Abstract
Binding of the N-terminus of fibronectin to assembly sites on the cell surface is an essential step in fibronectin fibrillogenesis. Fibronectin matrix assembly sites have customarily been quantified using an iodinated 70 kDa N-terminal fibronectin fragment. The 125I-70K fragment is a less than ideal reagent because its preparation requires large amounts of plasma fibronectin and it has a fairly short shelf life. An additional limitation is that the cells responsible for binding the 125I-70K cannot be quantified or identified directly but must be assessed in parallel cultures. To overcome these disadvantages, we developed an ELISA-based assay using a recombinant HA-tagged 70K fragment. This assay allows for the simultaneous quantification and localization of matrix assembly sites on the surface of adherent cells.
Keywords: Fibronectin, Vitronectin, Extracellular matrix, Fibronectin matrix assembly sites, Cell layer ELISA
1. Introduction
Fibronectin matrix assembly is a cell-dependent process that occurs on the surface of adherent cells and regulates cell behavior. Fibronectin matrix assembly is initiated through the binding of the N-terminus of fibronectin to matrix assembly sites (McKeown-Longo and Mosher, 1985). The molecular structure of fibronectin matrix assembly sites is not known but may consist of multi-protein complexes found in close association with β1 integrins and focal adhesions (Christopher et al., 1997; Hocking et al., 1996; Pankov et al., 2000; Zhang and Mosher, 1996).
The expression of assembly sites on the cell surface has typically been assessed using a radioiodinated 70 kDa fragment (125I-70K) derived from cathepsin digestion of plasma fibronectin. We have now developed a new assay to measure matrix assembly sites. This assay does not require human plasma or the use of radioactivity, thereby saving time as well as the need for oversight by Institutional Biosafety Committees. Understanding the regulation of assembly site expression will provide important insights into the etiology of pathologies associated with the deregulation of fibronectin deposition: tumor progression, organ fibrosis and abnormal wound healing.
2. Results and Discussion
To facilitate studies on matrix assembly site expression, we have developed a cell layer ELISA using a recombinant hemagglutinin-tagged 70K (HA-70K) as probe. The binding activity of HA-70K was compared with that of 125I-70K using competition assays on monolayers of either human dermal fibroblasts (A1-F) (Fig. 1A) or fibronectin-null mouse fibroblasts (5F) (Fig. 1B). As shown in Fig. 1, there was a dose-dependent inhibition of 125I-70K binding to cell layers by the addition of either the catheptic 70K fragment or the recombinant HA-70K. The inhibitory kinetics of both the proteolytic and recombinant 70K fragments on the binding of 125I-70K was similar, with nearly complete inhibition of binding seen within a hundred fold excess of competitor. These data suggest that recombinant HA-70K exhibits similar binding affinity for cell layers as does the proteolytic fragment of plasma fibronectin. The binding of HA-70K to cell layers was dose-dependent with a linear range between 2 to 20 μg/ml when measured directly by ELISA using antibody to the HA-tag (Fig. 1C). Another recombinant fibronectin fragment HA-40K served as a negative control. Earlier studies have shown that the catheptic 70K fragment localizes to focal contacts (Christopher et al., 1997). The localization of recombinant HA-70K to focal contacts was demonstrated using antibody to the HA-tag. Fig. 1D shows that matrix assembly sites are expressed by all cells in the field and that HA-70K localized in linear streaks which were closely associated with paxillin-containing focal contacts (yellow). These data suggest that recombinant HA-70K exhibited similar binding kinetics and subcellular localization as the 70K proteolytic fragment.
Fig. 1.
