Abstract
Diabetic retinopathy is a leading cause of vision loss. The primary clinical hallmarks are vascular changes that appear to contribute to the loss of sight. In a number of neurodegenerative disorders there is an appreciation that increased levels of excitatory amino acids are excitotoxic. The primary amino acid responsible appears to be the neurotransmitter glutamate. This review examines the nature of glutamatergic signaling at the retina and the growing evidence from clinical and animal model studies that glutamate may be playing similar excitotoxic roles at the diabetic retina.
1. INTRODUCTION
Diabetic retinopathy causes 12,000–24,000 new cases of blindness each year in the United States alone and is the leading cause of blindness in persons between the ages of 20–74 [1]. The incidence is increasing as the number of persons with diabetes mellitus rises. In 2005, it was estimated that 7% of the population in the United States, 20.8 million people, had diabetes mellitus [1]. Diabetic retinopathy is a major cause of disability with an estimated 40% of adults over the age of 40 years having some form of diabetic retinopathy and 8% having vision-impairing diabetic retinopathy [2]. The prevalence of diabetic retinopathy differs with racial characteristics with 50% of adult Hispanics with diabetes mellitus having some form of diabetic retinopathy [3].
Risk factors for the development of diabetic retinopathy have been well described and can be divided into systemic factors and local factors. The systemic factors include duration of diabetes, severity of diabetes as measured by hemoglobin A1c, hypertension, anemia, renal disease, and lipid levels [4]. The local protective factors include myopia, the presence of chorioretinal scars, and optic atrophy while local aggravating factors include inflammation and prior ischemia [5, 6]. Diabetic retinopathy is caused by an ischemic microvasculopathy and it is divided into nonproliferative and proliferative forms [7]. Both of these forms are presaged on damage to the capillaries and then the secondary response to the damage. In the nonproliferative form, there is evidence of leakage from the capillaries as well as drop out of capillaries. The leakage is manifest as swelling of the retina and deposition of lipoproteinaceous material in the retina (hard exudates) as well as microaneurysmal sacculations of the capillaries and intraretinal hemorrhages [8]. Following the loss of capillaries, there is a hypoxic response by the retina with release of vascular endothelial growth factor (VEGF) [9]. VEGF is in part what causes leakage from the remaining capillaries. It is important also to note that the normal retinal circulation is under autoregulation [10]. There is a compensatory increase or decrease in flow in the retinal circulation depending upon the physiologic demands from the retina. This autoregulation may be driven by local nitric oxide production [11]. What drives the autoregulation is at the present time speculative but may be intrinsically related to the excitatory amino acids because nitric oxide is associated with excitatory amino acids as will be discussed later [12]. Another aspect of diabetic retinopathy that is poorly recognized and occurs because of autoregulation is a compensatory increase in the flow through the remaining vessels [10].
With ischemia, there is a significant release of VEGF, which causes secondary growth of neovascular tissue on the surface of the retina. This neovascular tissue is comprised of very immature vessels that leaks further and bleeds readily. This is an important cause of visual loss. Ultimately, the vessels cause a secondary fibrotic response as well and this causes scarring on the surface of the retina.
Besides VEGF, there may be a panoply of other factors that may be associated with the changes noted. These factors may be proteins, peptides, and small molecules [13]. There is a marked increase in the number of proteins seen in the vitreous in both experimental as well as in clinical diabetic retinopathy [13, 14].
The vitreous is also affected by diabetes and the changed vitreous is involved in the development of diabetic retinop athy [15, 16]. The vitreous contracts probably because of nonenzymatic glycosylation [17]. The contraction of the vitreous then allows growth of the neovascular tissue onto its posterior surface and also causes the tissue to bleed.
2. EXCITATORY AMINO ACIDS
Amino acids or their metabolic products have been shown to be neurotransmitters [18]. Olney was the first to recognize that a group of these amino acids were excitatory [19, 20]. He labelled them as excitatory because the released amino acids cause rapid depolarization of glutamate sensitive cells. The number of amino acids that have been designated as excitatory has grown since Olney's initial studies and include glutamate, glycine, aspartate. Glutamate is a critical excitatory amino acid in the brain and the most important excitatory amino acid in the retina.
