Abstract
Purpose
To explore clinical/pathologic factors associated with prognosis of patients with locally advanced cervical carcinoma treated with weekly cisplatin and pelvic radiation.
Methods
We retrospectively reviewed data from 335 women who received weekly cisplatin and radiation while participating in similar arms of two GOG studies (protocols 120 and 165). Progression-free survival (PFS) and overall survival (OS) were evaluated for associations between clinical/pathologic factors and prognosis. Prognosis and selected toxicities were also compared between studies
Results
Four-year PFS and OS for stage II patients was 64.2% and 68.1%, respectively for those treated on GOG 120 and 65.8% and 73.9% for those treated on GOG 165, compared to 51.4% and 55.4% for stage III/IV patients respectively treated on GOG 120 and 37.7% and 42.7% respectively for those treated on GOG 165. In multivariate analysis, stage, tumor grade, race and age were independently predictive of PFS and OS (for all, p< .05). Prolonged (delayed for any cause) radiation was associated with poorer PFS (hazard ratio [HR], 1.98; 95% confidence interval [CI], 1.16–3.38; p=0.012) and OS (HR, 1.88; 95% CI, 1.08–3.26; p=0.024) in GOG 165 but not GOG 120.
Conclusions
FIGO stage, tumor grade, race and age are prognostic in patients with locally advanced cervical carcinoma treated with concurrent cisplatin and radiation. This exploratory analysis has generated a hypothesis that clinical staging (as per GOG 165) is less sensitive in detecting aortic nodal metastases compared to surgical staging (as per GOG 120) and may be associated with poorer prognosis particularly when radiation is prolonged. Prospective clinical studies are needed to test this hypothesis.
Keywords: cervical cancer, radiation, chemotherapy, clinicopathologic, prognosis
INTRODUCTION
In the United States, the majority of cervical carcinoma patients are diagnosed with early stage disease. Among the 13,458 staged patients with cervical carcinoma registered by the SEER program between 1973 and 1987, 71% were diagnosed with International Federation of Gynecology & Obstetrics (FIGO) stage I-IIA tumors1. Most of these women with early lesions are cured with surgery or radiation (RT) alone. However, patients with more advanced lesions are at greater risk of recurrence and account for the majority of cervical cancer deaths2.
The standard prescription for RT used to treat bulky or locally advanced cervical cancer has been dictated by common practice and Patterns of Care Studies3–5. In contrast, the addition of concomitant chemotherapy to RT has been studied in a number of randomized prospective trials6–10. When added to RT, cisplatin reduces the relative risk of death from cervical carcinoma by approximately 50% by decreasing local/pelvic failure and distant metastases. In 1999, weekly intravenous cisplatin 40 mg/m2 for 6 weeks in combination with RT was established as a new standard for the treatment of locally advanced cervical carcinoma6,7. This dose and schedule were favored because Gynecologic Oncology Group (GOG) protocol 120 showed it to be more convenient, equally efficacious and less toxic than other cisplatin regimens or combinations using 5-fluorouracil (5-FU) and/or hydroxyurea8. Indeed, all subsequent GOG randomized trials in this disease have used this dose and schedule as the standard arm, including GOG 1659 which compared protracted venous infusion 5-FU and RT to standard cisplatin and RT in patients with Stage IIB, IIIB and IVA cervical cancer.
There is a renewed interest and need to reevaluate the effects of common clinical and pathologic factors in today’s era of concomitant therapy; some of these were previously studied prior to the development of contemporary RT protocols that include chemotherapy10,11. The current report describes an exploratory analysis of selected patients on GOG 120 and GOG 165 who were treated with weekly cisplatin/pelvic RT in order to investigate the association between common clinical and pathological factors and progression-free survival (PFS) and overall survival (OS). We were also interested in assessing the association between RT duration and prognosis, stratified by these two protocols.