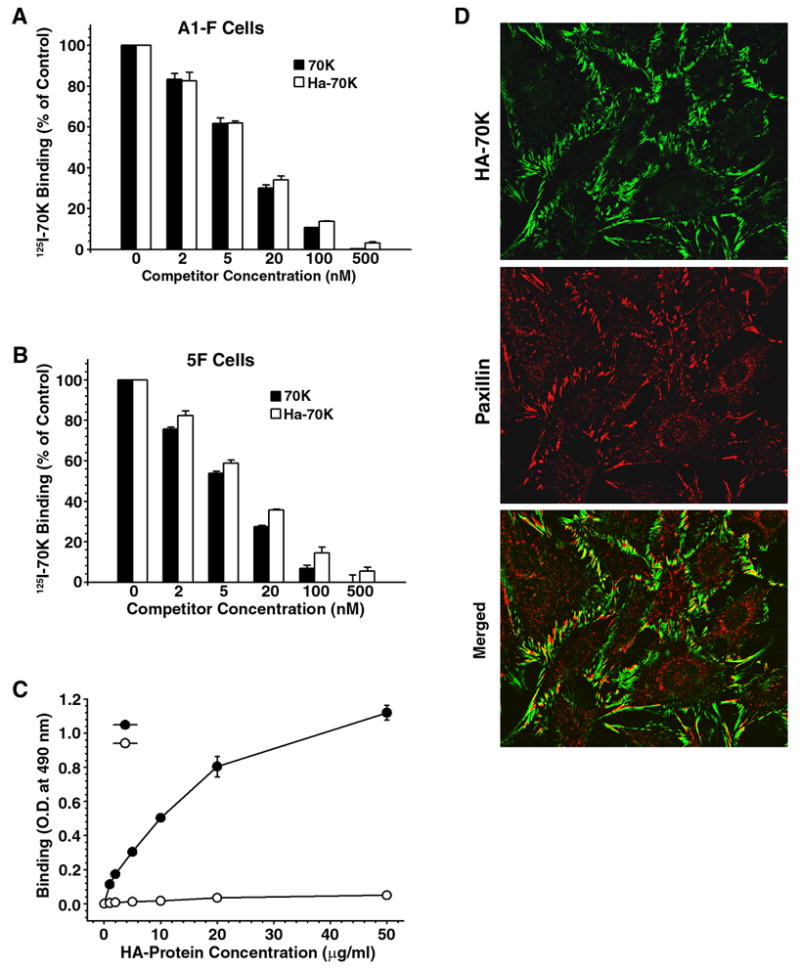
Binding and localization of HA-70K to adherent fibroblasts. Human fibroblasts, A1-F (Panel A) and FN-null mouse fibroblasts, 5F cells (Panel B–D) suspended in DMEM containing 0.1% heat-inactivated BSA were plated at confluent densities onto FN-coated plates and incubated overnight. A & B - Cell monolayers were incubated with 5 nM 125I-70K fragment and either unlabeled recombinant HA-70K or the 70K cathepsin fragment at the indicated concentration. 125I-70K binding in wells which did not receive any protein competitor were expressed as 100%. C - Cell monolayers on FN-coated wells were incubated with increasing concentrations of HA-70K or HA-40K. Bound HA-proteins were determined by ELISA as described under Experimental Procedures. (D) 5F cells on FN-coated coverslips were incubated with HA-70K. Cell layers were subjected to dual immunofluorescence staining: HA-70 kDa was visualized using Alexa-488 (green); paxillin was stained with Alexa-594 (red).
Previous studies have shown that vitronectin negatively regulates matrix assembly site expression (Hocking et al., 1999; Zhang et al., 1999). To determine whether similar results could be obtained using HA-70K as probe, fibronectin-null cells were seeded onto substrates comprised of either fibronectin or the GST fusion protein containing the integrin binding domain found in the connecting sequence (VNcs) of vitronectin. Fig. 2A shows that cells adherent to VNCS bound 90% less HA-70K than cells adherent to fibronectin. These results are consistent with earlier studies and indicate that cells adherent to vitronectin are unable to support matrix assembly. The addition of increasing amounts of VNCS to a fibronectin substrate did not suppress the binding of 70K to adherent cells. In fact, at low concentrations, VNCS elicited a slight increase in matrix assembly site expression. The stimulatory activity of VNCS on fibronectin matrix assembly is consistent with earlier experiments showing that vitronectin supported fibronectin assembly (Pankov et al., 2000). In contrast, Fig. 2B shows that fibronectin could rescue the binding of HA-70K on cells adherent to VNCS. The effect of fibronectin on matrix assembly site expression was dose-dependent, with full restoration of HA-70K binding at 10–20 nM fibronectin. These results, which are in agreement with earlier studies (Bae et al., 2004), indicate a vitronectin substrate is not permissive for matrix assembly and that the effect of fibronectin on the expression of matrix assembly sites is dominant.
Fig. 2.