The entries in Table 1 show the types and diversity of glutamate receptors. There are two classes of glutamate receptors, ionotropic and metabotropic. The ionotropic receptors work via ion channels. The metabotropic receptors are G-protein coupled receptors. There are three subclasses of ionotropic receptors: N-methyl-D-aspartate (NMDA), amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), and kainate type receptors. The NMDA receptors are the ones that are most associated with excitatory neurotoxicity and calcium entry into the cells. The calcium entry causes release of caspases from the mitochondria leading to apoptosis. NMDA receptors are made up of 3 different subunits, NR1, NR2A-D, and, in some cases, NR3A or B subunits. The receptor is probably composed of a tetramer of these subunits. Alternative splicing further helps in adding pharmacologic differences to the action of the receptors. There is a diversity of NMDA receptor types in different regions of the central nervous system.
Table 1.
Glutamate Receptors.
| Isoforms | Glutamate Response | ||
|
| |||
| Ionotropic | NMDA | NR1, NR2A-D and NR3A-B | Increase Ca2+/Na+ intracellular influx |
| AMPA | GluR1, GluR2, GluR3, and GluR4 | Increase Na+/(Ca2+) intracellular influx | |
| Kainate | GluR5, GluR6, GluR7, KA1, and KA2 | Increase Na+/(Ca2+) intracellular influx | |
|
| |||
| Metabotropic | Type I | mGluR1, mGluR5 | Increase intracellular inositol phosphate and diacylglycerol |
| Type II | mGluR2, mGluR3 | Decrease intracellular cyclic adenosine monophosphate | |
| Type III | mGluR4, mGluR6, mGluR7, and mGluR8 | Modulate intracellular cyclic adenosine monophosphate | |
There are at least eight metabotropic glutamate receptors (mGluR). These are subdivided into three subclasses. Type I metabotropic receptors are associated with intracellular phosphotidyl inositol metabolism. Type II and III receptors are associated with an inhibitory cAMP cascade as well as other postsynaptic cascades that lead to the release of Ca2+ from intracellular stores. There is some data to suggest that some of the type II mGluRs are neuroprotective.
There is a relationship between the metabolism of glutamate, glutamine, and GABA. GABA is a synthesized in the presynatic axons of certain neurons via the use of glutamate decarboxylase. It is the major inhibitory neurotransmitter in the brain and retina.
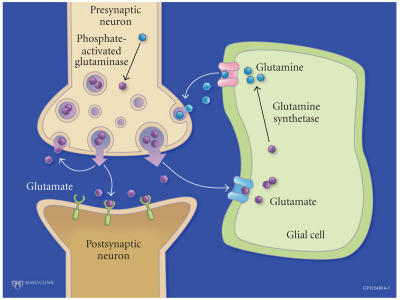
Because of the significant neurotoxic effects of glutamate, glutamate concentrations have to be very closely regulated in the synapse. Much of the released glutamate is taken up by the surrounding glial cells and converted into glutamine. The glutamine is then taken up by the presynaptic axon. Glutamine is deaminated and turned back into glutamate (Figure 1). Direct glutamate reuptake by the presynaptic neuron accounts for a small amount of the released glutamate. Another small amount actually escapes from the synaptic space and may have significant peripheral effects [21]. The amount that escapes appears to increase in pathologic conditions.
Figure 1.
Glutamate cycle in the synaptic space.
For glutamate, there are five known high-affinity excitatory amino acid transporters: EAAT1 (GLAST), EAAT2 (GLT-1), EAAT3 (EAAC1), EAAT4, and EAAT5 [22–26]. The transporters and their predominant locations are shown in Table 2. Uptake of glutamate into astrocytes is mediated by GLAST (also found in Müller cells) and GLT1 (or EAAT1 and 2) and into neurons by EAAC1 (or EAAT3), EAAT4, and EAAT5, of which the last primarily is found in the retinal photoreceptor cells. In addition, there are glutamine transporters that need to be synchronized to transport glutamine from the astrocytes into the neurons (Figure 1).
Table 2.
