Abstract
The application of boron neutron capture therapy to rheumatoid arthritis requires the selective delivery of the boron-10 isotope to the synovitic tissue. The use of liposomes as a boron delivery method has been explored through the measurement of the time course biodistribution of boron in rats with collagen-induced arthritis (CIA). Small unilamellar vesicles were composed of a 1:1 mixture of distearoylphosphatidylcholine and cholesterol, incorporated K[nido-7-CH3(CH2)15-7,8-C2B9H11] as an addend in the lipid bilayer and encapsulated Na3[a2-B20H17NH2CH2CH2NH2] in the aqueous core. The tissue concentration of boron delivered by liposomes was determined by inductively coupled plasma–atomic emission spectroscopy after intravenous injection of liposome suspensions into Louvain rats with CIA. With the low injected doses of boron used [13–18 mg of boron per kg (body weight)], the peak boron concentration observed in arthritic synovium was 29 μg of boron per g of tissue. The highest synovium/blood boron ratio observed was 3.0, when the synovial boron concentration was 22 μg of boron per g of tissue. In an attempt to increase the synovium/blood boron ratio by lowering the blood boron concentration, a liposomal formulation characterized by a shorter blood clearance time was examined. Thus, the biodistribution of liposomes with additional K[nido-7-CH3(CH2)15-7,8-C2B9H11] incorporated in the vesicle membrane not only demonstrated more rapid blood clearance and slightly higher synovium/blood boron ratios but also exhibited reduced boron uptake in synovial tissue. These studies with boron neutron capture therapy for CIA suggest that this form of therapy may be feasible in the treatment of rheumatoid arthritis.
Keywords: vesicles, unilamellar liposomes, synovitis
Rheumatoid arthritis (RA) afflicts between 1% and 2% of the adult population and is the most common cause of chronic inflammatory synovitis. The normal synovium consists of a lining that is two to four cell layers thick. In RA, the synovial pannus is markedly thickened and can destroy articular cartilage and bone (1). In a specific joint, surgical synovectomies are sometimes performed to relieve pain and reduce the potential for joint damage. Local, although temporary, control can sometimes be achieved with intraarticular corticosteroids. Chemical synovectomies, with such agents as thio-tepa or osmic acid, have been of marginal benefit. More recently, the local instillation of radioisotopes such as yttrium-90 or dysprosium-165 have shown modest, although temporary, benefits (2).
Because the synovium is the principal target in RA and behaves much like a local malignancy, the use of boron neutron capture therapy (BNCT) in combination with liposomal boron delivery offers an approach to synovectomy. BNCT was first proposed as a binary approach to cancer treatment by Locher (3) in 1936. BNCT arises from the propensity of the 10B nucleus to capture thermal neutrons. The resultant unstable 11B nucleus undergoes fission to produce a lithium ion and an α particle, as well as 0.48-MeV γ-radiation (1 eV = 1.620 × 10−19 J). These energetic fission products share 2.34 MeV of kinetic energy between them, which provides an effective path length of approximately one cell diameter. The limited range of these products localizes the associated ionization tracking, resulting cellular damage, and subsequent cytotoxicity to those cells that contain a sufficient concentration of 10B. Although most BNCT research has focused mainly on malignant tumor cells, this general approach is applicable to any diseased tissue of a localized nature as long as an effective targeting strategy for selective boron delivery can be implemented.
The application of BNCT to the treatment of arthritis (boron neutron capture synovectomy, BNCS) has been investigated by Yanch et al. (4). These studies have examined the uptake of boron (in the form of boric acid, particulate boron, or Na2B12H12) by synovial tissue in vitro to determine the feasibility of synovial boron delivery by intraarticular injection (4). In addition, significant progress has been reported on the development of neutron sources specifically designed for BNCS (5, 6).
A significant quantity of boron (>15 μg of boron per g of tissue) must be delivered site-specifically to the targeted tissue to ensure successful therapy and cell death (7). Liposomes have been shown to provide a highly selective boron delivery method for certain tumor cells. For example, small (50–80 nm in diameter) unilamellar liposomes encapsulating concentrated aqueous solutions of polyhedral borane anion salts have been shown to accumulate in tumor cells in vivo, delivering therapeutic amounts of boron to the malignant cells but leaving normal tissues, including blood, relatively free of boron (8–11).