METHODS
GOG 120 and GOG 165 were randomized prospective studies treating eligible patients with primary, previously untreated, histologically-confirmed invasive (Stage IIB to IVA) squamous cell carcinoma, adenocarcinoma or adenosquamous carcinoma of the uterine cervix. Patients on GOG 120 were enrolled in the study from April 1992 to April 1997 and patients on GOG 165 were enrolled in the study from October 1997 to July 2000. Arm 1 in each protocol consisted of intravenous cisplatin 40 mg/m2 weekly for 6 weeks in combination with RT. GOG 165 excluded patients with Stage IIIA disease or lower-third vaginal involvement because of the individualized RT techniques required for these patients. Patients were required to have adequate hematologic, renal and hepatic function, and GOG performance status of 0–3, to be eligible. Chemotherapy was identical in the two protocols, but between-study differences in RT included an increased Point A radiation dose and posterior field size, and shorter RT duration, in GOG 165. Additionally, surgical staging of the aortic nodes was required for GOG 120, but was optional on GOG 165.
The prognostic significance of age, ethnicity, GOG performance status, histology, FIGO stage, tumor grade, and tumor size was assessed by combining the two groups of women treated on GOG 120 and 165 (Table 2). This grouping is based on the assumption that these factors have similar associations with PFS and OS between studies. However, with the evolution of better imaging modalities making surgical staging no longer required on GOG 165 as it was on GOG 120, outcome data from these two clinical trials are presented separately with an emphasis on FIGO stage and the duration of RT. Survival (PFS and OS) probabilities were estimated using the Kaplan-Meier method. Hazard ratios were estimated with 95% confidence intervals using the Cox model, adjusted for covariates and protocol. In mutivariate analysis, all variables (age, race, GOG performance status, histology, FIGO stage, tumor grade, and tumor size) were categorical. The groups with a small number of patients were combined appropriately (GOG performance status, tumor grade). The tumor size was defined as a dummy variable by cutting at median (6 cm). In order to assess the association of RT duration with prognosis, 10 patients who terminated RT due to disease progression were excluded from the analysis, and PFS and OS were recalculated from the end of RT, rather than from the date of study entry. This strategy was used to minimize the bias caused by “outcome-cause” analysis or RT being a time-dependent variable. Since these two protocols were heterogeneous in staging as discussed above (surgical versus clinical) and because the dose and duration of RT were different, the analyses of PFS, OS and toxicity were conducted separately for the two protocols (Figures 1 – 4). Total RT duration was the elapsed time from the first day of RT until the completion of external and intracavitary therapy. RT delay was defined as RT ≥10 week for GOG 120 and RT ≥8 weeks for GOG 165, as prescribed by each protocol. All p values reported were two sided, with p< 0.05 interpreted as statistically significant. Analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
TABLE 2.
MULTIVARIATE ANALYSIS OF PROGNOSTIC FACTORS
| Progression-Free Survival
|
Overall Survival
|
|||||
|---|---|---|---|---|---|---|
| Prognostic Factor | HR | (95% CI) | p-value | HR | (95% CI) | p-value |
| Age (years) | ||||||
| ≤ 40 | Referent | Referent | ||||
| 41–50 | 0.87 | (0.57–1.31) | 0.496 | 0.76 | (0.49–1.17) | 0.210 |
| 51–60 | 0.57 | (0.34–0.94) | 0.027 | 0.56 | (0.34–0.95) | 0.031 |
| > 60 | 0.90 | (0.57–1.42) | 0.640 | 0.93 | (0.58–1.50) | 0.767 |
| Race | ||||||
| Caucasian | Referent | Referent | ||||
| African American | 1.09 | (0.74–1.61) | 0.657 | 0.90 | (0.60–1.36) | 0.623 |
| Other | 0.49 | (0.29–0.82) | 0.007 | 0.42 | (0.24–0.74) | 0.003 |
| Peformance Status | ||||||
| 0 | Referent | Referent | ||||
| 1 or 2 | 1.04 | (0.73–1.49) | 0.835 | 1.06 | (0.73–1.53) | 0.766 |
| Histology | ||||||
| Squamous | Referent | Referent | ||||
| Non-Squamous | 1.40 | (0.89–2.21) | 0.147 | 1.32 | (0.81–2.16) | 0.261 |
| FIGO Stage | ||||||
| II | Referent | Referent | ||||
| III/IV | 1.88 | (1.34–2.63) | <0.001 | 1.98 | (1.39–2.81) | <0.001 |
| Tumor Grade | ||||||
| 1 or 2 | Referent | Referent | ||||
| 3 | 1.81 | (1.30–2.54) | <0.001 | 2.01 | (1.42–2.85) | <0.001 |
| Tumor Size (cm) | ||||||
| < 6.0 | Referent | Referent | ||||
| ≤6.0 | 1.24 | (0.86–1.79) | 0.247 | 1.14 | (0.78–1.67) | 0.501 |
HR: Hazard Ratio; CI: 95% Confidence Interval.