Glutamate Transport Proteins.
| Predominant Retinal Localization | ||
|
| ||
| Glial | EAAT1, GLAST | Müller cells and astroglia |
| EAAT2, GLT1 | Bipolar and cone cells | |
|
| ||
| Neuronal | EAAT3, EAAC1 | Horizontal, amacrine, ganglion, and bipolar cells |
| EAAT4 | ||
| EAAT5 | Photoreceptor and bipolar cells | |
2.1. Excitatory amino acid and disease states
Recently, there has been a growing appreciation that disruption of this cycle leads to glutamate levels that may fall outside normal ranges and lead to tissue dysfunction and neuronal death [27]. This process has been implicated in hepatic failure-associated CNS dysfunction [28], HIV-associated dementia [29], ischemia [30], Alzheimer's [31], and Huntington's disease [32]. The detrimental effects of excess glutamate has largely been related to iontropic receptor overactivation [27, 30–35]. Treatments that have been proposed and tested then are aimed at interfering with these receptors though there is now data that implicates the metobotropic receptors for damage to the postsynaptic cells as well [27, 31–33]. With hepatic failure, there is an increase in ammonia. The ammonia increase causes a downregulation of GLT1 causing an accumulation of extracellular excitatory glutamate. This is thought to be one of the leading causes of the CNS derangements seen with hepatic failure [28]. Following ischemic stroke, there is a marked increase in extracellular glutamate as well [36]. Both clinical and animal studies have demonstrated an association between the increase in glutamate levels with worsening neurological deficits. How the increase of glutamate occurs, whether it is excessive release, poor reuptake or a problem in glutamate-glutamine cycling is still unknown. Hypoglycemia has also been associated with glutamate neurotoxicity in the central nervous system. Hypoglycemia has also been associated with glutamate neurotoxicity in the central nervous system [37]. This same neurotoxicity has also been reproduced by using iodoacetate which inhibits glycolysis. The neurotoxicity induced by hypoglycemia is inhibited by both glutamate inhibitors as well as pyruvate presumably by allowing progression of oxidative phosphorylation [38]. In vivo, potentiation of glutamate-mediated neuronal damage after chronic administration of the glycolysis inhibitor iodoacetate [38]. Hyperglycemia has not been associated with glutamate neurotoxicity in the central nervous system.
2.2. NMDA antagonists
MK-801 was the first NMDA antagonist that was used but clinically it was associated with coma and delirium. Amantadine, which was originally developed to interfere with influenza virus uptake and was then used in patients with Parkinson's disease, was noted to have NMDA receptor antagonism. Memantine, a derivative of amantidine, is a more efficacious NMDA receptor antagonist and has been approved for the treatment of Alzheimer's disease and is being investigated for possible use in other diseases including vascular dementia, neuropathic pain, and glaucoma [27].
2.3. Excitatory amino acids and the retina
The initial studies by Dreyer showing that glutamate was elevated in glaucoma were subsequently brought into question [39]. Subsequent studies by ourselves in this issue and others have shown that glutamate is elevated in the vitreous in glaucoma, diabetes mellitus, and retinal detachments and thus, similar to ischemic brain injury, glutamate may play an important role in these diseases [40–42].
Among the five excitatory amino acid transporters identified to date, four are found in the retina. EAAT1 (GLAST) is found in Müller cells and astrocytes [43]. EAAT2 (GLT1) is localized to cones and two types of bipolar cells [44]. EAAT3 (EAAC1) is found on horizontal, amacrine, and ganglion cells, and occasionally on bipolar cells [45]. EAAT5 is localized to photoreceptors and bipolar cells [46].
Glutamate receptors are present throughout the retina. The Müller cells in the retina act as the astrocytes in the brain. They have GLAST expression and are important in removing glutamate from the synaptic space of the retina [47]. GLAST1a is also expressed. In this form, exon 3 is not expressed which decreases the efficacy of glutamate uptake. This may be a method of regulating the efficacy of Müller cell glutamate uptake [48].
Bipolar cells have kainate, AMPA receptors, and NMDA receptors [21, 49]. For all bipolar cell types, the AMPA receptor subunits GluR2, 2/3, and 4 are the most common types while GluR1 are rare. The kainate GluR6/7 are predominantly associated with diffuse bipolar (DB6) and rod bipolar cells. The NMDA receptor, NR1C2, is seen in flat midget and DB3 axons. The kainate receptors are seen primarily in dendrites of off-bipolar cells associated with cone axons [21]. Horizontal receptors also have AMPA type receptors [50]. On-bipolar cells appear to use metabotropic receptors [51]. In the inner retina, glutamate receptors are present in the ganglion dendrites and amacrine cells. Metabotropic type II receptors are present on certain amacrine cells [52].
