Abstract
Background
As iron and lead promote oxidative damage, and hemochromatosis (HFE) gene polymorphisms increase body iron burden, HFE variant alleles may modify the lead burden and cognitive decline relationship.
Objective
Our goal was to assess the modifying effects of HFE variants on the lead burden and cognitive decline relation in older adults.
Methods
We measured tibia and patella lead using K-X-ray fluorescence (1991–1999) among participants of the Normative Aging Study, a longitudinal study of community-dwelling men from greater Boston. We assessed cognitive function with the Mini-Mental State Examination (MMSE) twice (1993–1998 and 1995–2000) and genotyped participants for HFE polymorphisms. We estimated the adjusted mean differences in lead-associated annual cognitive decline across HFE genotype groups (n = 358).
Results
Higher tibia lead was associated with steeper cognitive decline among participants with at least one HFE variant allele compared with men with only wild-type alleles (p interaction = 0.03), such that a 15 μg/g increase in tibia lead was associated with a 0.2 point annual decrement in MMSE score among HFE variant allele carriers. This difference in scores among men with at least one variant allele was comparable to the difference in baseline MMSE scores that we observed among men who were 4 years apart in age. Moreover, the deleterious association between tibia lead and cognitive decline appeared progressively worse in participants with increasingly more copies of HFE variant alleles (p-trend = 0.008). Results for patella lead were similar.
Conclusion
Our findings suggest that HFE polymorphisms greatly enhance susceptibility to lead-related cognitive impairment in a pattern consistent with allelelic dose.
Keywords: cognitive decline, epidemiology, HFE, lead, longitudinal studies, neuropsychologic tests
In the United States the population of persons age 65 years and older is projected to increase 2-fold to 75 million in the next 30 years, and a concomitant upsurge in the number of individuals with dementia is expected (U.S. Census Bureau 2000a, 2000b). Cognitive decline, a risk factor for dementia, may be a transition stage spanning normal cognition and onset of diseases associated with dementia (Bischkopf et al. 2002; Burns and Zaudig 2002; Pratico et al. 2002).
Lead has long been recognized as a neuro-toxicant; the associations between lead and cognitive impairment among workers in lead-related industries and poorer cognitive development in children have been well reproduced (Barth et al. 2002; Bleecker et al. 1997; Lanphear et al. 2000). The few studies that have been conducted among low-level lead-exposed older adults have generally reported inverse associations between lead burden and cognitive function (Muldoon et al. 1996; Nordberg et al. 2000; Payton et al. 1998; Weisskopf et al. 2004; Wright et al. 2003). With half-life estimates ranging from 5 to 20 years for cortical bone, and more than1 year for trabecular bone (Hu et al. 1998; Kim et al. 1997), lead levels in bone may better reflect long-term body burden of lead than blood lead, which has a half-life of approximately 30 days (Hu 2001). In addition, bone lead measures correlate well with measures of cumulative external lead levels and integrated blood lead levels, two commonly used indices of cumulative lead exposure (Bleecker et al. 1997).
Iron metabolism may play a critical role in neurodegenerative processes (Lee et al. 2006; Todorich and Connor 2004). Although iron is vital for cellular processes, plasma iron which is not bound to transferrin may be toxic. This nontransferrin-bound iron represents the portion of body iron likely to cause cellular oxidative damage, a purported mechanism in the pathogenesis of neurodegenerative diseases, and serve as a catalyst in the neuronal production of free radicals (Eaton and Qian 2002; Samson and Nelson 2000). Two variants in the hemochromatosis (HFE) gene, C282Y and H63D, are commonly found in the U.S.population, especially among whites, and are associated with hereditary hemochromatosis, a disease of iron overload. Several recent studies have reported an association between these HFE polymorphisms and neurodegenerative diseases such as Alzheimer disease (Berlin et al. 2004; Moalem et al. 2000; Sampietro et al. 2001). Furthermore, carriers of these polymorphisms who do not have clinical signs of iron overload are observed to have higher levels of nontransferrin-bound iron in addition to body iron measures higher than wild-types (Adams et al. 2005; Beutler et al. 2003; Datz et al. 1998; de Valk et al. 2000; Garry et al. 1997).
These studies have led to a growing interest in the interaction of iron and lead metabolism in the process of neurodegeneration. As HFE variant alleles are associated with neurodegenerative processes similar to those seen in lead toxicity, and the presence of iron enhances the oxidative effects of lead (Adonaylo and Oteiza 1999), HFE variant alleles may magnify the neurologic damage caused by lead. Therefore, we examined the modifying effect of the HFE alleles on the association between body lead burden and change in performance on the Mini-Mental State Examination (MMSE), a test of global cognitive function, in a cohort of older, community-dwelling men.
Materials and Methods
Participants in the current study were drawn from the Normative Aging Study (NAS), a community-based, prospective cohort study initiated in 1963 at the Veterans Affairs (VA) Outpatient Clinic in Boston to examine factors related to healthy aging (Bell et al. 1972). The cohort consisted of 2,280 men 21–81 years of age at the time of enrollment (1963–1968) who had successfully completed a screening process to ensure participants were free of known chronic medical conditions. Most cohort members were of northern European descent. Overall, their smoking and alcohol consumption patterns were similar to men of comparable age in the U.S.population. Every 3–5 years, study participants were asked to undergo extensive evaluations including medical and physical examinations and laboratory tests. They also completed questionnaires on smoking history, diet, and other factors potentially related to aging and health. To date, the annual attrition due to all causes has been less than 1%, and more than 80% have responded to mailed questionnaires supplementing on-site examinations (Hu et al. 1996). This study has been approved by the Human Subjects Committees of the Boston VA Medical Center, the Brigham and Women’s Hospital, and the Harvard School of Public Health.
