Abstract
The development of the devastating neurodegenerative condition, Alzheimer's disease, is strongly associated with amyloid-β (Aβ) deposition, neuronal apoptosis, and cell loss. Here, we provide evidence that implicates these same mechanisms in the retinal disease glaucoma, a major cause of irreversible blindness worldwide, previously associated simply with the effects of intraocular pressure. We show that Aβ colocalizes with apoptotic retinal ganglion cells (RGC) in experimental glaucoma and induces significant RGC apoptosis in vivo in a dose- and time-dependent manner. We demonstrate that targeting different components of the Aβ formation and aggregation pathway can effectively reduce glaucomatous RGC apoptosis in vivo, and finally, that combining treatments (triple therapy) is more effective than monotherapy. Our work suggests that targeting the Aβ pathway provides a therapeutic avenue in glaucoma management. Furthermore, our work demonstrates that the combination of agents affecting multiple stages in the Aβ pathway may be the most effective strategy in Aβ-related diseases.
Keywords: combination therapy, neuroprotection, retinal ganglion cell apoptosis
Although glaucoma, a major cause of blindness worldwide (1), is commonly linked to raised intraocular pressure (IOP) (2), the precise means by which IOP may lead to the irreversible destruction of retinal ganglion cells (RGCs, which are the nerve cells that transfer visual information from the eye to the brain) is far from clear. Indeed, interpretation of the mechanism is further complicated by the fact that damage can also occur at low IOP. Thus, for example, recent evidence indicates progressive visual-field loss in patients despite normalization of IOP with pressure-lowering treatment strategies (3, 4), which means that an alternative approach to the treatment of glaucoma is urgently needed. The principal step leading to irreversible loss of vision in glaucoma is RGC apoptosis, and the question is what mechanisms precede this cell death. Raised IOP in experimental glaucoma models can clearly precipitate RGC apoptosis (5–7) whatever the actual intervening steps. However, the presence of progressive glaucomatous damage in patients with normalized IOP has focused a growing body of work on alternative strategies to those regulating IOP and especially approaches targeting the cellular mechanisms leading to apoptosis.
Amyloid-β (Aβ) is the major constituent of senile plaques in Alzheimer's disease (AD), the formation of which, caused by abnormal processing of amyloid precursor protein (APP), has been involved in AD neuropathology, although the proximate cause of the neurodegeneration responsible for cognitive impairment is not clear (8). Aβ has recently been reported to be implicated in the development of RGC apoptosis in glaucoma, with evidence of caspase-3-mediated abnormal APP processing and increased expression of Aβ in RGCs in experimental glaucoma (9) and decreased vitreous Aβ levels (consistent with retinal Aβ deposition) in patients with glaucoma (10). Further evidence of a link between glaucoma and AD has emerged from studies showing that patients with AD have RGC loss associated with typical glaucomatous changes, such as optic neuropathy and visual functional impairment (11–14), as is also the case in Parkinson's disease (15). In addition, both diseases are chronic neurodegenerative conditions with a strong age-related incidence (16, 17). This finding is further supported by increasing evidence of similar pathological mechanisms involving Aβ leading to RGC loss as implicated in the brain (16, 18–20).
Here, we provide further strong evidence from an animal model of glaucoma supporting the involvement of Aβ in glaucoma-induced apoptosis of RGCs and show that the use of agents targeting multiple phases of the Aβ pathway raises the possibility of a neuroprotective approach to the treatment of glaucoma. By manipulating the Aβ pathway, we investigated three different approaches to targeting Aβ in experimental glaucoma and their combination effects: (i) reduction of Aβ formation by a β-secretase inhibitor; (ii) clearance of Aβ deposition by an anti-Aβ antibody (Aβab); and (iii) inhibition of Aβ aggregation and neurotoxic effects with Congo red (CR). In particular, we show that combined treatment (triple therapy) is more effective than either single- or dual-agent therapy.
Results
We have carried out four groups of experiments to explore the potential role of Aβ in glaucoma. Using an established model of glaucoma in rats, we first explored the way Aβ expression is associated with RGC apoptosis, then we assessed Aβ neurotoxicity on RGC cells in vivo, and finally, we explored the effectiveness of single- and combined-agent therapies, respectively, targeting Aβ in reducing RGC apoptosis in this model.
Aβ Expression and RGC Apoptosis in Experimental Glaucoma.