2.4. Retinal diseases associated with elevated glutamate levels in the vitreous
2.4.1. Glaucoma
It has been suggested that increased extracellular glutamate levels may be due, at least in part, to a failure of glutamate transporter buffering [53]. The data has to be carefully evaluated to look at the acute changes and the chronic changes following injury. For instance, following crush injury to the optic nerve, there appears to be an acute increase in GLT1 expression followed by a decrease to near normal levels over time [54].
In a chronic glaucoma model, both ionotropic and metabotropic antagonists appeared to be effective in limiting damage from glaucoma [55]. The NMDA antagonists appeared to have a greater effect in limiting damage than the metabotropic antagonists but this study shows that metabotropic receptors may be important in glutamate-induced damage as well. Previous studies using immunohistochemistry have demonstrated a reduced expression of EAAT1 in a rat glaucoma model [56]. It has been shown in rats that treatment with antisense-oligonucleotides to GLT-1 leads to increased vitreous glutamate levels and RGC death [33]. In addition, in human glaucoma the expression of this glutamate transporter is reduced at the protein level [57]. Following experimental glaucoma induction in rats, with subsequent optic nerve damage, Martin et al. [56] recently found no change in the expression of GLT1 by immunohistochemistry. However, Western blot analysis revealed a significant decrease in the levels of GLT1 protein. Interestingly, following optic nerve transsection, which leads to extensive RGC death, the GLT1 protein levels were found to be increased in this study [56].
As mentioned, the relationship between glaucoma and glutamate has been questioned. In fact, a recent article showed no relationship between glutatmate levels and glaucoma in persons with glaucoma requiring vitrectomy [58]. This is different from animal models and further human studies are required.
2.4.2. Retinal detachments and diabetic retinopathy
In clinical studies, there are mildly elevated levels of glutamate in the vitreous with rhegmatogenous retinal detachments [41] and markedly elevated in cases with diabetic retinopathy [40] though the number of studies is very limited and further studies are definitely warranted to confirm these findings. The levels of glutamate in the vitreous of patients with rhegmatogenous retinal detachment not associated with diabetic retinopathy was 25% higher than the glutamate levels in the vitreous of patients who underwent vitrectomies for other causes [59]. Rhegamtogenous retinal detachments are associated with mild retinal ischemia since neovascularization is rarely seen with rhegmatogenous retinal detachments and that may be the reason the glutamate levels were slightly elevated. The cases of diabetic retinopathy that have been associated with elevated glutamate levels were cases with proliferative diabetic retinopathy. In the study, Ambati and colleagues evaluated levels of glutamate in eyes with proliferative diabetic retinopathy and compared the levels to controls who underwent vitrectomies for other causes. They controlled for hemorrhage in the eyes with proliferative diabetic retinopathy by determining hemoglobin levels. Even controlling for elevated hemoglobin levels in the vitreous (as a marker for vitreous hemorrhage), the glutamate levels were at least twice as high as control eyes. Deng et al. also found elevated vitreous levels of glutamate in cases of proliferative diabetic retinopathy [60]. In this journal, our group using capillary electrophoresis, a low volume-sensitive method reports elevated levels of glutamate as well. On the contrary, Asensio Sanchez and his coauthors did not find elevated glutamate levels in the vitreous of patients with diabetes in comparison to their control patients [61]. Overall, there are more studies showing elevation in glutamate levels with proliferative diabetic retinopathy than studies showing no differences but further studies are needed in humans and if they do show elevations, studies to determine the use of glutamate inhibitors may then be warranted.