Although the mechanism of the tumor cell uptake of liposomes has not been precisely determined, the selective transport of liposomes from blood and into tumor cells is generally attributed to the increased level of neovascularization associated with rapidly growing tumors. New blood vessels tend to be leaky, in part because of vascular endothelial growth factor (also known as vascular endothelial permeability factor). This effect permits the access of liposomes to tumor cells, where they are internalized through endocytosis via an as yet undetermined mechanism such as the coated-pit/coated-vesicle process (12).
Several characteristics of tumor cells in which successful liposomal boron delivery has been achieved are shared by the RA synovium. The synovium is highly vascularized, with a high density of capillaries. These areas of angiogenesis allow diffusion of material both in and out of the surrounding pannus, particularly with increasing inflammation. In addition, the macrophage-like type A lining cells of the synovium are capable of phagocytosis/endocytosis (1). This synovial milieu should be ideal for the delivery of properly sized and formulated boron-containing liposomes to the pathologic pannus of RA. Subsequent exposure to a thermal neutron source would result in the destruction of the diseased synovial tissue. Because liposome targeting has been demonstrated in tumors, the ability of liposomes to deliver therapeutic quantities of boron to rats with collagen-induced arthritis (CIA), an animal model of RA, was examined.
MATERIALS AND METHODS
Materials.
Boron-containing compounds were prepared by published methods (10, 13). Distearoylphosphatidylcholine (DSPC) was obtained from Avanti Polar Lipids, and cholesterol (Ch) was supplied by Sigma. Type II collagen was obtained from Genzyme, and incomplete Freund’s adjuvant was obtained from Difco.
Physical Measurements.
The 11B Fourier transform-NMR spectra were obtained with a Bruker ARX-500 instrument at 160 MHz. Volume-weighted mean vesicle diameters were determined by dynamic light scattering with a Leeds & Northrup Microtrac ultrafine particle analyzer.
Vesicle Preparation.
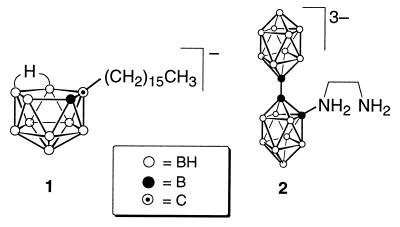
Liposome suspensions were prepared by probe sonication of a dried film (300 mg) composed of equimolar amounts of DSPC and Ch along with various amounts of K[nido-7-CH3(CH2)15-7,8-C2B9H11] (Fig. 1, species 1) with the hydrating solution (6 ml, 200 mM Na3[a2-1-(1′-B10H9)-2-NH2CH2CH2NH2B10H8]; Fig. 1, species 2) at 65°C for 23 min. Vesicles were separated from the remaining free borane salt by elution through a column of Sephadex G-25 with isotonic PBS (140 mM NaCl/10 mM sodium phosphate, pH 7.3) buffer. Liposomal preparations were diluted with buffer to achieve a lipid concentration of 23–24 mg/ml and sterilized by filtration through a 0.22-μm (pore size) membrane. The volume-weighted mean vesicle diameter (mv) of the liposomes reported herein (determined by dynamic light scattering) ranged from 65 to 88 nm.
Figure 1.
Structures of the lipophilic species 1 and the apical–apical (a2) isomer of species 2.
In Vivo Studies.
Biodistribution experiments used inbred female Louvain rats (127–153 g; total n = 45). Type II collagen was solubilized (1 mg/ml) in 0.1 M acetic acid overnight. The solution was emulsified with incomplete Freund’s adjuvant, 1:1, and injected intradermally into 15 sites. The final type II collagen dose was 0.5 mg per rat. CIA developed within 12–14 days in one or both hind legs. Injections of liposome emulsions (1.0 ml) were made in the lateral tail vein. At various time points, each rat was anesthetized with halothane before sacrifice and exsanguinated via cardiac puncture into heparinized syringes. Rats were euthanized with halothane. The synovium, liver, and spleen were dissected, weighed, and placed in nalgene tubes. Blood and tissue samples were stored frozen at −70°C until analyzed. Rat experiments were done in accordance with guidelines of the Animal Welfare Act. Boron analyses of tissues and of liposome emulsions were performed by inductively coupled plasma-atomic emission spectroscopy at the Idaho National Engineering Laboratory (14).