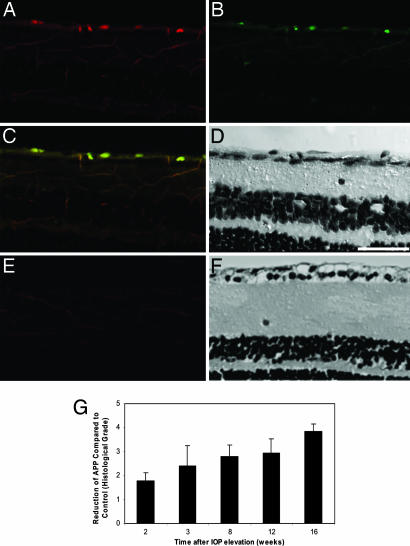
In the established model of glaucoma that we used (5, 6, 21, 22), chronic ocular hypertension (OHT) is surgically induced in one eye of each animal. In these experiments, we observed that the integral IOP, defined as the cumulative effect of IOP elevation over time, increased with time up to 16 weeks (P < 0.01). We sought to determine whether the apoptotic RGC cells resulting from the elevated IOP were linked to a change in the pattern of Aβ deposition. Histological analysis of the effects on the retina of IOP elevation revealed the colocalization of Aβ with apoptotic RGCs (Fig. 1 A–D) compared with normal control (Fig. 1 E and F). Quantitative analysis showed a significant increase of Aβ deposition in the retinal ganglion cell layer (RGCL) in OHT eyes at all of the time points observed, compared with control (P < 0.01), with a peak at 12 weeks (P < 0.01) after raised IOP. On the other hand, the amount of full-length APP expression was significantly decreased in the RGCL in OHT rats compared with control (Fig. 1G, P < 0.01). As a precursor protein, APP might be expected to decrease at a rate equal to the increased deposition of Aβ. The observations on Aβ provide a clear demonstration of Aβ colocalizing to apoptosing RGCs and suggest the potential involvement of Aβ neurotoxicity in the development of glaucomatous RGC death.
Fig. 1.
Aβ and RGC apoptosis. (A–D) Experimental glaucoma model. (E and F) Control. Aβ deposition was labeled by Aβ antibody (A and E, red) colocalized with RGC apoptosis labeled by fluorescent-labeled annexin 5 (B and E, green), from an OHT eye at 2 weeks (A–D). Composite (C and E) and transmission (D and F) images of the retinal cross-section show colocalization to retinal ganglion cell layer of Aβ deposition and RGC apoptosis only in the OHT eye. (G) APP deposition was found to decrease over time (P < 0.01) as assessed by histological grading. (Scale bar: 50 μm.)
Assessment of Intravitreal Aβ Neurotoxicity and RGC Apoptosis in Vivo.
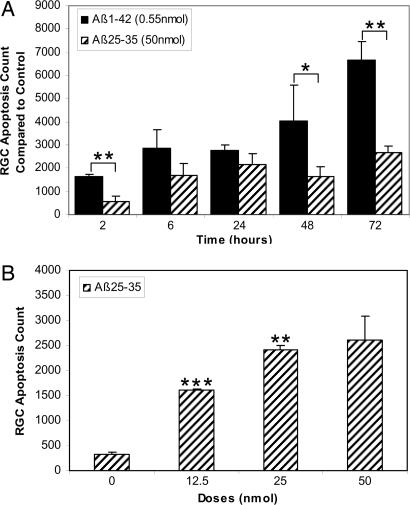
Because Aβ deposition was found to be associated with RGC apoptosis in our experimental glaucoma model, we next investigated the effects of exogenous Aβ peptide on RGC apoptosis in vivo. The Aβ1–40 peptide is known to be neurotoxic in the central nerve system (CNS), and the 25–35 portions (Aβ25–35) are known to contain the neurotoxic elements of Aβ1–40 (23, 24). Recent studies have suggested that the soluble Aβ1–42 peptide oligomer is the most potent neurotoxic form of Aβ in CNS-derived neuronal cultures (25), and recent data suggest that it is the nonfibrillar oligomeric aggregate form of Aβ that is neurocytotoxic (8). We found that both intravitreal Aβ1–42 and Aβ25–35 induced RGC apoptosis in the retina in a time-dependent manner. The peak of RGC apoptosis occurred at 72 h after treatment compared with control (Fig. 2A). We demonstrated significantly more RGC apoptosis with 0.55 nmol of Aβ oligomer compared with 50 nmol of Aβ25–35 (P < 0.05 or P < 0.01; Fig. 2A), consistent with the previous CNS findings that Aβ1–42 oligomer neurotoxicity is 10-fold greater than the insoluble fibrillar form and 40-fold greater than the unaggregated peptide (25). In addition to time-dependent effects [see also supporting information (SI) Movie 1], we also demonstrated dose-dependent effects (Fig. 2B). Aβ thus has a potent neurotoxic effect on RGCs, in line with its possible role in the precipitation of RGC loss in glaucoma.