Interestingly, even in animal models of diabetes, the determination of levels of glutamate in the eye is conflicting. In a study by Obrosova et al., that looked at retinas removed from STZ-induced diabetes within 6 weeks following induction of diabetes, showed that the levels of glutamate in the retinas were similar to those in the control eyes for diabetic mice while they were reduced compared to control eyes for diabetic rats [62]. In this study, they only determined the intraretinal levels and not the vitreous levels. Ward et al., in a similar animal model showed no difference in glutamate metabolism in the retinas [63]. Conversely, other studies have shown that glutamate levels are increased and it appears that glial cell metabolism is affected [34, 64]. Kerns showed that in their model of alloxan-induced diabetic rats, the glutamate levels in the retina were significantly elevated by 40% compared to control retinas after two months of hyperglycemia [65]. It may be that compared to the Obrosova study which did similar intraretinal glutamate sensing, the difference in comparing to normal after an extra 2.5 weeks of significant hyperglycemia in the Kerns study may have been the reason for Kerns finding a difference in glutamate levels while Obrosova did not. Lieth et al. showed that in ex vivo retinas of STZ-induced diabetes there was reduced glutamate oxidation by 38% compared to controls. Similarly, Puro used freshly dissected Müller cells from normal rat eyes and cells from STZ-induced diabetes eyes to demonstrate a statistically significant diminution in the glutamate transport in the diabetic group after four weeks of diabetes. After four weeks of diabetes, a statistically significant diminution could be seen in the glutamate transporter between these two groups. By 13 weeks, there was a 67% decrease in transporter function compared to controls. This is similar to other causes of retinal ischemia, where the GLAST function in the Müller cells is markedly diminished [66]. In addition, neural cells may have altered glutamate receptor function and calcium metabolism [67].
Besides direct neurotoxic effects, elevated glutamate levels may have other indirect retinotoxic effects as well. We have previously shown that nitrates are increased in diabetic retinopathy [68]. Glutamate elevation is associated with nitric oxide but how it is related is not completely understood. Kerns in his study using rats following 2 month of hyperglycemia showed that inhibiting glutamate levels decreased NO oxide production [65]. Subsequently, no other studies have been done to evaluate this. Considering that nitric oxide appears to be an important regulator of retinal vasculature autoregulation, further studies to understand the relationship between nitric oxide levels and glutamate are warranted. Finally, there are other pathways that are affected by excitotoxicity. Protein Kinase C (PKC) is activated by NMDA activation. PKC-ζ inhibitors prevent some of the damage from NMDA activation but PKC-β inhibitors do not inhibit neuronal death. PKC-β is activated in diabetes and appears to be related to diabetic retinopathy but the relationship between PKC-ζ and diabetes has not been established though it is interesting to note that PKC-ζ has been associated with upregulation of VEGF in other systems [69, 70].
3. SUMMARY
There appears to be a relationship between diabetic retinopathy and elevated glutamate levels similar to other cases of CNS ischemia and glaucoma, however, further studies are required to confirm this. Though part appears to be an effect of ischemia on the function of Müller cells, the exact pathophysiology of how diabetes causes elevated glutamate levels in the vitreous also has to be determined. Whether glutamate elevation is just a marker disease or actually adds to the complications of diabetes has not been established but from other diseases that cause elevation of glutamate in the CNS, treatment by glutamate inhibitors appears to decrease neurotoxicity. Results of clinical trials of memantine and glaucoma are ongoing and will help to determine the importance of glutamate in ocular pathologies. How glutamate affects other important factors, for instance nitric oxide and VEGF, have to be further elucidated.
ACKNOWLEDGMENTS
This work was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., NY.
References
- 1. National Diabetes Fact Sheet: United States 2005. Center for Disease Control. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf.