HR, CI and p-value reported from Cox model.
Figure 1.
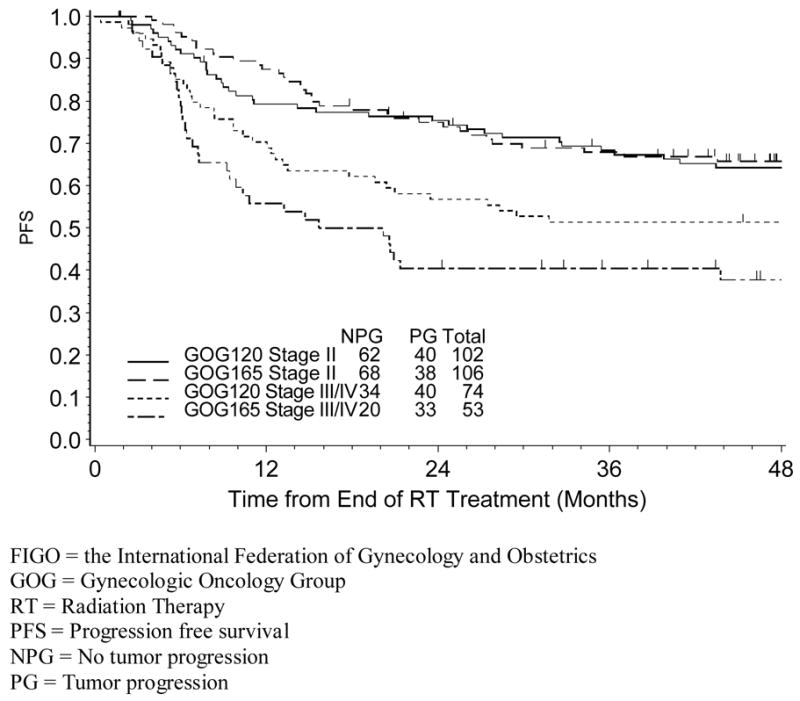
Estimated Progression-Free Survival for GOG 120 and GOG 165 by FIGO Stage
Figure 4.
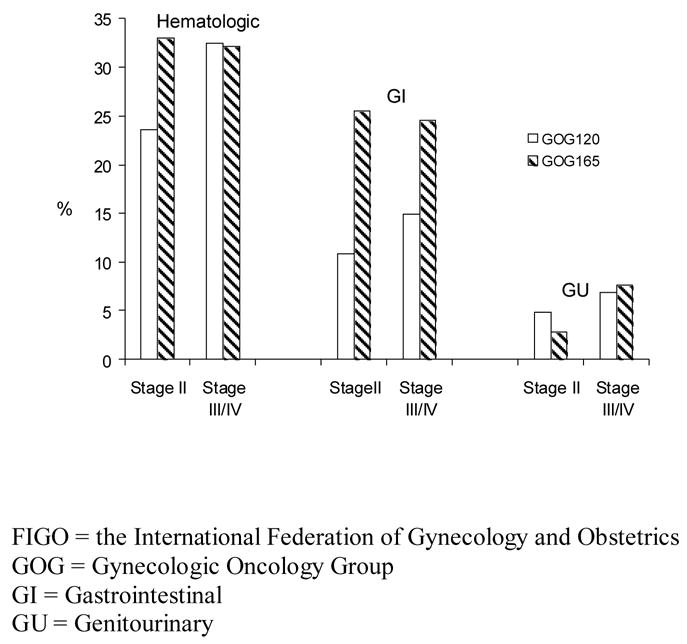
Grade 3 or 4 Toxicities by Protocol and FIGO stage
RESULTS
One hundred seventy-six patients treated on GOG 120 and 159 treated on GOG 165 who received weekly cisplatin plus RT were included for this study. Patient characteristics are shown in Table 1. Patient characteristics were comparable between the studies except that women on GOG 165 had better performance status than those on GOG 120. Additionally, there was a difference between protocols regarding staging methods, such that surgical staging was required in GOG 120 but optional in GOG 165 (performed in 18% of study subjects).