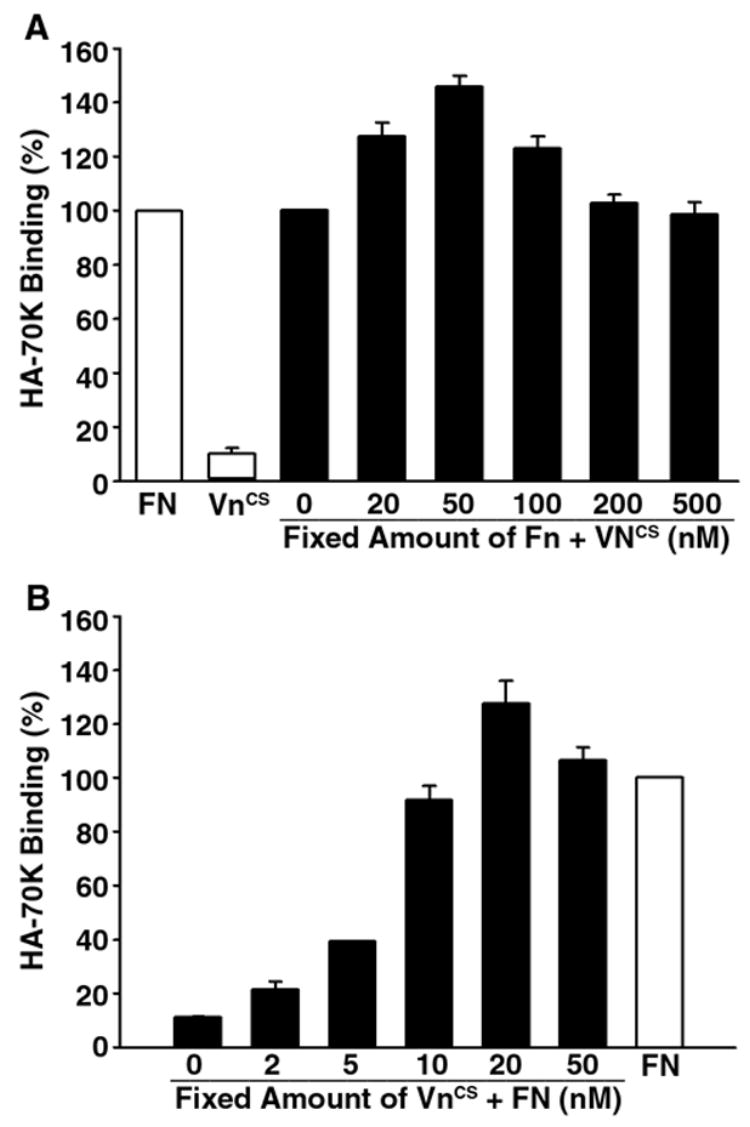
The effect of fibronectin on matrix assembly site expression is dominant. Panel A: 48-well suspension culture plates were coated with FN (15 nM, 0.15 ml/well) at 4°C overnight. Coated wells were washed with PBS and further coated with increasing concentrations of VNCS (from 20–500 nM). Some wells were coated with only VNCS (50 nM). Panel B: 48-well plates were coated with VNCS (40 nM, 0.15 ml/well) at 4°C overnight and further coated with increasing concentrations of FN (from 2–50 nM). Some wells were coated with only FN (20 nM). 5F cells were cultured in coated wells overnight in DMEM containing 0.1% heat-inactivated BSA. HA-70 kDa binding assays were performed as described under Fig. 1. Bound HA-70 kDa in wells coated with FN alone was set as 100%.
These studies describe a novel ELISA for the quantitation and localization of matrix assembly sites. Our results indicate that the recombinant HA-70K probe is as effective as the radioiodinated-70K catheptic fragment of fibronectin in detecting matrix assembly sites. The ELISA has advantages over earlier methods in that it does not require the use of radioactivity, allows for visualization of bound ligands and direct normalization of bound 70K to cell number.
3. Experimental Procedures
3.1 Cell culture
Human foreskin dermal fibroblasts (A1-F) and fibronectin-null (FN −/−) mouse fibroblasts (5F) and MCF7-α18 cells were maintained as previously described (McLeskey et al., 1993; Saoncella et al., 1999; Zheng and McKeown-Longo, 2002).
3.2 Purification of proteins
Preparation of human plasma fibronectin (FN) and N-terminal 70K fragment of FN has been previously described (Zheng and McKeown-Longo, 2002). Recombinant 70K N-terminal region of fibronectin (aa Gln-35 through Ser-608; bases 23–1744; EMBL: X02761) was generated through PCR amplification of the human fibronectin cDNA clone pFH100 (Hocking et al., 1994). An HA-tag was introduced on the C-terminus of 70K, and the human fibronectin signal sequence (aa Met-1 through Gln-32; bases 567–672; EMBL: M15801) was engineered on the N-terminus. Recombinant 40K segment of fibronectin was generated from the C-terminal region of 70K (amino acids Gln-295 through Ser-608; bases 803–1744). The DNAs were cloned into expression vector pcDNA6/Myc-His (Invitrogen, Rockville, MD) and introduced into the MCF7-α18 cells (McLeskey et al., 1993). Stable transfectants were established from the blasticidin S-resistant clones. HA-70K in serum-free culture supernatant was bound to gelatin-agarose and eluted with 6 M urea. HA-70K fraction was further bound to heparin-agarose and eluted with 20–300 mM gradient NaCl. HA-40K containing serum-free culture supernatant was dialyzed against 10 mM phosphate buffer and applied to a heparin-agarose column. HA-40K in the pass-through fraction was purified by gelatin-agarose chromatography as described above. The procedure can be applied to purified HA-70K and HA-40K from culture supernatant with serum without contamination with intact FN. Human fibronectin connecting sequence (VNCS), cDNA encoding aa Lys-40 through Pro-131 was cloned into bacterial expression vector pGEX-2T. GST-VNCS fusion protein was expressed in BL21 bacteria and purified from bacterial lysates.