- 2.Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Archives of Ophthalmology. 2004;122(4):552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 3.Varma R, Torres M. Prevalence of lens opacities in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(8):1449–1456. doi: 10.1016/j.ophtha.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. British Medical Journal. 2006;333(7566):475–480. doi: 10.1136/bmj.38922.650521.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malinowski SM, Pulido JS, Flickinger RR. The protective effect of the tilted disc syndrome in diabetic retinopathy. Archives of Ophthalmology. 1996;114(2):230–231. doi: 10.1001/archopht.1996.01100130224028. [DOI] [PubMed] [Google Scholar]
- 6.Dev S, Pulido JS, Tessler HH, et al. Progression of diabetic retinopathy after endophthalmitis. Ophthalmology. 1999;106(4):774–781. doi: 10.1016/S0161-6420(99)90166-5. [DOI] [PubMed] [Google Scholar]
- 7.Pulido JS. Retina, Choroid, and Vitreous. St. Louis, Mo, USA: Mosby; 2002. [Google Scholar]
- 8.Cusick M, Chew EY, Chan C-C, Kruth HS, Murphy RP, Ferris FL., III Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110(11):2126–2133. doi: 10.1016/j.ophtha.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New England Journal of Medicine. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 10.Brown SM, Jampol LM. New concepts of regulation of retinal vessel tone. Archives of Ophthalmology. 1996;114(2):199–204. doi: 10.1001/archopht.1996.01100130193015. [DOI] [PubMed] [Google Scholar]
- 11.Izumi N, Nagaoka T, Mori F, Sato E, Takahashi A, Yoshida A. Relation between plasma nitric oxide levels and diabetic retinopathy. Japanese Journal of Ophthalmology. 2006;50(5):465–468. doi: 10.1007/s10384-006-0344-y. [DOI] [PubMed] [Google Scholar]
- 12.Milusheva EA, Kuneva VI, Itzev DE, Kortezova NI, Sperlagh B, Mizhorkova ZN. Glutamate stimulation of acetylcholine release from myenteric plexus is mediated by endogenous nitric oxide. Brain Research Bulletin. 2005;66(3):229–234. doi: 10.1016/j.brainresbull.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Shires TK, Faeth JA, Pulido JS. Protein levels in the vitreous of rats with streptozotocin-induced diabetes mellitus. Brain Research Bulletin. 1993;30(1-2):85–90. doi: 10.1016/0361-9230(93)90042-a. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi M, West K, Crabb JW, Kinoshita S, Kamei M. Proteomic analysis of vitreous from diabetic macular edema. Experimental Eye Research. 2005;81(2):176–182. doi: 10.1016/j.exer.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Sebag J, Nie S, Reiser K, Charles MA, Yu N-T. Raman spectroscopy of human vitreous in proliferative diabetic retinopathy. Investigative Ophthalmology and Visual Science. 1994;35(7):2976–2980. [PubMed] [Google Scholar]
- 16.Shires TK, Faeth JA, Pulido JS. Nonenzymatic glycosylation of vitreous proteins in vitro and in the streptozotocin-treated diabetic rat. Retina. 1990;10(2):153–158. doi: 10.1097/00006982-199004000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Pulido JS. Experimental nonenzymatic glycosylation of vitreous collagens occurs by two patients. Transactions of the American Ophthalmological Society. 1996;94:1029–1072. [PMC free article] [PubMed] [Google Scholar]
- 18.Werman R. Amino acids as central neurotransmitters. Research Publications - Association for Research in Nervous and Mental Disease. 1972;50:147–180. [PubMed] [Google Scholar]
- 19.Olney JW. Glutaate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. Journal of Neuropathology and Experimental Neurology. 1969;28(3):455–474. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Olney JW, Ho OL. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature. 1970;227(5258):609–611. doi: 10.1038/227609b0. [DOI] [PubMed] [Google Scholar]
- 21.DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28(3):847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 22.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 24.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 25.Pines G, Danbolt NC, Bjoras M, et al. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360(6403):464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 26.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(22):10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton SA. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Current Alzheimer Research. 2005;2(2):155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 28.Vaquero J, Butterworth RF. The brain glutamate system in liver failure. Journal of Neurochemistry. 2006;98(3):661–669. doi: 10.1111/j.1471-4159.2006.03918.x. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka M, Bradley WG, Shapshak P, et al. Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases. Advances in Neuroimmunology. 1995;5(3):335–358. doi: 10.1016/0960-5428(95)00012-q. [DOI] [PubMed] [Google Scholar]
