP. Human B lymphocytes and B lymphomas express PPAR-γ and are killed by PPAR-γ agonists. Clinical Immunology. 2002;103(1):22–33. doi: 10.1006/clim.2001.5181. [DOI] [PubMed] [Google Scholar]
- 31.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARγ, and PPARγ agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104(5):1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 32.Simon DM, Arikan MC, Srisuma S, et al. Epithelial cell PPARγ is an endogenous regulator of normal lung maturation and maintenance. Proceedings of the American Thoracic Society. 2006;3(6):510–511. doi: 10.1513/pats.200603-034MS. [DOI] [PubMed] [Google Scholar]
- 33.Simon DM, Arikan MC, Srisuma S, et al. Epithelial cell PPARγ contributes to normal lung maturation. The FASEB Journal. 2006;20(9):1507–1509. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- 34.Marian E, Baraldo S, Visentin A, et al. Up-regulated membrane and nuclear leukotriene B4 receptors in COPD. Chest. 2006;129(6):1523–1530. doi: 10.1378/chest.129.6.1523. [DOI] [PubMed] [Google Scholar]
- 35.Culver DA, Barna BP, Raychaudhuri B, et al. Peroxisome proliferator-activated receptor γ activity is deficient in alveolar macrophages in pulmonary sarcoidosis. American Journal of Respiratory Cell and Molecular Biology. 2004;30(1):1–5. doi: 10.1165/rcmb.2003-0304RC. [DOI] [PubMed] [Google Scholar]
- 36.Bonfield TL, Farver CF, Barna BP, et al. Peroxisome proliferator-activated receptor-γ is deficient in alveolar macrophages from patients with alveolar proteinosis. American Journal of Respiratory Cell and Molecular Biology. 2003;29(6):677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Current Opinion in Pharmacology. 2006;6(4):421–427. doi: 10.1016/j.coph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Sher T, Yi H-F, McBride OW, Gonzalez FJ. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32(21):5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- 39.Cuzzocrea S. Peroxisome proliferator-activated receptors and acute lung injury. Current Opinion in Pharmacology. 2006;6(3):263–270. doi: 10.1016/j.coph.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Toyama T, Nakamura H, Harano Y, et al. PPARα ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochemical and Biophysical Research Communications. 2004;324(2):697–704. doi: 10.1016/j.bbrc.2004.09.110. [DOI] [PubMed] [Google Scholar]
- 41.Svegliati-Baroni G, Candelaresi C, Saccomanno S, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-α and n-3 polyunsaturated fatty acid treatment on liver injury. American Journal of Pathology. 2006;169(3):846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39(5):1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 43.Ogata T, Miyauchi T, Sakai S, Irukayama-Tomobe Y, Goto K, Yamaguchi I. Stimulation of peroxisome-proliferator-activated receptor α (PPARα) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clinical Science. 2002;103(supplement 48):284S–288S. doi: 10.1042/CS103S284S. [DOI] [PubMed] [Google Scholar]
- 44.Iglarz M, Touyz RM, Viel EC, et al. Peroxisome proliferator-activated receptor-α and receptor-γ activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension. 2003;42(4):737–743. doi: 10.1161/01.HYP.0000083511.91817.B1. [DOI] [PubMed] [Google Scholar]
- 45.Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrology Dialysis Transplantation. 2006;21(9):2399–2405. doi: 10.1093/ndt/gfl212. [DOI] [PubMed] [Google Scholar]
- 46.Reynders V, Loitsch S, Steinhauer C, Wagner T, Steinhilber D, Bargon J. Peroxisome proliferator-activated receptor α (PPARα) down-regulation in cystic fibrosis lymphocytes. Respiratory Research. 2006;7:104. doi: 10.1186/1465-9921-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuzzocrea S, Mazzon E, Di Paola R, et al. The role of the peroxisome proliferator-activated receptor-α (PPAR-α) in the regulation of acute inflammation. Journal of Leukocyte Biology. 2006;79(5):999–1010. doi: 10.1189/jlb.0605341. [DOI] [PubMed] [Google Scholar]
- 48.Thatcher TH, Sime PJ, Barth RK. Sensitivity to bleomycin-induced lung injury is not moderated by an antigen-limited T-cell repertoire. Experimental Lung Research. 2005;31(7):685–700. doi: 10.1080/01902140591007218. [DOI] [PubMed] [Google Scholar]