3.3 Quantification of matrix assembly sites
To quantify matrix assembly sites by ELISA, cells were plated onto 24-well and 48-well suspension culture plates coated with adhesion proteins. Cell layers were incubated with either HA-70K or HA-40K in DMEM containing 0.2% heat-inactivated BSA for 1 h. Coated wells without cells were incubated with HA-tagged fragments and used as the blank control. Wells were washed and fixed in 3.7% paraformaldehyde and blocked with 5% nonfat milk. Bound HA-tagged proteins were detected by incubation of the cell layers with 1:1000 dilution of rabbit anti-HA polyclonal IgG (Santa Cruz Biotechnology) at room temperature for 2 h. After washing, the cell layers were incubated with 1:1500 dilution of HRP-conjugated goat anti-rabbit IgG (Bio-Rad) at room temperature for 1 h. Color was developed with o-phenylenediamine solution, and O.D. at 490 nm was measured. After ELISA, the cell layers were washed with dH2O, stained with 0.05% toluidine blue and destain with dH2O. Color was extracted with 10% acetic acid and O.D. at 650 nm was measured to determine the cell number. Measurement of matrix assembly sites using 125I-70K has been described previously (McKeown-Longo and Mosher, 1985).
3.4 Fluorescence microscopy
Cell layers grown on glass coverslips were fixed in PBS containing 3.7% paraformaldehyde, permeabilized with 0.2% Triton X-100 and blocked with 3% BSA. The cell layers were incubated with primary antibody diluted in blocking buffer, followed by fluorescence conjugated secondary antibody. The cell layers were examined using an Olympus BMX-60 microscope equipped with a cooled CCD sensi-camera (Cooke). Images were acquired using Slidebook software (Intelligent Imaging Innovation).
The success of the protocol described here does not depend on the use of a particular cell line, such as the line MCF-7α18 used in this study. One can prepare recombinant HA-70K in any mammalian cell line routinely used for protein expression. We will make the HA-70K plasmid available to anyone who requests ipt.
Acknowledgments
The authors thank Dr. Richard O. Hynes (Massachusetts Institute of Technology, Cambridge, MA) for providing fibronectin-null mouse fibroblasts, Dr. Fran Kern (Southern Research Institute, Birmingham, AL) for providing MCF7α18 cells, and Ms. Carol Horzempa for technical assistance. This work was supported by R01 CA-69612 and CA-58626 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells is dependent on the adhesive substrate. J Biol Chem. 2004;279:35749–35759. doi: 10.1074/jbc.M406283200. [DOI] [PubMed] [Google Scholar]
- Christopher RA, Kowalczyk AP, McKeown-Longo PJ. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci. 1997;110:569–581. doi: 10.1242/jcs.110.5.569. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–19191. [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Reho T, Fassler R, McKeown-Longo PJ. Inhibition of fibronectin matrix assembly by the heparin-binding domain of vitronectin. J Biol Chem. 1999;274:27257–27264. doi: 10.1074/jbc.274.38.27257. [DOI] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of the 70 kilodalton amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeskey SW, Kurebayashi J, Honig SF, Zwiebel J, Lippman MEDRB, Kern FG. Fibroblast growth factor 4 transfection of MCF-7 cells produces cell lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude mice. Cancer Res. 1993;53:2168–2177. [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Dehnez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Mosher DF. Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. J Biol Chem. 1996;271:33284–33292. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sakai T, Nowlen J, Hayashi I, Fassler R, Mosher DF. Functional β1-integrins release the suppression of fibronectin matrix assembly by vitronectin. J Biol Chem. 1999;274:368–375. doi: 10.1074/jbc.274.1.368. [DOI] [PubMed] [Google Scholar]
- Zheng M, McKeown-Longo PJ. Regulation of HEF1 expression and phosphorylation by TGF-β1 and cell adhesion. J Biol Chem. 2002;277:39599–39608. doi: 10.1074/jbc.M202263200. [DOI] [PubMed] [Google Scholar]