Study population
Beginning in 1991, bone lead measurements were taken using K-X-ray fluorescence (KXRF) among active participants who gave written informed consent. In 1993, cognitive function assessments were initiated. At the time of the present study, 1,055 study participants had completed at least one cognitive assessment; whereas 540 men had two or more assessments. The first and second cognitive assessments were on average 3.2 years apart. Of the men with two cognitive measures, 420 had at least one bone lead measurement. In 2000, NAS participants (n = 730) were genotyped for two HFE polymorphisms based on archived blood samples. In all, our analyses included the 358 men with at least two cognitive assessments, complete covariate information, HFE genotyping data, and at least one measure of bone lead.
Bone lead KXRF measurement
In vivo bone measurements were taken using a K-X-ray fluorescence (KXRF) instrument (ABIOMED, Inc., Danvers, MA) at the mid-tibia (shin bone) and the patella (knee cap bone) (Aro et al. 2000). The sites were chosen to be representative of the two predominant bone types: cortical bone (tibia) and trabecular bone (patella). These measurements had units of micrograms of lead per gram bone mineral. The bone measurement taken closest in time to the baseline cognitive assessment served as a proxy for tissue lead burden. The instrument also provides an estimate of the uncertainty for each measurement equivalent to the standard deviation of repeated measurements. Lead estimates with uncertainty values > 10 μg/g for tibia and > 15 μg/g for patella were excluded as unreliable, a standard protocol in analyses of bone lead (Hu et al. 1998). Negative estimates of bone lead concentrations may occur for lead values close to zero. As recoding the negative values to the minimum detectable limit may induce bias and reduce efficiency in the statistical analyses, KXRF-measured bone lead concentration estimates were used in the analysis without recoding (Kim et al. 1997).
HFE genotyping
We genotyped participants for both the C282Y and H63D polymorphisms of the HFE gene (GenBank accession no. Z92910; http://www.ncbi.nlm.nih.gov/sites/entrez) using archived blood. Puregene DNA isolation kits (Gentra Systems, Inc., Minneapolis, MN) were used to extract the DNA from the blood sample. The H63D polymorphism was genotyped by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) analysis as previously described (Cardoso et al. 1998; Wright et al. 2004). Similarly, the C282Y polymorphism was genotyped by separate PCR and RFLP procedures (Cardoso et al. 1998; Feder et al. 1996).
As a quality control measure, 10% of samples were randomly selected and run in duplicate. Genotypes were also determined on control blood known to be from persons homozygous for the wild-type genotype and heterozygous and homozygous for each HFE variant genotype. The full data set was anonymized after genotyping to protect our study members and to conform to current Institutional Review Board policies.
Assessment of cognitive function
One of the tests included in the cognitive assessment battery was the MMSE, a global examination of cognitive function that assesses orientation, immediate and short-term recall, verbal and written skills, and attention and ability to follow commands (Crum et al. 1993; Folstein et al. 1975). The test is commonly used in epidemiologic studies to evaluate cognitive status (Farmer et al. 1995; Izaks et al. 1995; Knopman et al. 2003). Scores range from 0 to 30, with higher score denoting better cognitive performance, although in our analysis the highest possible score was 29 because of deletion of the question “What county are we in?” from our tally. Other studies have reported that most Massachusetts residents do not know in which county they reside as counties in Massachusetts do not have strong governmental function (Tombaugh and McIntyre 1992).
Statistical analysis
Because of small sample sizes in some strata of HFE genotypes, we classified HFE genotypes in two different manners: binary [wild-type (having only HFE wild-type alleles), any HFE variant allele]; and dose (wild-type, one HFE variant allele, two HFE variant alleles). Our measure of change in cognition was the average annual rate of decline in MMSE score, defined as (MMSE score at second visit – MMSE score at baseline visit)/(years between assessments), for each participant.
We analyzed lead levels in tibia and patella separately. To assess effect modification, we fitted multiple linear regression models of average annual rate of decline in MMSE score, in which we included a term for the lead bio-marker, indicator variables for the HFE genotype classification, and cross-product terms between HFE genotype and lead biomarker, along with terms for age, years of education, smoking status (current, never, past), pack-years smoked, nondrinker, alcohol consumption (grams/day), English as first language (yes, no), computer experience (yes, no) and diabetes (diagnosis or fasting glucose > 126 mg/dL). Values of covariates used in the analyses were those reported at the baseline MMSE assessment. Stroke and Alzheimer disease predict MMSE score, but as so few men in our study population had such conditions, these conditions were not considered in our analyses. We assessed the linearity of the association between lead and annual rate of cognitive decline within class of HFE genotype by fitting a penalized spline for the lead biomarker and adjusting for covariates using the generalized additive models function in R software (http://www.r-project.org/). A penalized spline is a technique for flexibly modeling dose–response by dividing the range of exposure into intervals, and fitting a separate cubic polynomial within each interval. A penalty term is added to the log likelihood that is proportional to how “wiggly” the resulting dose–response curve is, which prevents excessive nonlinearity (Wood and Augustin 2002). The optimal degree of smoothing was determined by the generalized cross-validation criterion, which is, in practice, an approximation of Akaike’s information criterion (Wood and Augustin 2002).
To assess whether participants with C282Y and H63D alleles have different lead-associated cognitive changes, we also conducted exploratory analyses to evaluate the association of lead on cognitive decline by HFE genotype groups (e.g., wild-type, H63D homozygotes, C282Y heterozygotes). Finally, to assess the robustness of our results, we first restricted our analyses to white participants, then repeated the analyses after removing outliers identified by the generalized extreme studentized deviation (ESD) method (Rosner 1983). All statistical analyses were conducted using SAS (version 8.2; SAS Inc., Cary, NC) and R version 2.1.1. We used partial F-tests and likelihood ratio tests for statistical hypothesis testing. The p-value of significance was < 0.05.