RESULTS
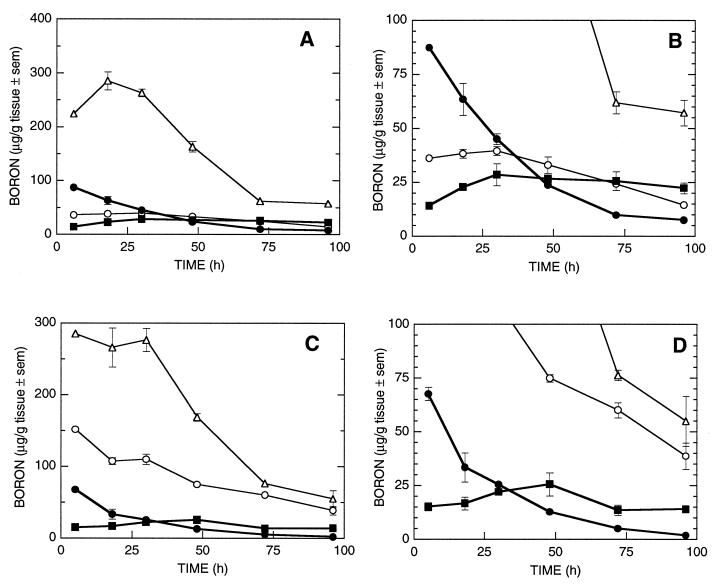
Liposomes (mv = 65 nm) incorporating species 1 in the vesicle bilayer and encapsulating a solution of species 2 were prepared from a lipid mixture of DSPC/Ch/species 1, molar ratio 3:3:1. The time-dependent biodistribution of these liposomes is shown in Fig. 2 A and B [injected dose, 1.6 mg of boron or 13 mg of boron per kg (body weight)]. The concentration of boron found in the synovial tissue increased from 14 μg of boron per g of tissue at 6 h to a maximum value of 29 μg of boron per g of tissue at 30 h. The synovial boron concentration remained relatively constant, dropping to 26 ppm at 72 h at which time a synovium/blood boron ratio of 2.6 was observed. At 96 h after injection, the boron concentration of the synovium was 22 ppm and the synovium/blood boron ratio was 3.0.
Figure 2.
Murine tissue boron concentrations from delivery of borane salts by liposomes: •, blood; ▪, synovium; ○, liver; ▵, spleen. (A and B) Liposomes formulated with 3:3:1 DSPC/Ch/species 1 in the lipid bilayer and encapsulating species 2 at 1.6 mg of boron injected dose [13 mg of boron per kg (body weight)]. (C and D) Liposomes formulated with 3:3:2 DSPC/Ch/species 1 in the lipid bilayer and encapsulating species 2 at 2.3 mg of boron injected dose [18 mg of boron per kg (body weight)].
The time-dependent biodistribution of liposomes (mv = 88 nm) incorporating a larger amount of species 1 in the bilayer and encapsulating a solution of species 2 is shown in Fig. 2 C and D [injected dose, 2.3 mg of boron or 18 mg of boron per kg (body weight)]. Liposomes were prepared from a lipid mixture of 3:3:2 DSPC/Ch/species 1. The concentration of boron found in the synovium increased steadily from 15 μg of boron per g of tissue at 6 h to the observed maximum at 26 μg of boron per g of tissue at 48 h. The observed synovium/blood boron ratio at 48 h was 2.0. After 48 h, the amount of boron found in the synovial tissue slowly decreased.
DISCUSSION
The primary requirement for potential use of BNCT as a method to involute pannus is to identify a technique that delivers sufficiently high concentrations of boron to the synovium to ensure successful therapy. Liposomes (small unilamellar vesicles) have been shown to deliver therapeutic concentrations of boron to tumor cells in vivo (9–11). Similarities between rapidly growing tumor cells and rheumatoid synovium, both with leaky neovascularization and endocytotic capacity, suggest that the use of liposomes as a boron delivery system to arthritic synovium could be efficacious.