TABLE 1.
PATIENT CHARACTERISTICS
| GOG 120 (n=176)
|
GOG 165 (n=159)
|
Total (n=335)
|
|
|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) |
| Age (years); Median (Range) | 47 (25–80) | 48 (27–78) | 47 (20–80) |
| ≤ 40 | 56 (31.8) | 34 (21.4) | 90 (26.9) |
| 41–50 | 50 (28.4) | 52 (32.7) | 102 (30.5) |
| 51–60 | 34 (19.3) | 35 (22.0) | 69 (20.6) |
| > 60 | 36 (20.5) | 38 (23.9) | 74 (22.1) |
| Race | |||
| Caucasian | 101 (57.4) | 98 (61.6) | 199 (59.4) |
| African American | 45 (25.6) | 29 (18.2) | 74 (22.1) |
| Hispanic | 24 (13.6) | 22 (13.8) | 46 (13.7) |
| Asian/Pacific Island | 5 (2.8) | 10 (6.3) | 15 (4.5) |
| Other | 1 (0.6) | 0 (0.0) | 1 (0.3) |
| GOG Performance Status | |||
| 0 | 104 (59.1) | 129 (81.1) | 233 (69.6) |
| 1 | 63 (35.8) | 26 (16.4) | 89 (26.6) |
| 2 | 9 (5.1) | 4 (2.5) | 13 (3.9) |
| Histology | |||
| Squamous | 157 (89.2) | 135 (84.9) | 292 (87.2) |
| Adenosquamous | 13 (7.4) | 10 (6.3) | 23 (6.9) |
| Adenocarcinoma | 5 (2.8) | 10 (6.3) | 15 (4.5) |
| Other | 1 (0.6) | 4 (2.5) | 5 (1.5) |
| Tumor Grade | |||
| 1 | 15 (8.5) | 12 (7.6) | 27 (8.1) |
| 2 | 116 (65.9) | 87 (54.7) | 203 (60.6) |
| 3 | 45 (25.6) | 60 (37.7) | 105 (31.3) |
| FIGO Stage | |||
| IIB | 102 (58.0) | 106 (66.7) | 208 (62.1) |
| IIIA | 3 (1.7) | 0 (0.0) | 3 (0.9) |
| IIIB | 67 (38.1) | 47 (29.6) | 114 (34.0) |
| IVA | 4 (2.3) | 6 (3.8) | 10 (3.0) |
| Surgical Staging | |||
| Yes | 176 (100.0) | 29 (18.2) | 205 (61.2) |
| No | 0 (0.0) | 130 (81.8) | 130 (38.8) |
| Tumor Size (cm); Median (Range) | 6.0 (2.0–12.0) | 6.0 (2.0–12.0) | 6.0 (2.0–12.0) |
|
| |||
| ≤ 4.0 | 25 (14.2) | 33 (20.8) | 58 (17.3) |
| 4.1–5.0 | 22 (12.5) | 32 (20.1) | 54 (16.1) |
| 5.1–6.0 | 49 (27.8) | 34 (21.4) | 83 (24.8) |
| 6.1–7.0 | 27 (15.3) | 26 (16.4) | 53 (15.8) |
| 7.1–8.0 | 30 (17.1) | 24 (15.1) | 54 (16.1) |
| > 8.0 | 23 (13.1) | 10 (6.3) | 33 (9.9) |
At the time of this analysis, the median follow-up time for survival was 75 months for women on GOG 120, with 80 (46%) patients having progressed or died compared with 45 months for patients on GOG 165, with 71 (45%) women having progressed or died. The 4-year PFS and OS for stage II patients was 64.2% and 68.1%, respectively for those treated on GOG 120, and 65.8% and 73.9% for those treated on GOG 165, compared to 51.4% and 55.4% for stage III/IV patients respectively treated on GOG 120; and 37.7% and 42.7% respectively for those treated on GOG 165. Interestingly, between-study comparisons of PFS and OS estimates (stratified by FIGO stage) demonstrated no differences in PFS or OS for FIGO stage II patients. However, a difference in PFS was suggested in FIGO stage III/IV patients (Figure 1), with advanced stage patients on GOG 165 experiencing decreased PFS compared to those on GOG 120 (p=0.071 for log-rank test).