Fig. 2.
Effects of Aβ on RGC apoptosis in vivo. Both Aβ1–42 and Aβ25–35 induced time- (A) and dose- (B) dependent levels of RGC apoptosis in vivo. (A) Aβ1–42 appeared more toxic to RGCs than Aβ25–35 at much reduced concentrations (0.55 versus 50 nmol, respectively). (B) RGC apoptosis count was found to increase with increasing doses of Aβ25–35. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Single-Agent Therapies Targeting Aβ in Glaucoma.
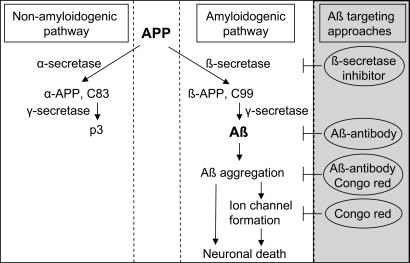
The findings that Aβ is elevated in experimental glaucoma and that exogenous Aβ induces RGC apoptosis suggest that Aβ could be a factor mediating the apoptotic changes in RGC cells in our glaucoma model. If so, we hypothesized that interventions that target Aβ production or its site of action might be expected to reduce the levels of RGC apoptosis. We used several different methods for reducing the effectiveness of Aβ (Fig. 3), including a β-secretase inhibitor (βSI), CR and Aβab. β-Secretase, a membrane-anchored aspartic protease, is responsible for the initial step of APP cleavage in the amyloidgenic pathway leading to the generation of Aβ; βSIs therefore inhibit the production of Aβ, with evidence of in vitro and in vivo efficacy in AD-related models (26, 27). It has been shown that CR completely blocks Aβ aggregation and toxicity in rat hippocampal neuron cultures (28), and it is believed that its inhibitory effects are the result of an interference with Aβ protein misfolding, fibril formation, and aggregation and possibly an action on channel formation (Fig. 3) (28, 29). Aβabs are thought to work by not only blocking Aβ aggregation (30) but also by increasing Aβ clearance in AD-related animal models (31).
Fig. 3.
Approaches for targeting Aβ. APP, a transmembrane protein, has two identified processing pathways: a nonamyloidogenic pathway, where APP is cleaved by α- and γ-secretases producing α-APP and p3, respectively, and an amyloidogenic pathway associated with β- and γ-secretase-mediated cleavage of APP. Aβ may aggregate, deposit, and form ion channels in cell plasma membrane, leading to neuronal death. The βSI is believed to block Aβ formation by inhibiting β-secretase activity. The Aβab is not only able to clear preexisting Aβ but also to block further Aβ aggregation. CR is thought to block Aβ aggregation and neurotoxicity by interfering with protein misfolding and preventing ion channel formation. ⊢, blocking effects.
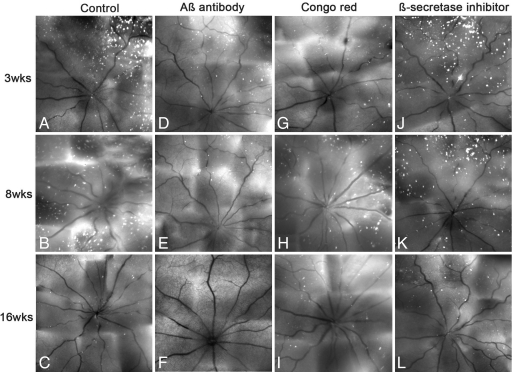
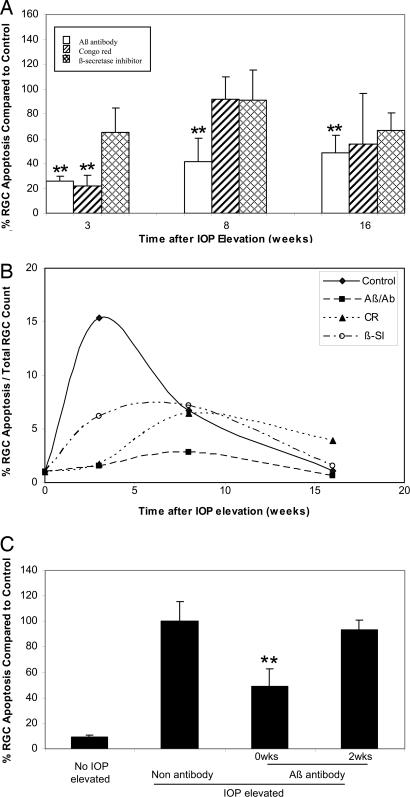
In control experiments for the use of Aβab, we found no significant difference between the level of RGC apoptosis seen with a saline control compared with the IgG1 (null antibody) at any time point. On the other hand, we did see differences between Aβab and control. Detection of apoptosing retinal cells (DARC) real-time images (5) (Fig. 4A–L) and quantitative analysis (Fig. 5A) showed that RGC apoptosis (white spots) was significantly lower than control (Figs. 4 A–C and 5A) with Aβab treatment at all of the time points studied, i.e., 3 (P < 0.01), 8 (P < 0.01), and 16 (P < 0.01) weeks (Figs. 4 D–F and 5A). When we used CR, we only observed a significant reduction in RGC apoptosis at 3 (P < 0.01) weeks (Figs. 4G and 5A). The βSI showed a modest reduction of RGC apoptosis at 3 weeks but did not reach significance compared with control (Figs. 4J and 5A). There were no significant effects with CR or βSI at 8 and 16 weeks compared with control (Figs. 4 H, I, K, and L and 5A).