Results
Median concentrations of bone lead in our study population were 19 and 23 μg/g for tibia and patella, respectively. Participants who were younger, more educated, native English speakers, or who had computer experience on average had lower bone lead levels (Table 1).Bone lead concentrations were slightly higher among men with lower baseline cognitive scores. Thirty-six percent of men had at least one HFE variant allele.Both genotype distributions conformed to Hardy-Weinberg expected frequencies (C282Y: χ2 =0.53, p = 0.47; H63D:χ2 =0.18, p = 0.67). Nine participants in our population were heterozygous for both C282Y and H63D polymorphisms (known as compound heterozygotes). Although bone lead levels appeared higher in the four participants who were C282Y homozygotes, these measures were not significantly different than the bone lead levels among wild-types. Characteristics of men with only HFE wild-type alleles were similar to those of variant allele carriers with the exception of report of computer experience (Table 2). Compared with men in the larger NAS cohort who had only one MMSE score, men in our study population had similar average baseline MMSE scores and only slightly lower mean bone lead levels (data not shown). The distribution of HFE genotypes was also similar in these two groups. Men with and without bone lead measures had similar baseline characteristics, distribution of HFE genotypes, and MMSE scores (data not shown).
Table 1.
Baseline characteristics of study participants (n = 358) by bone lead measures [median μg/g (IQR)].
| Tibia lead | Patella lead | |
|---|---|---|
| Age (years) | ||
| < 65 | 15 (9–20) | 18 (13–27.5) |
| 65–70 | 20 (14–28) | 25 (17–34) |
| ≥71 | 25 (18–35) | 28 (17–52) |
| Education | ||
| Never finished high school | 30 (14–36) | 33.5 (23–46) |
| High school graduate | 21 (14–29) | 27 (16–44) |
| Some college | 19 (13–28) | 25 (15–37) |
| College graduate | 17 (11–22) | 19 (13–29) |
| Smoking status | ||
| Never | 17 (11–27) | 21.5 (13–33) |
| Former | 20 (14–29) | 24 (16–36) |
| Current | 19 (13.5–23.5) | 27 (15–34) |
| Alcohol consumption | ||
| Yes | 19 (13–26) | 23 (15–35) |
| No | 20 (12.5–30) | 24 (17–36) |
| History of diabetesa | ||
| Yes | 23 (14–35) | 27 (17–38) |
| No | 19 (13–27) | 23 (15–35) |
| English as first language | ||
| Yes | 19 (12–27) | 22.5 (15–34) |
| No | 24 (15–30) | 27 (17–39) |
| Computer experience | ||
| Yes | 15 (10–22) | 20 (13.5–30) |
| No | 21 (14–30) | 26 (16–39) |
| Baseline MMSE scoreb | ||
| < 26 | 20.5 (13–30) | 26.5 (15–39) |
| 26–27 | 21 (13.5–27.5) | 24 (15–38) |
| ≥28 | 16 (11–22.5) | 21 (15–31) |
| HFE genotype | ||
| Wild-type (n = 228) | 19 (13–29) | 24 (15–37) |
| One or more HFE variant alleles | 19.5 (12–26) | 22 (14–34) |
| H63D heterozygotes (n = 69) | 20.5 (11.5–25.5) | 22 (14–33) |
| H63D homozygotes (n = 8) | 17 (12.5–27) | 23.5 (13–34) |
| C282Y heterozygotes (n = 40) | 17 (13–21) | 22 (14–34) |
| C282Y homozygotes (n = 4) | 32.5 (21–39) | 39 (16–61.5) |
| Compound heterozygotes (n = 9) (C282Y and H63D alleles) | 15 (11–20) | 21 (12–27) |
IQR, interquartile range.
History of diabetes defined as having reported diagnosis of diabetes or having fasting glucose above 126 mg/dL.
Highest possible MMSE score in our analysis was 29 because of deletion of the question “What county are we in?”
Table 2.
Baseline characteristics of participants by HFE genotype (n = 358).
| HFE wild-type (n = 228) | HFE variant allele (n = 130) | |
|---|---|---|
| Age [median years (IQR)] | 67.2 (62.6–71.8) | 67.7 [63.7–71.3] |
| Education [n (%)] | ||
| Never finished high school | 19 (8.3) | 9 (6.9) |
| High school graduate | 64 (28.1) | 36 (27.7) |
| Some college | 64 (28.1) | 33 (25.4) |
| College graduate | 81 (35.5) | 52 (40.0) |
| Smoking status [n (%)]a | ||
| Never | 76 (33.3) | 46 (35.4) |
| Former | 138 (60.5) | 81 (62.3) |
| Current | 14 (6.1) | 3 (2.3) |
| Alcohol consumption [median g/day (IQR)] | 5.8 [0.4–18.7) | 6.0 [0–16.7) |
| History of diabetesb [n (%)] | 24 (10.5) | 13 (10.0) |
| English as first language [n (%)] | 199 (87.3) | 118 (90.8) |
| Computer experience [n (%)] | 87 (38.2) | 65 (50.0) |
| Patella lead [median μg/g (IQR)] | 24 (15, 37) | 22 (14, 34) |
| Tibia lead [median μg/g (IQR)] | 19.0 (13, 29) | 19.5 (12, 26) |
| Baseline MMSE score [median (IQR)]c | 27 (25, 28) | 27 (26, 28) |
IQR, interquartile range.
Percentages may not add up to 100 because of rounding.
History of diabetes defined as having reported diagnosis of diabetes or having fasting glucose above 126 mg/dL.