Pooled data from the two studies was used to assess the relationship between clinical-pathologic factors and prognosis. After adjusting for other covariates and protocol, non-Caucasian/non-African American women demonstrated a better prognosis (PFS, HR: 0.49, p=0.007 and OS, HR: 0.42, p=0.003) compared to Caucasians, while patients with FIGO stage III/IV disease (PFS, HR: 1.88, p<0.001 and OS, HR: 1.98, p<0.001) compared to FIGO stage II and those with poorly differentiated (grade 3) tumors (PFS, HR: 1.81, p<0.001 and OS, HR: 2.01, p<0.001) compared to grade 1 or grade 2, had an unfavorable prognosis. In multivariate analysis (Table 2), patients with non-squamous cell histology did not have a statistically significant difference in outcome compared with those with squamous type (PFS, HR: 1.40, p=0.147 and OS, HR: 1.32, p=0.261). Interestingly, the association between age and survival appeared non-linear, with women aged 51–60 years having the best prognosis (PFS, HR: 0.57, p=0.027 and OS, HR: 0.56, p=0.031, compared to those ≤ 40 years).
For patients treated on GOG 120, the median RT duration was 60 days and 65 days respectively, for stage II and stage III/IV; 17% of stage II patients and 42% of stage III/IV patients had RT delay (longer than 10 weeks as defined by the protocol); there was no evidence that RT prolongation was associated with worse prognosis (PFS, HR:1.09, p=0.764 and OS, HR:1.06, p=0.843); the estimated PFS (since the end of RT) stratified by RT duration and stage is shown in figure 2. For patients treated with GOG 165, the median RT duration was 51 days for stage II patients and 53 days for stage III/IV patients, respectively; 16% of stage II patients and 45% stage III/IV patients had RT delay (longer than 8 weeks as defined by the protocol); RT delay was associated with worse prognosis (PFS, HR: 1.98, p=0.012 and OS, HR: 1.88, p=0.024) and the results were consistent for both early and advanced stage patients; the estimated PFS, stratified by RT duration and stage can be seen in figure 3. In summary, duration of RT was not associated with clinical outcome after stratifying for stage among patients treated on GOG 120, whereas the association of RT duration was evident in both stage II and stage III/IV patients treated on GOG 165.
Figure 2.
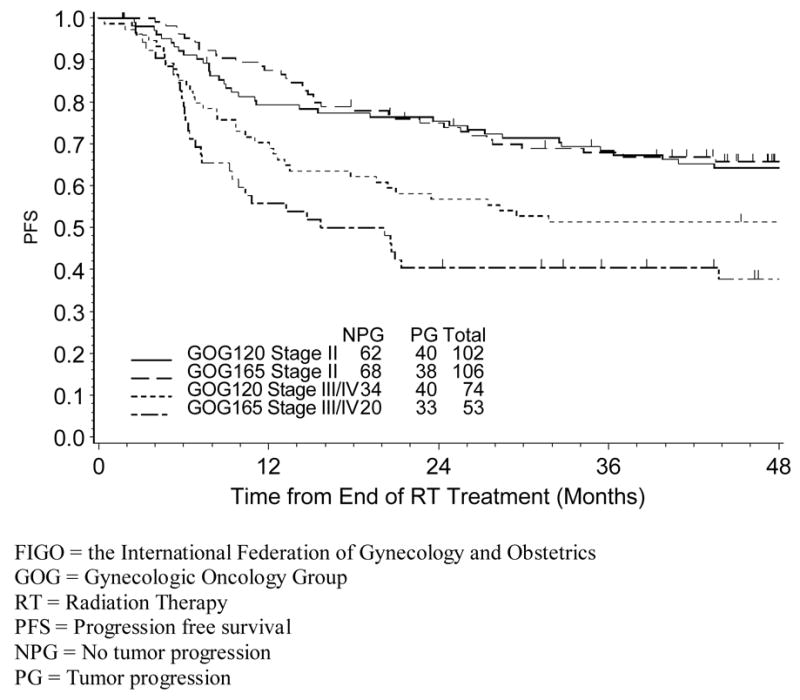
Estimated Progression-Free Survival for GOG 120 by FIGO Stage and RT Duration
Figure 3.