The rat CIA model was chosen because of its clinical and pathologic similarities to RA. It exhibits a reliable onset and incidence of disease, accelerated disease course, neovascular dependency, and disease activity similar to that of human RA (15, 16). Biodistribution experiments with this rat model determined the selected tissue boron concentrations at six times over a 96-h period. The synovium was examined to determine whether therapeutic quantities of boron could be delivered by liposomes and subsequently retained for sufficient time to allow the blood to clear of boron. Boron concentrations in the liver and spleen were examined because these tissues compete with diseased tissue for circulating liposomes. Successful liposomal boron delivery requires that the boron-containing species be retained by the synovial cells over time and that boron in the blood is cleared. The selective delivery of boron to synovium may be estimated by the synovium/blood boron ratio.
Liposomes containing species 1 embedded in the lipid bilayer at a 3:3:1 DSPC/Ch/species 1 ratio and species 2 encapsulated in the aqueous core of the liposomes produced the time course biodistribution shown in Fig. 2 A and B (injected dose, 1.6 mg of boron). The synovial tissue accumulates boron at therapeutic levels (>15 μg of boron per g of tissue) at all times examined after 6 h. The maximum synovial boron concentration 29 μg of boron per g of tissue is observed at 30 h and remains fairly constant throughout the remainder of the experiment, dropping only to 22 μg of boron per g of tissue at 96 h. This prolonged retention by synovium provides sufficient time for extensive boron clearance from other tissues. At 96 h, the synovium/blood boron ratio was 3.0.
The observed boron concentrations in the spleen were quite high, >150 μg of boron per g of tissue, throughout the initial time points of the study. However, the spleen did reach more moderate levels after 72 h. Several control rats that did not have CIA were injected with the liposome formulation above by using an injected dose of 1.6 mg of boron as before. These animals exhibited boron levels in the blood, liver, and spleen that were similar to those of the diseased rats. The reason for these unusually high spleen values is not clear, although it probably is secondary to reticular endothelial clearance. Previously measured spleen concentrations in mice have been comparable to or less than simultaneous liver concentrations (9–11). Nonetheless, because RA typically involves the peripheral joints, it should be possible to readily shield internal organs at the time of irradiation with thermal or epithermal neutrons, depending on the desired depth of treatment.
In an effort to accelerate the clearance of boron from the blood, the serum stability of the liposomes was lowered by increasing the proportion of species 1 embedded in the lipid bilayer. The biodistribution of liposomes formulated with a 3:3:2 DSPC/Ch/species 1 bilayer and with species 2 encapsulated in the aqueous core is shown in Fig. 2 C and D (injected dose, 2.3 mg of boron). The accumulated boron in the synovium increased from 15 μg of boron per g of tissue at 6 h to the observed maximum of 26 μg of boron per g of tissue at 48 h (synovium/blood boron ratio of 2). After 48 h, the synovial boron concentration slowly decreased. Although boron concentration in the synovium at 96 h (14 μg of boron per g of tissue) was slightly lower than the desired therapeutic level, the observed synovium/blood boron ratio was 7.5. The use of these less-stable liposomes also greatly increased the amount of boron found in both the liver and the spleen. The observed boron concentrations were >150 μg of boron per g of tissue in both organs at all time points prior to 48 h. These tissues cleared to more moderate boron concentration levels after 72 h.
The clearance of boron from the blood was more rapid when the less-stable liposomes formulated to contain a 3:3:2 DSPC/Ch/species 1 ratio of species in the lipid bilayer were used. After the initial time point, the blood boron concentrations when the 3:3:2 DSPC/Ch/species 1 formulation were used were approximately one-half that of the corresponding boron concentrations observed in the blood when the 3:3:1 DSPC/Ch/species 1 liposomes were used. However, the synovial boron concentrations were more constant over time for the 3:3:1 DSPC/Ch/species 1 liposomes, ending with a 96-h synovial boron concentration (22 μg of boron per g of tissue) higher than the corresponding 3:3:2 DSPC/Ch/species 1 concentration (14 μg of boron per g of tissue).
CONCLUSION
Small unilamellar liposomes provide a viable method for the selective delivery of boron to arthritic synovium, allowing the accretion of therapeutic concentrations of boron suitable for BNCT. Although the injected boron dose was relatively low [12–18 mg of boron per kg (body weight)], biodistribution experiments with liposomes containing species 1 embedded in the bilayer and species 2 encapsulated in the aqueous core demonstrated selective delivery of boron to synovial tissue in therapeutic amounts (>15 μg of boron per g of tissue). Once the boron species are delivered to the synovium by the liposomes, the synovial boron is retained although the boron concentrations in other tissues, particularly the blood, decrease. This study demonstrates the feasibility of using the neovascularization found in pannus as a target for liposomal boron and subsequent BNCT.