Highest possible MMSE score in our analysis was 29 because of deletion of the question “What county are we in?”
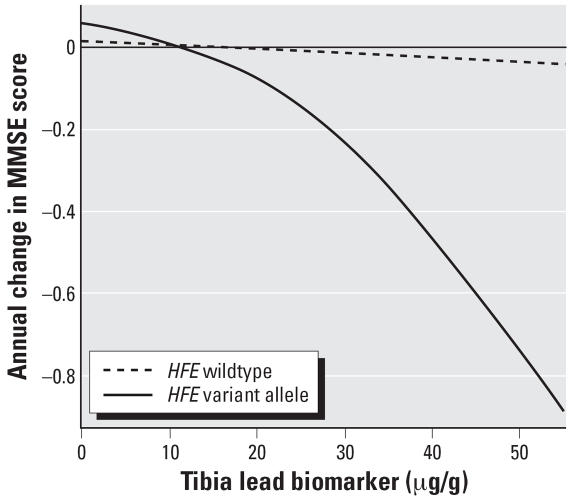
The average annual rate of decline in MMSE scores was modestly though not significantly worse among HFE variant allele carriers than among men with only HFE wild-type alleles {difference in annual rate of change: –1.77 points/year [95% confidence interval (CI), –3.88 to 0.35]}, results not shown). Higher tibia lead was associated with a steeper decline in MMSE scores among participants with at least one HFE variant allele compared with wild-types (p = 0.03; Table 3, model 1). Among participants with any HFE variant allele, an interquartile range (IQR) increment in tibia lead (15 μg/g) was associated with a 0.22-point annual decrement in MMSE score (95% CI, –0.39 to –0.05). This annual change in MMSE score among variant allele carriers was approximately equivalent to the difference in baseline MMSE scores between men in our study population who were 4 years apart in age. In contrast, although tibia lead level was associated with decline in MMSE score among wild-types as well, this association was smaller in magnitude and not statistically significant. We used covariate-adjusted penalized splines to explore the linearity of the association between tibia lead level and change in MMSE score by HFE genotype, as shown in Figure 1. Even though the association of lead burden with cognitive decline among men with only HFE wild-type alleles appeared linear (optimal degree of smoothing: 1, p = 0.72), the association appeared curvilinear, specifically, steeper with greater lead burden in the variant allele carriers. The test for deviation from linearity for the tibia lead–cognitive change association among the variant allele carriers was nearly significant (optimal degree of smoothing: 1.68, p = 0.08), suggesting that, among variant allele carriers, a unit increase in lead burden may be associated with disproportionately greater cognitive decline at high lead burden than at low lead burden.
Table 3.
Association with an interquartile (15 μg/g) increase in tibia lead biomarkers on change in MMSE score by class of HFE genotype.
| Model/class of HFE genotype | Unadjusted mean difference in annual rate of change in MMSE (95% CI) | Adjusteda mean difference in annual rate of change of MMSE (95% CI) | p-Value interaction | p-Value trend |
|---|---|---|---|---|
| Model 1: binary | 0.03b | NA | ||
| Wild-type | –0.02 (–0.10 to 0.05) | –0.02 (–0.10 to 0.07) | ||
| Any HFE variant allele | –0.23 (–0.40 to –0.07) | –0.22 (–0.39 to –0.05) | ||
| Model 2: dose | < 0.01c | < 0.01 | ||
| Wild-type | –0.02 (–0.10 to 0.05) | –0.02 (–0.10 to 0.07) | ||
| One HFE variant allele | –0.15 (–0.33 to 0.03) | –0.14 (–0.33 to 0.04) | ||
| Two HFE variant alleles | –0.62 (–1.03 to –0.22) | –0.63 (–1.04 to –0.21) |
NA, not applicable.
Adjusted for age, years of education, nonsmoker, former smoker, pack-years, nondrinker, alcohol consumption, English as first language, computer experience, and diabetes.
p-Value for tibia lead and any HFE variant allele interaction.
p-Value for tibia lead and two HFE variant alleles interaction.
Figure 1.
Exploration of nonlinear association of tibia lead concentration with annual rate of cognitive decline, by class of HFE genotype. The lines indicate curvilinear trends estimated from the penalized spline method. Among HFE wild-types, the optimal degree of smoothing was 1, meaning that the association between tibia lead and annual cognitive decline was nearly linear, but among variant allele carriers, the association tended to deviate from linearity (p = 0.08), with an optimal 1.68 degree of smoothing. The model was adjusted for age, years of education, nonsmoker, former smoker, pack-years, nondrinker, alcohol consumption, English as first language, computer experience, and diabetes.
Interestingly, the detrimental association between tibia lead and decline in MMSE was progressively larger with increasing number of HFE variant alleles: each IQR increment in tibia lead was associated with a –0.02-point/year change in MMSE score among wild-types, –0.14-point/year change among men with one HFE variant allele, and –0.63 point/year change among men with two HFE variant alleles (p < 0.01, Table 3, model 2). The pronounced difference in lead-associated cognitive decline between men with two variant alleles and men with only wild-type alleles was significant (p < 0.01, Table 3, model 2). This difference in change in MMSE scores among participants with two HFE variant alleles was comparable to the difference in baseline MMSE scores that we observed among men who were 11 years apart in age.
We also examined the modification of the lead association with annual change in MMSE score by HFE genotype groups. The magnitudes of effect modification among H63D heterozygotes and C282Y heterozygotes were very similar to the magnitude of effect modification among one variant allele carriers. Similarly, the results for H63D homozygotes and C282Y homozygotes were similar to the results for two variant allele carriers (results not shown).