- 49.Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Laboratory Investigation. 1984;50(5):487–488. [PubMed] [Google Scholar]
- 50.Genovese T, Mazzon E, Di Paola R, et al. Role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor α in the development of bleomycin-induced lung injury. Shock. 2006;24(6):547–555. doi: 10.1097/01.shk.0000190825.28783.a4. [DOI] [PubMed] [Google Scholar]
- 51.Dyrøy E, Yndestad A, Ueland T, et al. Antiinflammatory effects of tetradecylthioacetic acid involve both peroxisome proliferator-activated receptor α-dependent and -independent pathways. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(7):1364–1369. doi: 10.1161/01.ATV.0000171982.57713.96. [DOI] [PubMed] [Google Scholar]
- 52.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. Journal of Biological Chemistry. 1997;272(6):3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 53.Michalik L, Desvergne B, Tan NS, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. Journal of Cell Biology. 2001;154(4):799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan NS, Michalik L, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor-β as a target for wound healing drugs. Expert Opinion on Therapeutic Targets. 2004;8(1):39–48. doi: 10.1517/14728222.8.1.39. [DOI] [PubMed] [Google Scholar]
- 55.Ali FY, Egan K, FitzGerald GA, et al. Role of prostacyclin versus peroxisome proliferator-activated receptor β receptors in prostacyclin sensing by lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology. 2006;34(2):242–246. doi: 10.1165/rcmb.2005-0289OC. [DOI] [PubMed] [Google Scholar]
- 56.Matsusue K, Peters JM, Gonzalez FJ. PPARβ/δ potentiates PPARγ-stimulated adipocyte differentiation. The FASEB Journal. 2004;18(12):1477–1479. doi: 10.1096/fj.04-1944fje. [DOI] [PubMed] [Google Scholar]
- 57.Huang TH-W, Razmovski-Naumovski V, Kota BP, Lin DS-H, Roufogalis BD. The pathophysiological function of peroxisome proliferator-activated receptor-γ in lung-related diseases. Respiratory Research. 2005;6:102. doi: 10.1186/1465-9921-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-Δ12,14 -prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 59.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 60.McIntyre TM, Pontsler AV, Silva AR, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schopfer FJ, Lin Y, Baker PRS, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 63.Place AE, Suh N, Williams CR, et al. The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clinical Cancer Research. 2003;9(7):2798–2806. [PubMed] [Google Scholar]
- 64.Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor γ agonists as therapy for chronic airway inflammation. European Journal of Pharmacology. 2006;533(1–3):101–109. doi: 10.1016/j.ejphar.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 65.Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor γ, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflammation Research. 2005;54(11):464–470. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- 66.Inoue K-I, Takano H, Yanagisawa R, et al. Effect of 15-deoxy-Δ12,14-prostaglandin J2 on acute lung injury induced by lipopolysaccharide in mice. European Journal of Pharmacology. 2003;481(2-3):261–269. doi: 10.1016/j.ejphar.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Ward JE, Fernandes DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARγ ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulmonary Pharmacology and Therapeutics. 2006;19(1):39–46. doi: 10.1016/j.pupt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Honda K, Marquillies P, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptor γ is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. Journal of Allergy and Clinical Immunology. 2004;113(5):882–888. doi: 10.1016/j.jaci.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 69.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 70.Wang ACC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-γ regulates airway epithelial cell activation. American Journal of Respiratory Cell and Molecular Biology. 2001;24(6):688–693. doi: 10.1165/ajrcmb.24.6.4376. [DOI] [PubMed] [Google Scholar]