- 30.Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. Journal of Biochemistry and Molecular Biology. 2002;35(1):67–86. doi: 10.5483/bmbrep.2002.35.1.067. [DOI] [PubMed] [Google Scholar]
- 31.Wenk GL. Neuropathologic changes in Alzheimer's disease: potential targets for treatment. Journal of Clinical Psychiatry. 2006;67(supplement 3):3–7. [PubMed] [Google Scholar]
- 32.Chuang D-M. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Critical Reviews in Neurobiology. 2004;16(1-2):83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- 33.Kuehn MH, Fingert JH, Kwon YH. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmology Clinics of North America. 2005;18(3):383–395. doi: 10.1016/j.ohc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Lieth E, Barber AJ, Xu B, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 35.Mali RS, Cheng M, Chintala SK. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB Journal. 2005;19(10):1280–1289. doi: 10.1096/fj.04-3403com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunimatsu T, Asai S, Kanematsu K, Kohno T, Misaki T, Ishikawa K. Effects of glutamate receptor agonist on extracellular glutamate dynamics during moderate cerebral ischemia. Brain Research. 2001;923(1-2):178–186. doi: 10.1016/s0006-8993(01)02989-4. [DOI] [PubMed] [Google Scholar]
- 37.Tasker RC, Coyle JT, Vornov JJ. The regional vulnerability to hypoglycemia-induced neurotoxicity in organotypic hippocampal culture: protection by early tetrodotoxin or delayed MK-801. The Journal of Neuroscience. 1992;12(11):4298–4308. doi: 10.1523/JNEUROSCI.12-11-04298.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massieu L, Gómez-Román N, Montiel T. In vivo potentiation of glutamate-mediated neuronal damage after chronic administration of the glycolysis inhibitor iodoacetate. Experimental Neurology. 2000;165(2):257–267. doi: 10.1006/exnr.2000.7481. [DOI] [PubMed] [Google Scholar]
- 39.Dreyer EB, Zhang D, Lipton SA. Transcriptional or translational inhibition blocks low dose NMDA-mediated cell death. NeuroReport. 1995;6(6):942–944. doi: 10.1097/00001756-199504190-00029. [DOI] [PubMed] [Google Scholar]
- 40.Ambati J, Chalam KV, Chawala DK, et al. Elevated γ-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Archives of Ophthalmology. 1997;115(9):1161–1166. doi: 10.1001/archopht.1997.01100160331011. [DOI] [PubMed] [Google Scholar]
- 41.Diederen RMH, La Heij EC, Deutz NEP, et al. Increased glutamate levels in the vitreous of patients with retinal detachment. Experimental Eye Research. 2006;83(1):45–50. doi: 10.1016/j.exer.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Archives of Ophthalmology. 1996;114(3):299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 43.Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. Journal of Neuroscience Research. 1995;42(1):131–143. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- 44.Rauen T, Kanner BI. Localization of the glutamate transporter GLT-1 in rat and macaque monkey retinae. Neuroscience Letters. 1994;169(1-2):137–140. doi: 10.1016/0304-3940(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 45.Wiessner M, Fletcher EL, Fischer F, Rauen T. Localization and possible function of the glutamate transporter, EAAC1, in the rat retina. Cell and Tissue Research. 2002;310(1):31–40. doi: 10.1007/s00441-002-0612-1. [DOI] [PubMed] [Google Scholar]
- 46.Pow DV, Barnett NL. Developmental expression of excitatory amino acid transporter 5: a photoreceptor and bipolar cell glutamate transporter in rat retina. Neuroscience Letters. 2000;280(1):21–24. doi: 10.1016/s0304-3940(99)00988-x. [DOI] [PubMed] [Google Scholar]
- 47.Rauen T, Taylor WR, Kuhlbrodt K, Wiessner M. High-affinity glutamate transporters in the rat retina: a major role of the glial glutamate transporter GLAST-1 in transmitter clearance. Cell and Tissue Research. 1998;291(1):19–31. doi: 10.1007/s004410050976. [DOI] [PubMed] [Google Scholar]
- 48.Macnab LT, Williams SM, Pow DV. Expression of the exon 3 skipping form of GLAST, GLAST1a, in brain and retina. NeuroReport. 2006;17(18):1867–1870. doi: 10.1097/WNR.0b013e328010b898. [DOI] [PubMed] [Google Scholar]
- 49.Grünert U, Lin B, Martin PR. Glutamate receptors at bipolar synapses in the inner plexiform layer of primate retina: light microscopic analysis. Journal of Comparative Neurology. 2003;466(1):136–147. doi: 10.1002/cne.10862. [DOI] [PubMed] [Google Scholar]