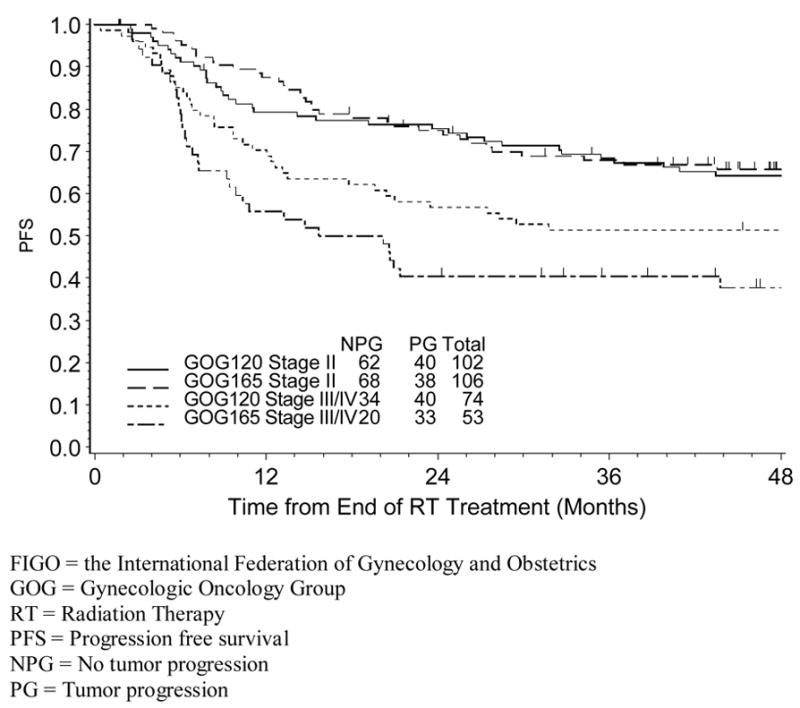
Estimated Progression-Free Survival for GOG 165 by FIGO Stage and RT Duration
Cisplatin administration (40 mg/m2 I.V. weekly × 6) was identical in both protocols; however, only 50% of GOG 120 patients and 52% of GOG 165 patients completed all 6 cycles of chemotherapy. There was no statistically significant difference in the PFS or OS between those who received 6 cycles compared to 5 cycles for protocols 120 and 165 but this was not a prospective comparison.
Hematologic, gastrointestinal (GI) and genitourinary (GU) adverse effects for both studies were also compared. Stage II patients on GOG 165 (having a higher RT dose and larger lateral radiation port) experienced more grade 3–4 GI toxicity than those on GOG 120 (stage II: 26% vs 11%, p=0.006; stage III/IV: 25% vs 15%, p=0.170). There was a trend for patients with stage II tumors treated on GOG 165 to have more grade 3–4 hematologic toxicity than stage II patients treated on GOG 120 (33% vs 24%) but this was not statistically significant (p=0.129). Genitourinary toxicity was rare and similar between studies. These results are shown in figure 4.
DISCUSSION
According to the National Comprehensive Cancer Network12, the current standard treatment of women with FIGO stage IB2 to IVA cervical carcinoma with clinically negative aortic nodes is pelvic RT (external and internal techniques delivering approximately 85 Gy to Point A) plus concurrent chemotherapy (cisplatin 40 mg/m2 weekly for 6 cycles)6. Although the importance of adding cisplatin to RT is well established, there have been no randomized trials investigating variations in the dose or schedule of chemotherapy or the RT dose, schedule or technique; nor has the relationship between common pathologic or clinical factors and this combination regimen been investigated. Given this, we sought to generate hypotheses regarding the complex interactions between these factors by analyzing and updating patient data from two recently published GOG trials that used this combination regimen. This analysis was not intended to change standard practice nor contradict the prospective objectives or conclusions of either of the treatment studies, but to explore questions which could be considered for prospective study in future randomized trials.
GOG 1208 and GOG 1659 included as Arm 1 the previously described standard chemotherapy. These two groups represent women treated similarly in two controlled clinical trials in which pathologic and treatment data had been independently reviewed for accuracy and quality.