Fig. 4.
Targeting Aβ in experimental glaucoma. In vivo DARC images show the effects of different approaches targeting Aβ on RGC apoptosis in OHT rats. Eyes were assessed at 3 (A, D, G, and J), 8 (B, E, H, and K), and 16 (C, F, I, and L) weeks after IOP elevation with treatments of Aβab (D–F), CR (G–I), and βSI (J–L), respectively, compared with control (IgG1, no antibody; A–C). The white spots represent apoptotic RGCs labeled by annexin 5.
Fig. 5.
Effects of targeting Aβ on RGC apoptosis. (A) All treatments reduced levels of RGC apoptosis at 3 weeks. Aβ antibody (Aβ/Ab) resulted in a significant reduction of RGC apoptosis at 3, 8, and 16 weeks compared with control. CR showed a significant reduction of RGC apoptosis at 3 but not 8 and 16 weeks. The βSI showed a modest (no significant) decrease of RGC apoptosis at 3 but not 8 and 16 weeks. (B) RGC apoptosis as a percentage of total RGC count with time after IOP elevation. All three treatments delayed peak RGC apoptosis from 3 to 8 weeks with reduced peak levels of RGC apoptosis from 15% to at least 3%. (C) Comparing time of administration in terms of efficacy, OHT animals treated with the Aβ/Ab at the time of IOP elevation (0 weeks) as opposed to 2 weeks later showed a significant reduction in RGC apoptosis at 16 weeks after IOP elevation. **, P < 0.01.
All three treatments appeared to alter the profile of RGC apoptosis in a temporal manner (Fig. 5B) by delaying the development of peak RGC apoptosis as well as influencing the peak level of RGC apoptosis. Hence, untreated OHT eyes were found to have peak levels of RGC apoptosis at 3 weeks (15%) compared with 8 weeks for βSI, CR, and Aβab (7%, 6%, and 3%), respectively (Fig. 5B). It appears that the main effect of the treatments is to suppress the early peak of RGC apoptosis over the first 3 weeks.
In an attempt to assess whether timing of treatment administration affected the development of RGC apoptosis and its profile, we next assessed a group of animals treated with the Aβab at the time of IOP elevation (0 weeks) as opposed to 2 weeks later (2 weeks). Compared with control and 2 weeks, the 0 weeks group showed a significant reduction in RGC apoptosis at 16 weeks after IOP elevation (Fig. 5C, P < 0.01). Thus, it is likely that a protective agent given at the time of IOP elevation would be more effective than when given at 2 weeks later. The Aβ-targeting therapies in this work may act directly by reducing the initial injury of RGCs and subsequently decreasing the secondary effects of the primary injured RGCs (32), especially those produced by oxidative stress mechanisms. This theory probably explains the shift in the peak RGC apoptosis time point in all treatment groups from 3 to 8 weeks. It is further supported by our finding that Aβab treatment at the time of IOP elevation (0 weeks) was more effective than when given at 2 weeks. Basically, we propose that Aβ neurotoxicity may be involved soon after IOP elevation occurs and that inhibiting Aβ production and aggregation at the early stages of glaucoma may offer maximal protection to RGCs.