- 71.Feldon SE, O'Loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. American Journal of Pathology. 2006;169(4):1183–1193. doi: 10.2353/ajpath.2006.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leclercq IA, Sempoux C, Stärkel P, Horsmans Y. Limited therapeutic efficacy of pioglitazone on progression of hepatic fibrosis in rats. Gut. 2006;55(7):1020–1029. doi: 10.1136/gut.2005.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochemical and Biophysical Research Communications. 2004;315(1):187–195. doi: 10.1016/j.bbrc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 74.Uto H, Nakanishi C, Ido A, et al. The peroxisome proliferator-activated receptor-γ agonist, pioglitazone, inhibits fat accumulation and fibrosis in the livers of rats fed a choline-deficient, L-amino acid-defined diet. Hepatology Research. 2005;32(4):235–242. doi: 10.1016/j.hepres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Buckingham RE. Thiazolidinediones: pleiotropic drugs with potent anti-inflammatory properties for tissue protection. Hepatology Research. 2005;33(2):167–170. doi: 10.1016/j.hepres.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 76.Lansang MC, Coletti C, Ahmed S, Gordon MS, Hollenberg NK. Effects of the PPAR-γ agonist rosiglitazone on renal haemodynamics and the renin-angiotensin system in diabetes. Journal of the Renin-Angiotensin-Aldosterone System. 2006;7(3):175–180. doi: 10.3317/jraas.2006.028. [DOI] [PubMed] [Google Scholar]
- 77.Genovese T, Cuzzocrea S, Di Paola R, et al. Effect of rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2 on bleomycin-induced lung injury. European Respiratory Journal. 2005;25(2):225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- 78.Barak Y, Nelson MC, Ong ES, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 79.Burgess HA, Daugherty LE, Thatcher TH, et al. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. American Journal of Physiology. 2005;288(6):L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 80.Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. Journal of Biological Chemistry. 2000;275(46):35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 81.Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Archiv. 1997;430(3):195–207. doi: 10.1007/BF01324802. [DOI] [PubMed] [Google Scholar]
- 82.Bissell DM. Connective tissue metabolism and hepatic fibrosis: an overview. Seminars in Liver Disease. 1990;10(1):iii–iv. doi: 10.1055/s-2008-1040491. [DOI] [PubMed] [Google Scholar]
- 83.Marra F, Efsen E, Romanelli RG, et al. Ligands of peroxisome proliferator-activated receptor γ modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119(2):466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Wen X, Spataro BC, Hu K, Dai C, Liu Y. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-γ agonists. Journal of the American Society of Nephrology. 2006;17(1):54–65. doi: 10.1681/ASN.2005030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masamune A, Satoh K, Sakai Y, Yoshida M, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-γ induce apoptosis in AR42J cells. Pancreas. 2002;24(2):130–138. doi: 10.1097/00006676-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. Journal of the American Society of Nephrology. 2005;16(3):638–645. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- 87.Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARγ agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. American Journal of Physiology. 2005;289(5):F1153–F1158. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]
- 88.Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochemical and Biophysical Research Communications. 2006;350(2):385–391. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SJ, Yang EK, Kim SG. Peroxisome proliferator-activated receptor-γ and retinoic acid X receptor α represses the TGFβ1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: role for Zf9 dephosphorylation. Molecular Pharmacology. 2006;70(1):415–425. doi: 10.1124/mol.106.022954. [DOI] [PubMed] [Google Scholar]