Overall, the associations pertaining to patella lead were similar to although smaller in magnitude than those pertaining to tibia lead (results not shown). Results from analyses in which we excluded extreme values of bone lead were also similar, as were results in analyses in which we restricted the study population to whites (results not shown).
Discussion
In our population of older men, the deleterious association between long-term lead burden and rate of decline in cognitive function was significantly worse among HFE variant allele carriers than among wild-types. Furthermore, the detrimental association of lead with cognitive decline was magnified among participants with a greater number of either variant alleles (H63D or C282Y ); the largest drop in MMSE scores associated with lead burden was observed in men carrying two variant alleles. Of note, the magnitude of effect modification was linked to number and not to type of HFE variant alleles. Our study is the first to provide evidence that the neurodegenerative effects of these variants during aging may be in part due to genetic susceptibility to non-iron metals such as lead.
Several studies have addressed cognitive function among adults who experienced chronic low-level lead exposure (Muldoon et al. 1996; Weisskopf et al. 2004; Wright et al. 2003). Among the studies that used bone lead measures, tibia lead was more strongly associated with cognitive decline than were patella and blood leads (Payton et al. 1998; Weisskopf et al. 2004), suggesting that lead in tibia serves as a superior proxy for effective lead dose in the brain. As lead in the tibia has a substantially longer half-life than lead in the patella or blood (Hu 1998), these results indicate that long-term, chronic lead exposure may be more predictive of cognitive changes. Interestingly, the strongest interaction between lead and HFE variant alleles in our study was also observed with tibia lead measures.
We are not aware of any other study that has evaluated the modifying effect of the HFE genotype on the association between lead and change in cognitive function. The HFE protein appears to regulate metal transport across cell membranes (Chung and Wessling-Resnick 2003), although its role in transporting iron and non-iron divalent metals across the blood brain barrier is unknown. Two common polymorphisms in the HFE gene, the C282Y and the H63D, have been implicated in hereditary hemochromatosis, a disease associated with excess iron absorption (Feder et al. 1996; Hanson et al. 2001). In addition, persons who carry the HFE variant alleles but who lack clinical signs of hemochromatosis disease are reported to have significantly higher values of serum iron, transferrin saturation, and non-transferrin-bound iron than individuals with only HFE wild-type alleles (Beutler et al. 2003; Datz et al. 1998; Garry et al. 1997; Moura et al. 1998). HFE polymorphisms have also been reported to affect lead uptake (Bannon et al. 2003; Barton et al. 1994; Onalaja and Claudio 2000; Wright et al. 2004). In our analysis, men who were homozygous for C282Y appeared to have higher bone lead burden than men with other HFE genotypes, although we were not able to detect a statistically significant difference with our small sample size of C282Y homozygotes. This subject matter merits further examination in larger study populations.
Although the relationship between HFE alleles and neurodegenerative diseases is not fully established, several recent studies have found positive associations between HFE variant alleles and Alzheimer disease. Researchers have reported the C282Y and H63D polymorphisms to be significantly more prevalent in subjects with Alzheimer disease compared with controls, and that persons with Alzheimer disease and HFE variant alleles were on average 6 years younger at the time of diagnosis than individuals with Alzheimer disease but with only the wild-type alleles (Moalem et al. 2000; Pulliam et al. 2003; Sampietro et al. 2001). Another study also observed earlier age of onset of Alzheimer disease but only among H63D homozygotes (Berlin et al. 2004). In contrast, others have found no association between HFE alleles and Alzheimer disease (Berlin et al. 2004; Guerreiro et al. 2006). The discrepancies across studies may reflect that HFE polymorphisms do not independently impart cognitive risk but instead enhance the neurotoxicity of agents such as lead. Interestingly, although the C282Y and H63D functional polymorphisms may differentially alter iron and divalent metal metabolism (Bomford 2002; Lyon and Frank 2001; Townsend and Drakesmith 2002), we observed these two polymorphisms to have comparable magnitudes of effect modification on the relation between lead and cognitive decline.
It is not known how the HFE variant alleles may accelerate cognitive decline in the presence of lead. Lead and free iron are independently capable of promoting oxidative damage, a purported mechanism in the pathogenesis of neurodegenerative disease (Jellinger 1999; Jenner 1993; Samson and Nelson 2000; Winterbourn 1995). In vitro studies have suggested synergistic oxidative effects between lead and iron; lead appears to increase lipid oxidation in the presence of iron (Adonaylo and Oteiza 1999). Therefore, our findings may reflect the complex relationship between iron and lead metabolism where the metals interact to further increase damage and, consequently, cognitive decline. Unfortunately, we were not able to measure body iron status or magnitude of oxidative stress for our study population.
Our current findings are an interesting juxtaposition to our previous report in which HFE variant alleles were associated with lower levels of internal lead dose biomarkers in the same cohort (Wright et al. 2004). If the HFE variant alleles affect cognition predominantly by lowering lead accumulation in the body tissues, one might expect the variant alleles to be associated with better cognitive performance. Instead, we found a suggestive association between HFE variant alleles and poorer cognitive function that is consistent with the prior reports linking HFE variant alleles to neurode-generation. In addition, because the HFE variant alleles are not known to affect the relation of lead levels in tibia to lead levels in the brain, one would anticipate similar magnitudes of change in MMSE scores per unit increase in body lead burden among wild-types compared with variant allele carriers. Our data suggest quite the opposite, indicating that HFE variant alleles augment the toxicity of lead that is absorbed.
There were several limitations to this study. Although the associations observed were significant, it is essential to attempt to reproduce such associations in larger populations. The mean interval between the two cognitive tests was 3.2 years. With a longer interval between testing or with additional MMSE assessments, we may be able to better describe the relationship between lead and cognitive changes by HFE genotype.