Overall, the Aβab appeared the most effective in the prevention of RGC apoptosis compared with CR and the βSI. The application of a single dose of the Aβab delayed peak RGC apoptosis and appeared to have prolonged effects, with a reduction of RGC apoptosis up to 16 weeks. These effects may be related to the Aβab not only clearing preexisting Aβ deposition but also blocking Aβ aggregation (Fig. 3) (30). Clinical trials with antibodies to Aβ have also shown effective clearance of Aβ plaques, improved cognitive function, and decreased cerebral volume in AD patients (31). In comparison, CR dramatically reduced RGC apoptosis at 3 weeks and resulted in a delayed peak of RGC apoptosis at 8 weeks after raised IOP. However, compared with the Aβab, CR appeared to have a shorter window of protection against RGC apoptosis.
Although the effects of the βSI were not significant, they did appear to delay the peak RGC apoptosis. There are grounds for expecting the βSI to be more effective (27), and our findings may reflect the low dose we used.
Effect of Combining Agents Targeting Aβ in Glaucoma.
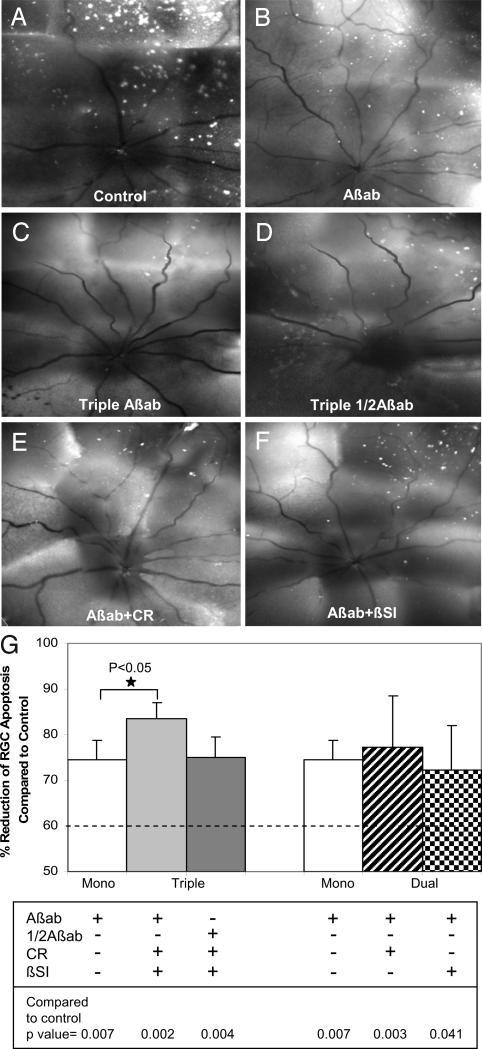
Because each of the agents used above targets different and multiple stages of the Aβ pathway, we checked their effectiveness in combination to see how these differences affected the outcome. Using the same OHT model described before, we assessed different combinations of the single agents, with the same doses as in the monotherapy. In addition, a half-dose of Aβab was also used in the triple-therapy combination. In combination, the neuroprotective effect of all three agents (triple-Aβab therapy) was significantly improved at 3 weeks after IOP elevation compared with Aβab alone (P < 0.05, Fig. 6 A–G). The triple-Aβab therapy resulted in 84% mean reduction of RGC apoptosis compared with 74% by Aβab alone (Fig. 6G). Compared with βSI alone, dual therapy of βSI combined with Aβab showed a significant protective effect on RGC apoptosis (P < 0.05). All other combination therapies showed a significant reduction of RGC apoptosis compared with control, although the results were not significantly better than with Aβab monotherapy (Fig. 6G). Our results suggest that combination therapy targeted at different points of the Aβ pathway may provide the most promising approach to prevent glaucomatous RGC apoptosis. Thus, the Aβab and its use in combination therapy may have great potential in glaucoma treatment.
Fig. 6.
Effect of combining agents targeting Aβ in glaucoma. In vivo DARC images show the effects of triple (C and D) and dual (E and F) therapies on prevention of RGC apoptosis at 3 weeks after IOP elevation compared with control (A) and Aβab monotherapy (B). (G) Triple therapy (triple Aβab) significantly reduced RGC apoptosis compared with Aβab alone (*, P < 0.05). In fact, the triple therapy resulted in 84% mean reduction of RGC apoptosis compared with 74% by Aβab monotherapy. All other combining therapies showed significant reduction of RGC apoptosis compared with control, although there was no statistic significance compared with Aβab monotherapy. For comparison, the dashed line represents the results of our previous study, where we had combined two different glutamate modulators (MK801 and an mGlut agonist) (22) and shown a 60% reduction of RGC apoptosis at 3 weeks after IOP elevation.