- 50.Pan F, Massey SC. Rod and cone input to horizontal cells in the rabbit retina. Journal of Comparative Neurology. 2007;500(5):815–831. doi: 10.1002/cne.21127. [DOI] [PubMed] [Google Scholar]
- 51.Rentería RC, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. The Journal of Neuroscience. 2006;26(46):11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen RJ. Activation of group II metabotropic glutamate receptors reduces directional selectivity in retinal ganglion cells. Brain Research. 2006;1122(1):86–92. doi: 10.1016/j.brainres.2006.08.119. [DOI] [PubMed] [Google Scholar]
- 53.Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends in Pharmacological Sciences. 2001;22(4):174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 54.Mawrin C, Pap T, Pallas M, Dietzmann K, Behrens-Baumann W, Vorwerk CK. Changes of retinal glutamate transporter GLT-1 mRNA levels following optic nerve damage. Molecular Vision. 2003;9:10–13. [PubMed] [Google Scholar]
- 55.Guo L, Salt TE, Maass A, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Investigative Ophthalmology and Visual Science. 2006;47(2):626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Investigative Ophthalmology and Visual Science. 2002;43(7):2236–2243. [PubMed] [Google Scholar]
- 57.Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investigative Ophthalmology and Visual Science. 2000;41(7):1940–1944. [PubMed] [Google Scholar]
- 58.Honkanen RA, Baruah S, Zimmerman MB, et al. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Archives of Ophthalmology. 2003;121(2):183–188. doi: 10.1001/archopht.121.2.183. [DOI] [PubMed] [Google Scholar]
- 59.Diederen RMH, La Heij EC, Deutz NEP, et al. Increased glutamate levels in the vitreous of patients with retinal detachment. Experimental Eye Research. 2006;83(1):45–50. doi: 10.1016/j.exer.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 60.Deng J, Wu DZ, Gao R. Detection of glutamate and γ-aminobutyric acid in vitreous of patients with proliferative diabetic retinopathy. Yan Ke Xue Bao. 2000;16(3):199–202. [PubMed] [Google Scholar]
- 61.Asensio Sanchez VM, Corral AA, Aragón AB, De Paz GM. Sarcoidosis of the optic nerve. Archivos de la Sociedad Espanola de Oftalmologia. 2003;78(3):165–168. [PubMed] [Google Scholar]
- 62.Obrosova IG, Drel VR, Kumagai AK, Szábo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006;49(10):2525–2533. doi: 10.1007/s00125-006-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward MM, Jobling AI, Kalloniatis M, Fletcher EL. Glutamate uptake in retinal glial cells during diabetes. Diabetologia. 2005;48(2):351–360. doi: 10.1007/s00125-004-1639-5. [DOI] [PubMed] [Google Scholar]
- 64.Puro DG. Diabetes-induced dysfunction of retinal Müller cells. Transactions of the American Ophthalmological Society. 2002;100:339–352. [PMC free article] [PubMed] [Google Scholar]
- 65.Kowluru RA, Engerman RL, Case GL, Kerns TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochemistry International. 2001;38(5):385–390. doi: 10.1016/s0197-0186(00)00112-1. [DOI] [PubMed] [Google Scholar]
- 66.Barnett NL, Pow DV, Bull ND. Differential perturbation of neuronal and glial glutamate transport systems in retinal ischaemia. Neurochemistry International. 2001;39(4):291–299. doi: 10.1016/s0197-0186(01)00033-x. [DOI] [PubMed] [Google Scholar]
- 67.Santiago AR, Rosa SC, Santos PF, Cristóvão AJ, Barber AJ, Ambrósio AF. Elevated glucose changes the expression of ionotropic glutamate receptor subunits and impairs calcium homeostasis in retinal neural cells. Investigative Ophthalmology & Visual Science. 2006;47(9):4130–4137. doi: 10.1167/iovs.06-0085. [DOI] [PubMed] [Google Scholar]
- 68.Gao L, Pulido JS, Hatfield RM, Dundervill RF, III, McCannel CA, Shippy S. Capillary electrophoretic assay for nitrate levels in the vitreous of proliferative diabetic retinopathy. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2007;847(2):300–304. doi: 10.1016/j.jchromb.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koponen S, Kurkinen K, Åkerman KEO, Mochly-Rosen D, Chan PH, Koistinaho J. Prevention of NMDA-induced death of cortical neurons by inhibition of protein kinase Cζ . Journal of Neurochemistry. 2003;86(2):442–450. doi: 10.1046/j.1471-4159.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- 70.Neid M, Datta K, Stephan S, et al. Role of insulin receptor substrates and protein kinase C-ζ in vascular permeability factor/vascular endothelial growth factor expression in pancreatic cancer cells. Journal of Biological Chemistry. 2004;279(6):3941–3948. doi: 10.1074/jbc.M303975200. [DOI] [PubMed] [Google Scholar]