As with any aging cohort, there was a potential for selection bias mainly because of differential attrition and survival. We were somewhat reassured, as the lead biomarker levels and first MMSE scores in persons who had completed only baseline cognitive assessment were similar to those found in our study population. The frequencies of HFE genotypes were also similar in the two groups. Moreover, baseline characteristics and MMSE scores did not differ in men with and without bone lead measures.
The potential for misclassification should be considered. Although there may have been some measurement error in our bone lead data, such errors would most likely be nondifferential, and thus, bias our association estimates toward the null. It is possible that the modifying effects observed were caused by other polymorphisms in the HFE gene or polymorphisms in a proximal gene that is in tight linkage disequilibrium with these HFE polymorphisms, although we believe this is unlikely, as we did not find other genes known to regulate iron metabolism in this genomic region.
As in any observational study, confounding is a concern. We accounted for known strong predictors of cognitive function. As only one participant had suffered a stroke and none had been diagnosed with Alzheimer disease, we did not adjust for these factors in our final model. Of the men in our study population, 99% were white, making population stratification an unlikely confounder. Overall, crude and adjusted comparisons of MMSE change were similar, suggesting that strong confounding from an unmeasured source is unlikely.
The MMSE is widely used to screen for dementia and has frequently been used to assess cognitive status and track longitudinal changes in cognitive function (Farmer et al. 1995; Izaks et al. 1995; Knopman et al. 2003). However, it is a relatively easy test for which a learning effect has been reported (Jacqmin-Gadda et al. 1997). A low degree of variability in MMSE scores among more highly educated persons is often observed (Crum et al. 1993). Therefore, the MMSE may have had low sensitivity in detecting cognitive impairment in our participants. Despite these limitations, the MMSE is among the most extensively characterized and most widely used tests of cognitive status for older adults, and performs with a reasonable degree of reproducibility and validity. Past studies have found high correlations between MMSE scores and scores on other well-described cognitive tests, such as the Blessed Information-Memory-Concentration test and reasonable sensitivity and specificity in delineating individuals with and without dementia (Crum et al. 1993; Stuss et al. 1996; Tombaugh and McIntyre 1992). Finally, persons with mild cognitive impairment are found to have significantly worse MMSE scores than cognitively normal individuals (Bennett et al. 2002).
Although we were not yet able to assess development of dementia in our population, cognitive decline is a strong predictor of subsequent dementia. Cognitively impaired individuals have a 10–15% annual risk of developing dementia compared with a 1–2% annual risk among healthy controls (Bischkopf et al. 2002; Knopman et al. 2003; Morris et al. 2001). Cognitive impairment has also been associated with a 3.1- to 5-fold increase in risk of developing Alzheimer disease, the most common cause of age-related dementia (Tuokko et al. 2003).
Our findings suggest that cumulative lead exposure may be particularly detrimental to the cognitive well-being of HFE variant allele carriers as they age. Given the high prevalence of variant allele carriers in North American and European populations and the long retention of lead in the body, our results indicate that lead-related cognitive impairment experienced by a large subset of older adults is likely more substantial than currently recognized. Although these early cognitive changes may have slight consequences on many affected individuals, these small decrements pose a major public health concern, translating into a much larger proportion of older individuals who are considered clinically impaired, a societal burden that is projected to grow substantially, given that older persons make up one of the fastest growing segments of our population (U.S. Census Bureau 2000a, 2000b). Our findings provide insight on the mechanisms and pathways of cognitive decline. As there is currently no cure for dementia, elucidating the biological mechanisms may facilitate the development of preventive measures and treatments to hinder the rate of cognitive decline. As long-term chronic lead exposure appears to be most detrimental to cognitive health in the later years, our findings stress the continued importance of public health interventions aimed at reducing occupational and environmental exposure to lead in younger populations including lead abatement efforts and lead exposure prevention programs.
In summary, we have found the HFE polymorphisms to significantly modify the association between lead burden and the rate of cognitive decline. Persons with more copies of HFE variant alleles experienced greater cognitive decline per unit increase in bone lead biomarker level.
Footnotes
This work was supported in part by the Cognition and Health in Aging Men Project (CHAMP) and the VA Normative Aging Study (NAS). CHAMP is supported by the Research Services of the U.S. Department of Veterans Affairs, the National Institutes of Health (NIH; AA08941, AG13006, AG14345, AG18436, ES05947, ES05257), and the U.S. Department of Agriculture, Agricultural Research Service (contract 53–K06–510). VA NAS is supported by the Cooperative Studies Program/ERIC, U.S. Department of Veterans Affairs, and is a research component of the Massachusetts Veterans Epidemiology Research and Information Center. F.T.W was supported by NIH grants CCT110421 and ES007155.