Discussion
We have shown here that Aβ is strongly implicated in the development of RGC apoptosis in experimental glaucoma. We also demonstrate in vivo that Aβ peptide induces significant RGC apoptosis. We provide evidence that targeting Aβ and blocking its effects with combination therapy may represent an effective treatment strategy in glaucoma. Our ability to monitor the effects of Aβ therapy in vivo with DARC (5) highlights the potential of this imaging technology in assessing the clinical value of glaucoma treatments.
Non-IOP-lowering treatments have become a key research area in glaucoma because the control of IOP has been shown to be inadequate in the prevention of progressive glaucomatous damage (3, 4). Yet at present, all medical clinical treatments in glaucoma is pressure-lowering, with an estimated cost of $5 billion per annum in America alone by 2011 (33).
Currently, the most widely advocated neuroprotective agents in RGC degeneration are modifiers of the glutamate pathways because excessive activation of glutamate receptors (such as the NMDA receptor) is strongly implicated in the development of RGC apoptosis and loss in glaucoma (22, 34, 35). Memantine is the only clinically approved neuroprotective NMDA antagonist and is currently in a phase III clinical trial of glaucoma (34), although early results suggest that just as in the CNS (36), its clinical effectiveness has failed to live up to expectations (Allergan press release, January 2007).
Our work demonstrates a potential neuroprotective strategy for glaucoma. At present in the field of glaucoma, there is a clear lack of therapies that target the actual causative cellular processes of glaucoma. Targeting Aβ specifically in the eye will provide a localized therapy limiting generalized side effects associated with systemic administration. Furthermore, it will provide treatment only to those areas with degenerating activity.
In conclusion, we have shown that Aβ is a likely mediator of pressure-induced RGC death and that neutralizing antibodies to Aβ can significantly delay and attenuate RGC apoptosis in experimental glaucoma. Perhaps the most exciting finding of the work has been that combination therapy, targeting three different aspects of the Aβ pathway, produced the maximal reduction of RGC apoptosis (>80%). These findings suggest that by manipulating the signaling pathways that drive RGC apoptosis, it may be possible to protect RGCs from degeneration and loss in glaucoma. In this context, we have shown that Aβ could be a particularly suitable target for therapeutic intervention in the eye, in an area in clear need of novel and cellular-based neuroprotective strategies.
Materials and Methods
Animal Experiments.
All procedures were in accordance with the regulations of U.K. Home Office and the statement of Association for Research in Vision and Ophthalmology for the use of animals in research and were performed under general anesthesia. Adult male Dark Agouti rats (150–200 g) were used in this work. All animals were imaged in vivo with fluorescent-labeled annexin 5 with our recently established imaging technique DARC (5, 22).
RGC Identification.
For identification of RGCs, rats had RGCs retrogradely labeled by the application of 1,1′-dioctadecy1–3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil; DiIC18) (3) (Molecular Probes, Eugene, OR) to both superior colliculi as described previously (5, 6, 37). Ten days after the Dil labeling, the rats underwent either surgery to elevate IOP or Aβ intravitreal application.
Experimental OHT.
Unilateral elevation of IOP was surgically induced in left eyes of 25 rats by injection of hypertonic saline (1.80 M) into episcleral veins (5, 6, 21). Contralateral unoperated eyes served as a control. The IOP of both eyes in each rat was measured at regular intervals with a Tonopen XL, and the integral IOP was calculated (5, 6). Animals were imaged with DARC at 2, 3, 8, 12, and 16 weeks (n = 5 at each time point) and killed for histology immediately after imaging.
Aβ-induced RGC Apoptosis.
Aβ25–35 (Sigma–Aldrich, St. Louis, MO) dissolved in sterilized water (12.5–50 nmol) or freshly made Aβ1–42 oligomers (Sigma–Aldrich, 0.55 nmol) (25) were intravitreally injected in Dark Agouti rats (Aβ25–35, n = 32; Aβ1–42, n = 20). Animals were imaged with DARC at 2, 6, 24, 48, and 72 h, with at least four animals at each time point being killed for histology soon after imaging. The contralateral eyes were used as controls and injected with vehicle (sterilized water).