- 90.Teresi RE, Shaiu C-W, Chen C-S, Chatterjee VK, Waite KA, Eng C. Increased PTEN expression due to transcriptional activation of PPARγ by Lovastatin and Rosiglitazone. International Journal of Cancer. 2006;118(10):2390–2398. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- 91.White ES, Atrasz RG, Hu B, et al. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome 10) American Journal of Respiratory and Critical Care Medicine. 2006;173(1):112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.White ES, Thannickal VJ, Carskadon SL, et al. Integrin α4β1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. American Journal of Respiratory and Critical Care Medicine. 2003;168(4):436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) α expression by C/EBPβ during adipogenesis requires a peroxisome proliferator-activated receptor-γ-associated repression of HDAC1 at the C/ebpα gene promoter. Journal of Biological Chemistry. 2006;281(12):7960–7967. doi: 10.1074/jbc.M510682200. [DOI] [PubMed] [Google Scholar]
- 94.Chung SS, Choi HH, Cho YM, Lee HK, Park KS. Sp1 mediates repression of the resistin gene by PPARγ agonists in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2006;348(1):253–258. doi: 10.1016/j.bbrc.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 95.Atsmon J, Sweetman BJ, Baertschi SW, Harris TM, Roberts LJ., II Formation of thiol conjugates of 9-deoxy-Δ9 ,Δ12(E)-prostaglandin D2 and Δ12 (E)-prostaglandin D2 . Biochemistry. 1990;29(15):3760–3765. doi: 10.1021/bi00467a023. [DOI] [PubMed] [Google Scholar]
- 96.Shibata T, Yamada T, Ishii T, et al. Thioredoxin as a molecular target of cyclopentenone prostaglandins. Journal of Biological Chemistry. 2003;278(28):26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- 97.Shimizu K, Shiratori K, Kobayashi M, Kawamata H. Troglitazone inhibits the progression of chronic pancreatitis and the profibrogenic activity of pancreatic stellate cells via a PPARγ- independent mechanism. Pancreas. 2004;29(1):67–74. doi: 10.1097/00006676-200407000-00058. [DOI] [PubMed] [Google Scholar]
- 98.Kim H-J, Kim J-Y, Meng Z, et al. 15-deoxy-Δ12,14-prostaglandin J2 inhibits transcriptional activity of estrogen receptor-α via covalent modification of DNA-binding domain. Cancer Research. 2007;67(6):2595–2602. doi: 10.1158/0008-5472.CAN-06-3043. [DOI] [PubMed] [Google Scholar]
- 99.Issemann I, Prince RA, Tugwood JD, Green S. The peroxisome proliferator-activated receptor: retinoid X receptor heterodimer is activated by fatty acids and fibrate hypolipidaemic drugs. Journal of Molecular Endocrinology. 1993;11(1):37–47. doi: 10.1677/jme.0.0110037. [DOI] [PubMed] [Google Scholar]
- 100.Ahuja HS, Szanto A, Nagy L, Davies PJA. The retinoid X receptor and its ligands: versatile regulators of metabolic function, cell differentiation and cell death. Journal of Biological Regulators and Homeostatic Agents. 2003;17(1):29–45. [PubMed] [Google Scholar]
- 101.Germain P, Chambon P, Eichele G, et al. International union of pharmacology. LXIII. Retinoid X receptors. Pharmacological Reviews. 2006;58(4):760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 102.Hellemans K, Verbuyst P, Quartier E, et al. Differential modulation of rat hepatic stellate phenotype by natural and synthetic retinoids. Hepatology. 2004;39(1):97–108. doi: 10.1002/hep.20015. [DOI] [PubMed] [Google Scholar]
- 103.Ohata M, Lin M, Satre M, Tsukamoto H. Diminished retinoic acid signaling in hepatic stellate cells in cholestatic liver fibrosis. American Journal of Physiology. 1997;272(3, part 1):G589–G596. doi: 10.1152/ajpgi.1997.272.3.G589. [DOI] [PubMed] [Google Scholar]
- 104.Wen X, Li Y, Hu K, Dai C, Liu Y. Hepatocyte growth factor receptor signaling mediates the anti-fibrotic action of 9-cis-retinoic acid in glomerular mesangial cells. American Journal of Pathology. 2005;167(4):947–957. doi: 10.1016/S0002-9440(10)61185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chinetti G, Fruchart J-C, Staels B. Peroxisome proliferator-activated receptors and inflammation: from basic science to clinical applications. International Journal of Obesity and Related Metabolic Disorders. 2003;27(supplement 3):S41–S45. doi: 10.1038/sj.ijo.0802499. [DOI] [PubMed] [Google Scholar]