References
- Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- Adonaylo VN, Oteiza PI. Pb2+ promotes lipid oxidation and alterations in membrane physical properties. Toxicology. 1999;132:19–32. doi: 10.1016/s0300-483x(98)00134-6. [DOI] [PubMed] [Google Scholar]
- Aro A, Amarasiriwardena C, Lee ML, Kim R, Hu H. Validation of K x-ray fluorescence bone lead measurements by inductively coupled plasma mass spectrometry in cadaver legs. Med Phys. 2000;27:119–123. doi: 10.1118/1.598863. [DOI] [PubMed] [Google Scholar]
- Bannon DI, Abounader R, Lees PS, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am J Physiol Cell Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- Barth A, Schaffer AW, Osterode W, Winker R, Konnaris C, Valic E, et al. Reduced cognitive abilities in lead-exposed men. Int Arch Occup Environ Health. 2002;75:394–398. doi: 10.1007/s00420-002-0329-1. [DOI] [PubMed] [Google Scholar]
- Barton JC, Patton MA, Edwards CQ, Griffen LM, Kushner JP, Meeks RG, et al. Blood lead concentrations in hereditary hemochromatosis. J Lab Clin Med. 1994;124:193–198. [PubMed] [Google Scholar]
- Bell B, Rose CL, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Aging Hum Dev. 1972;3:5–17. [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Berlin D, Chong G, Chertkow H, Bergman H, Phillips NA, Schipper HM. Evaluation of HFE (hemochromatosis) mutations as genetic modifiers in sporadic AD and MCI. Neurobiol Aging. 2004;25:465–474. doi: 10.1016/j.neurobiolaging.2003.06.008. [DOI] [PubMed] [Google Scholar]
- Beutler E, Felitti V, Gelbart T, Waalen J. Haematological effects of the C282Y HFE mutation in homozygous and heterozygous states among subjects of northern and southern European ancestry. Br J Haematol. 2003;120:887–893. doi: 10.1046/j.1365-2141.2003.04215.x. [DOI] [PubMed] [Google Scholar]
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- Bleecker ML, Lindgren KN, Ford DP. Differential contribution of current and cumulative indices of lead dose to neuro-psychological performance by age. Neurology. 1997;48:639–645. doi: 10.1212/wnl.48.3.639. [DOI] [PubMed] [Google Scholar]
- Bomford A. Genetics of haemochromatosis. Lancet. 2002;360:1673–1681. doi: 10.1016/S0140-6736(02)11607-2. [DOI] [PubMed] [Google Scholar]
- Burns A, Zaudig M. Mild cognitive impairment in older people. Lancet. 2002;360:1963–1965. doi: 10.1016/S0140-6736(02)11920-9. [DOI] [PubMed] [Google Scholar]
- Cardoso EM, Stal P, Hagen K, Cabeda JM, Esin S, de Sousa M, et al. HFE mutations in patients with hereditary haemochromatosis in Sweden. J Intern Med. 1998;243:203–208. doi: 10.1046/j.1365-2796.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- Chung J, Wessling-Resnick M. Molecular mechanisms and regulation of iron transport. Crit Rev Clin Lab Sci. 2003;40:151–182. doi: 10.1080/713609332. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- Datz C, Haas T, Rinner H, Sandhofer F, Patsch W, Paulweber B. Heterozygosity for the C282Y mutation in the hemochromatosis gene is associated with increased serum iron, transferrin saturation, and hemoglobin in young women: a protective role against iron deficiency? Clin Chem. 1998;44:2429–2432. [PubMed] [Google Scholar]
- de Valk B, Addicks MA, Gosriwatana I, Lu S, Hider RC, Marx JJ. Non-transferrin-bound iron is present in serum of hereditary haemochromatosis heterozygotes. Eur J Clin Invest. 2000;30:248–251. doi: 10.1046/j.1365-2362.2000.00628.x. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32:833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Kittner SJ, Rae DS, Bartko JJ, Regier DA. Education and change in cognitive function. The Epidemiologic Catchment Area Study. Ann Epidemiol. 1995;5:1–7. doi: 10.1016/1047-2797(94)00047-w. [DOI] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician”. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garry PJ, Montoya GD, Baumgartner RN, Liang HC, Williams TM, Brodie SG. Impact of HLA-H mutations on iron stores in healthy elderly men and women. Blood Cells Mol Dis. 1997;23:277–287. doi: 10.1006/bcmd.1997.0144. [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Bras JM, Santana I, Januario C, Santiago B, Morgadinho AS, et al. Association of HFE common mutations with Parkinson’s disease, Alzheimer’s disease and mild cognitive impairment in a Portuguese cohort. BMC Neurol. 2006;6:24. doi: 10.1186/1471-2377-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. Am J Epidemiol. 2001;154:193–206. doi: 10.1093/aje/154.3.193. [DOI] [PubMed] [Google Scholar]
- Hu H. Bone lead as a new biologic marker of lead dose: recent findings and implications for public health. Environ Health Perspect. 1998;106(suppl 4):961–967. doi: 10.1289/ehp.98106s4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. Progress in lead toxicity research: peeling the onion. Biomedicine. 2001;21:1–17. [Google Scholar]
- Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, et al. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA. 1996;275:1171–1176. [PubMed] [Google Scholar]
- Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaks GJ, Gussekloo J, Dermout KM, Heeren TJ, Ligthart GJ. Three-year follow-up of Mini-Mental State Examination score in community residents aged 85 and over. Psychol Med. 1995;25:841–848. doi: 10.1017/s0033291700035091. [DOI] [PubMed] [Google Scholar]
- Jacqmin-Gadda H, Fabrigoule C, Commenges D, Dartigues JF. A 5-year longitudinal study of the Mini-Mental State Examination in normal aging. Am J Epidemiol. 1997;145:498–506. doi: 10.1093/oxfordjournals.aje.a009137. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The role of iron in neurodegeneration: prospects for pharmacotherapy of Parkinson’s disease. Drugs Aging. 1999;14:115–140. doi: 10.2165/00002512-199914020-00004. [DOI] [PubMed] [Google Scholar]