Treatments for OHT Rats.
OHT rats were treated by different therapies targeting Aβ. Rats at the time of IOP elevation (0 weeks) were given intravitreal injections (5 μl total volume) with the following Aβ targets: monoclonal Aβab IgG1k (0.5 mg/ml, n = 5; Biosource, Camarillo, CA), βSI (10 μg/ml, Z-VLL-CHO, n = 5; Calbiochem, San Diego, CA), CR (1.46 mg/ml, n = 5; Sigma–Aldrich), and IgG1k-purified protein (null antibody, 0.5 mg/ml, n = 5; AbD serotec, Raleigh, NC) and saline (n = 5) as control. All treated animals were imaged with DARC at baseline and 3, 8, and 16 weeks and then killed immediately for histology. A further study was performed to investigate the effects of timing administration of Aβ target on prevention of RGC apoptosis. Briefly, OHT rats were intravitreally injected with Aβab (0.5 mg/ml, 5 μl) at 0 (n = 5) or 2 (n = 5) weeks after IOP elevation or with null antibody (0.5 mg/ml, 5 μl) as control. For combination therapy, individual treatments were combined with the same doses as in the monotherapy. In addition, a half-dose of Aβab (1/2Aβab) was also used for triple combination. Five OHT rats were used in the each combination treatment.
Histology.
After the animals were killed, their eyes were enucleated and fixed immediately in 4% fresh paraformaldehyde overnight, after which the eyes were dissected at the equator, the lens and vitreous were removed, and whole mount retinas were collected.
Confocal Microscopy.
Fluorescent retinas were assessed with a confocal laser scanning microscope (CLSM 510 META; Zeiss, Gottingen, Germany) with LSM software. This process allowed visualizing annexin 5-labeled RGC apoptosis, Dil-labeled RGCs, and Cy3- or Cy5-labeled Aβ and APP in both retina whole mounts and cross-sections. For the whole-mount retina (magnification, ×16), we assessed 81 adjacent fields (each measuring 0.329 mm2) radiating outward from the optic nerve head in the rat, and accounting for 40% of the whole retina. A retinal montage was then constructed for each whole retina (5, 6, 22, 38).
Aβ and APP Localization.
For immunohistochemical localization of Aβ, paraffin cross-sections were incubated with mouse monoclonal Aβab (1:500 in PBS) (39), followed by incubation with Cy3- or Cy5-conjugated donkey anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA) (6). The same procedure was performed for APP immunolabeling with mouse anti-full-length APP monoclonal antibody (1:1,000 in PBS; Chemicon, Temecula, CA).
Image Analysis.
The number of apoptotic RGCs labeled by annexin 5 and RGCs labeled by DiI was counted manually with MetaMorph software (Universal Imaging Corp., West Chester, PA) by observers masked to treatment protocols. The amount of RGC apoptosis was presented either by density (number per area) or RGC apoptosis as percentage of the total RGC count. Aβ and APP immunolabeling on paraffin cross-sections was assessed by four independent and masked observers who used a grading system and analysis methods well known to our group (6, 40, 41). (Scale: −4 to + 4, where 0 = same as control eye, 1 = 1–25% of control, 2 = 26–50% of control, 3 = 51–75% of control, 4 = >75% of control; prefix + is more than, and prefix − less than). Statistical analysis was performed with Student's t test, ANOVA, and Pearson's correlation.
Supplementary Material
Acknowledgments
This work was supported by Wellcome Trust Grants GR063658 and GR076947 (to L.G.).
Abbreviations
- Aβ
amyloid-β
- Aβab
antibody to Aβ
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- βSI
β-secretase inhibitor
- CR
Congo red
- DARC
detection of apoptosing retinal cells
- IOP
intraocular pressure
- OHT
ocular hypertension
- RGC
retinal ganglion cell.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703707104/DC1.