Acknowledgments
We thank W. F. Bauer (Idaho National Engineering Laboratory) for the inductively coupled plasma–atomic emission spectroscopy boron analyses. M.F.H. is a member of the Scientific Advisory Board of Neutron Therapies, Inc. This research was performed under the auspices of the Office of Energy Research, U.S. Department of Energy (Contract DE-FG03-95ER61975) and National Institutes of Health Grant AR-42200. Additional support for R.W.C. was provided by National Institutes of Health Grant GM08496 and for E.B. was provided by the Bertram A. Maltz, M.D., Laboratory of Molecular Rheumatology.
ABBREVIATIONS
- RA
rheumatoid arthritis
- BNCT
boron neutron capture therapy
- CIA
collagen-induced arthritis
- DSPC
distearoylphosphatidylcholine
- Ch
cholesterol
References
- 1.Harris E D. Rheumatoid Arthritis. Philadelphia: Saunders; 1997. p. xix. and 12–13. [Google Scholar]
- 2.Kelley W N, Harris E D, Ruddy S, Sledge C B. Textbook of Rheumatology. Philadelphia: Saunders; 1989. pp. 1934–1953. [Google Scholar]
- 3.Locher G L. Am J Roentogenol Radium Ther. 1936;36:1–13. [Google Scholar]
- 4.Johnson L S, Yanch J C, Shortkroff S, Sledge C B. In: Cancer Neutron Capture Therapy. Mishima Y, editor. New York: Plenum; 1996. pp. 183–188. [Google Scholar]
- 5.Binello E, Shefer R E, Yanch J C. In: Advances in Neutron Capture Therapy. Larsson B, Crawford J, Weinreich R, editors. I. Amsterdam: Elsevier Science; 1997. pp. 459–463. [Google Scholar]
- 6.Harling O K, Yanch J C, Choi J R, Solares G R, Rogus R D, Moulin D J, Johnson L S, Olmez I, Wirdzek S, Bernard J A, et al. Nucl Sci Eng. 1992;110:330–348. [Google Scholar]
- 7.Fairchild R G, Bond V P. J Radiat Oncol Biol Phys. 1985;11:831–840. doi: 10.1016/0360-3016(85)90318-9. [DOI] [PubMed] [Google Scholar]
- 8.Hwang K J. In: Liposomes: From Biophysics to Therapeutics. Ostro M J, editor. New York: Dekker; 1987. pp. 109–156. [Google Scholar]
- 9.Shelly K, Feakes D A, Hawthorne M F, Schmidt P G, Krisch T A, Bauer W F. Proc Natl Acad Sci USA. 1992;89:9039–9043. doi: 10.1073/pnas.89.19.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feakes D A, Shelly K, Knobler C B, Hawthorne M F. Proc Natl Acad Sci USA. 1994;91:3029–3033. doi: 10.1073/pnas.91.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feakes D A, Shelly K, Hawthorne M F. Proc Natl Acad Sci USA. 1995;92:1367–1370. doi: 10.1073/pnas.92.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straubinger R M, Papahadjopoulos D, Hong K. Biochemistry. 1990;29:4929–4939. doi: 10.1021/bi00472a025. [DOI] [PubMed] [Google Scholar]
- 13.Georgiev E M, Shelly K, Feakes D A, Kuniyoshi J, Romano S, Hawthorne M F. Inorg Chem. 1996;35:5412–5416. doi: 10.1021/ic960171y. [DOI] [PubMed] [Google Scholar]
- 14.Johnson D A, Siemer D D, Bauer W F. Anal Chim Acta. 1992;270:223–230. [Google Scholar]
- 15.Peacock D J, Banquerigo M L, Brahn E. Cell Immun. 1995;160:178–184. doi: 10.1016/0008-8749(95)80025-e. [DOI] [PubMed] [Google Scholar]
- 16.Peacock D J, Banquerigo M L, Brahn E. J Exp Med. 1992;175:1135–1138. doi: 10.1084/jem.175.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]