- Jenner P. Altered mitochondrial function, iron metabolism and glutathione levels in Parkinson’s disease. Acta Neurol Scand. 1993;146(suppl):6–13. [PubMed] [Google Scholar]
- Kim R, Landrigan C, Mossmann P, Sparrow D, Hu H. Age and secular trends in bone lead levels in middle-aged and elderly men: three-year longitudinal follow-up in the Normative Aging Study. Am J Epidemiol. 1997;146:586–591. doi: 10.1093/oxfordjournals.aje.a009318. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78:1290–1308. doi: 10.4065/78.10.1290. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115:521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Andersen JK, Kaur D. Iron dysregulation and neurodegeneration: the molecular connection. Mol Interv. 2006;6:89–97. doi: 10.1124/mi.6.2.6. [DOI] [PubMed] [Google Scholar]
- Lyon E, Frank EL. Hereditary hemochromatosis since discovery of the HFE gene. Clin Chem. 2001;47:1147–1156. [PubMed] [Google Scholar]
- Moalem S, Percy ME, Andrews DF, Kruck TP, Wong S, Dalton AJ, et al. Are hereditary hemochromatosis mutations involved in Alzheimer disease? Am J Med Genet. 2000;93:58–66. doi: 10.1002/1096-8628(20000703)93:1<58::aid-ajmg10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Moura E, Noordermeer MA, Verhoeven N, Verheul AF, Marx JJ. Iron release from human monocytes after erythrophagocytosis in vitro: an investigation in normal subjects and hereditary hemochromatosis patients. Blood. 1998;92:2511–2519. [PubMed] [Google Scholar]
- Muldoon SB, Cauley JA, Kuller LH, Morrow L, Needleman HL, Scott J, et al. Effects of blood lead levels on cognitive function of older women. Neuroepidemiology. 1996;15:62–72. doi: 10.1159/000109891. [DOI] [PubMed] [Google Scholar]
- Nordberg M, Winblad B, Fratiglioni L, Basun H. Lead concentrations in elderly urban people related to blood pressure and mental performance: results from a population-based study. Am J Ind Med. 2000;38:290–294. doi: 10.1002/1097-0274(200009)38:3<290::aid-ajim7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Onalaja AO, Claudio L. Genetic susceptibility to lead poisoning. Environ Health Perspect. 2000;108(suppl 1):23–28. doi: 10.1289/ehp.00108s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton M, Riggs KM, Spiro A, III, Weiss ST, Hu H. Relations of bone and blood lead to cognitive function: the VA Normative Aging Study. Neurotoxicol Teratol. 1998;20:19–27. doi: 10.1016/s0892-0362(97)00075-5. [DOI] [PubMed] [Google Scholar]
- Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Pulliam JF, Jennings CD, Kryscio RJ, Davis DG, Wilson D, Montine TJ, et al. Association of HFE mutations with neurode-generation and oxidative stress in Alzheimer’s disease and correlation with APOE. Am J Med Genet. 2003;119B:48–53. doi: 10.1002/ajmg.b.10069. [DOI] [PubMed] [Google Scholar]
- Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- Sampietro M, Caputo L, Casatta A, Meregalli M, Pellagatti A, Tagliabue J, et al. The hemochromatosis gene affects the age of onset of sporadic Alzheimer’s disease. Neurobiol Aging. 2001;22:563–568. doi: 10.1016/s0197-4580(01)00219-6. [DOI] [PubMed] [Google Scholar]
- Samson FE, Nelson SR. The aging brain, metals and oxygen free radicals. Cell Mol Biol (Noisy-le-grand) 2000;46:699–707. [PubMed] [Google Scholar]
- Stuss DT, Meiran N, Guzman DA, Lafleche G, Willmer J. Do long tests yield a more accurate diagnosis of dementia than short tests? A comparison of 5 neuropsychological tests. Arch Neurol. 1996;53:1033–1039. doi: 10.1001/archneur.1996.00550100119021. [DOI] [PubMed] [Google Scholar]
- Todorich BM, Connor JR. Redox metals in Alzheimer’s disease. Ann NY Acad Sci. 2004;1012:171–178. doi: 10.1196/annals.1306.014. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Townsend A, Drakesmith H. Role of HFE in iron metabolism, hereditary haemochromatosis, anaemia of chronic disease, and secondary iron overload. Lancet. 2002;359:786–790. doi: 10.1016/S0140-6736(02)07885-6. [DOI] [PubMed] [Google Scholar]
- Tuokko H, Frerichs R, Graham J, Rockwood K, Kristjansson B, Fisk J, et al. Five-year follow-up of cognitive impairment with no dementia. Arch Neurol. 2003;60:577–582. doi: 10.1001/archneur.60.4.577. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Projections of the Total Resident Population by 5-Year Age Groups, and Sex with Special Age Categories: Middle Series, 2001 to 2005. 2000a. [[accessed 18 May 2005]]. Available: http://www.census.gov/population/projections/nation/summary/np-t3-b.pdf.
- U.S. Census Bureau. Projections of the Total Resident Population by 5-Year Age Groups, and Sex with Special Age Categories: Middle Series, 2025 to 2045. 2000b. [[accessed 18 May 2005]]. Available: http://www.census.gov/population/projections/nation/summary/np-t3-f.pdf.
- Weisskopf MG, Wright RO, Schwartz J, Spiro A, III, Sparrow D, Aro A, et al. Cumulative lead exposure and prospective change in cognition among elderly men: the VA Normative Aging Study. Am J Epidemiol. 2004;160:1184–1193. doi: 10.1093/aje/kwh333. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82–83:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- Wood SN, Augustin NH. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecolog Modelling. 2002;157:157–177. [Google Scholar]
- Wright RO, Tsaih SW, Schwartz J, Spiro A, III, McDonald K, Weiss ST, et al. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology. 2003;14:713–718. doi: 10.1097/01.EDE.0000081988.85964.db. [DOI] [PubMed] [Google Scholar]
- Wright RO, Silverman EK, Schwartz J, Tsaih SW, Senter J, Sparrow D, et al. Association between hemochro-matosis genotype and lead exposure among elderly men: the Normative Aging Study. Environ Health Perspect. 2004;112:746–750. doi: 10.1289/ehp.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]