References
- 1.Quigley HA, Broman AT. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer A. Am J Ophthalmol. 1989;107:186–188. doi: 10.1016/0002-9394(89)90221-3. [DOI] [PubMed] [Google Scholar]
- 3.Oliver JE, Hattenhauer MG, Herman D, Hodge DO, Kennedy R, Fang-Yen M, Johnson DH. Am J Ophthalmol. 2002;133:764–772. doi: 10.1016/s0002-9394(02)01403-4. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Normal-Tension Glaucoma Study Group T. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 5.Cordeiro MF, Guo L, Luong V, Harding G, Wang W, Jones HE, Moss SE, Sillito AM, Fitzke FW. Proc Natl Acad Sci USA. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Invest Ophthalmol Visual Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Invest Ophthalmol Visual Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 8.Pepys MB. Annu Rev Med. 2006;57:223–241. doi: 10.1146/annurev.med.57.121304.131243. [DOI] [PubMed] [Google Scholar]
- 9.McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H, et al. Invest Ophthalmol Visual Sci. 2002;43:1077–1087. [PubMed] [Google Scholar]
- 10.Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Jpn J Ophthalmol. 2005;49:106–108. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 11.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Clin Neurophysiol. 2001;112:1860–1867. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 12.Iseri PK, Altinas O, Tokay T, Yuksel N. J Neuroophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 13.Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH. Neurobiol Aging. 1996;17:385–395. doi: 10.1016/0197-4580(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 14.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Neurobiol Aging. 1996;17:377–384. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 15.Bayer AU, Keller ON, Ferrari F, Maag KP. Am J Ophthalmol. 2002;133:135–137. doi: 10.1016/s0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. Proc Natl Acad Sci USA. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wostyn P. Med Hypotheses. 2004;62:925–930. doi: 10.1016/j.mehy.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Archer S, Hirano J, Diss JK, Fraser SP, Djamgoz MB. Neuroreport. 1998;9:2049–2056. doi: 10.1097/00001756-199806220-00026. [DOI] [PubMed] [Google Scholar]
- 19.Loffler KU, Edward DP, Tso MO. Invest Ophthalmol Visual Sci. 1995;36:24–31. [PubMed] [Google Scholar]
- 20.Vickers JC, Lazzarini RA, Riederer BM, Morrison JH. Exp Neurol. 1995;136:266–269. doi: 10.1006/exnr.1995.1104. [DOI] [PubMed] [Google Scholar]
- 21.Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 22.Guo L, Salt TE, Maass A, Luong V, Moss SE. Invest Ophthalmol Visual Sci. 2006;47:626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowall NW, McKee AC, Yankner BA, Beal MF. Neurobiol Aging. 1992;13:537–542. doi: 10.1016/0197-4580(92)90053-z. [DOI] [PubMed] [Google Scholar]
- 24.Yankner BA, Duffy LK, Kirschner DA. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 25.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 26.Citron M. J Neurosci Res. 2002;70:373–379. doi: 10.1002/jnr.10393. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto R, Yoneda S, Hara H. Neurosci Lett. 2004;370:61–64. doi: 10.1016/j.neulet.2004.07.087. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzo A, Yankner BA. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirakura Y, Lin MC, Kagan BL. J Neurosci Res. 1999;57:458–466. [PubMed] [Google Scholar]
- 30.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 31.Vasilevko V, Cribbs DH. Neurochem Int. 2006;49:113–126. doi: 10.1016/j.neuint.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Levkovitch-Verbin H, Quigley HA, Martin KR, Zack DJ, Pease ME, Valenta DF. Invest Ophthalmol Visual Sci. 2003;44:3388–3393. doi: 10.1167/iovs.02-0646. [DOI] [PubMed] [Google Scholar]
- 33.Lee PP, Walt JG, Doyle JJ, Kotak SV, Evans SJ, Budenz DL, Chen PP, Coleman AL, Feldman RM, Jampel HD, et al. Arch Ophthalmol. 2006;124:12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Lipton SA. J Alzheimers Dis. 2004;6:S61–S74. doi: 10.3233/jad-2004-6s610. [DOI] [PubMed] [Google Scholar]
- 35.Farlow MR. Geriatrics. 2004;59:22–27. [PubMed] [Google Scholar]
- 36.Ringman JM, Cummings JL. Behav Neurol. 2006;17:5–16. doi: 10.1155/2006/315386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naskar R, Wissing M, Thanos S. Invest Ophthalmol Visual Sci. 2002;43:2962–2968. [PubMed] [Google Scholar]
- 38.Guo L, Tsatourin V, Luong V, Podoleanu AG, Jackson DA, Fizke FW, Cordeiro MF. Br J Ophthalmol. 2005;89:1210–1216. doi: 10.1136/bjo.2004.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, et al. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordeiro MF, Reichel MB, Gay JA, D'Esposita F, Alexander RA, Khaw PT. Invest Ophthalmol Visual Sci. 1999;40:1975–1982. [PubMed] [Google Scholar]
- 41.Cordeiro MF, Gay JA, Khaw PT. Invest Ophthalmol Visual Sci. 1999;40:2225–2234. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.