GOG 165 was developed after GOG 120 was completed, using a more aggressive RT regimen consisting of a 4 Gy (5%) increase in the Point A dose and a reduced overall RT treatment time (from 10 to 8 weeks). In addition, the lateral beam was increased to more adequately encompass the posterior pelvis. These changes to RT were made in an effort to increase the therapeutic index by improving local control without adding to toxicity.
Only patients with negative aortic lymph nodes were eligible for these protocols. However, surgical staging required on GOG 120 was made optional on GOG 165. The rationale for this modification was that radiographic imaging had developed (circa 1997) to the point that surgical staging could be considered of limited benefit13. Clearly, this protocol-specific distinction may explain why GOG 165 patients with stage III/IV disease had a worse prognosis than their GOG 120 counterparts (Figure 1), and emphasizes the notion that standard tomographic imaging (CT and MRI) may not be as accurate as surgical biopsies of the aortic lymph nodes14. Whether positron emission tomography using fluoro-deoxyglocuse (FDG-PET) may finally obviate the need for surgical staging remains unsettled, but this new technique, not studied in these protocols, appears more sensitive and specific than CT or MRI.15
In 1991, Stehman and colleagues performed an analysis of 626 women with locally advanced cervical cancer treated with RT alone or RT plus non-platinum chemotherapy on one of three GOG trials conducted between 1977 and 198511. None of these studies showed a statistically significant difference in PFS or OS between the RT alone and chemotherapy-containing experimental arms. Using multivariate analysis they showed that patient age, performance status, aortic lymph node status, tumor size, and pelvic node status were independently associated with PFS. While the current study excluded women with suspicious aortic nodes and pelvic node status was not collected, it suggests (like the 1991 study) that age remains an important prognostic factor. However, unlike the 1991 Stehman study that simply showed a lower risk of recurrence and death for older patients, the current study demonstrated a bimodal risk with both younger and older patients having a worse outcome and women aged 51–60 having the most favorable outcome. This association appears to be independent of other prognostic factors in multivariate analysis. Interestingly, we found performance status and tumor size less important than was reported in 1991, which may be explained by the smaller sample size in the current report or by differences in the populations studied. Clearly, clinical assessment of tumor size/volume based on physical exam as used in all of these studies is less accurate than more objective imaging techniques. In both the Stehman paper and the current analysis, FIGO stage was an important independent predictor of outcome, which is consistent with almost all studies in this disease1,14,16.
Although assigning histological grade is subject to variability among pathologists, a significant independent difference between grades 1–2 and grade 3 tumors relative to PFS and OS was shown in the current report., This is consistent with most clinicopathologic studies of invasive cervical carcinoma14,16,17. Although histologic subtype has often been considered a poor prognostic factor14,16, one study has shown that adding cisplatin to RT may temper the poor prognosis associated with glandular cancers17. In the current report, although adenocarcinoma and adenosquamous histologies represented only about 10% of cases, there was not a significant difference in prognosis compared to squamous cell tumors.
The association between ethnicity and prognosis after therapy for cervical cancer is controversial. Clearly, a disproportionate number of cervical cancer deaths occur among racial/ethnic minorities, particularly African Americans18,19. The current study suggests that African American women can achieve the same, or better, level of survival compared to Caucasian women, and that other minorities including Asians and Hispanics have the best prognosis, apparently independent of other prognostic factors. The explanation for the latter finding is unclear but may be related to differences in comorbidities including vascular disease, smoking or nutrition.
The foregoing factors (age, ethnicity, histological cell type, tumor grade, tumor stage, primary lesion size) cannot be altered at the time of diagnosis; in contrast, it is possible that other modifiable factors such as variations in RT or chemotherapy administration may improve survival or decrease toxicity. Although every effort was made to strictly adhere to the prescribed treatment algorithm of each protocol, unavoidable minor variations in chemotherapy (e.g. number of cycles) and RT (e.g. dose, field size, duration) occurred in GOG 120 and GOG 165, raising important questions regarding the impact of these deviations. While the complex interaction between these factors is difficult to determine, some interesting observations can be made.
Although the goal of standard practice is to minimize toxicity and maximize outcome, at times one may be sacrificed for the other20,21. For instance, logic would suggest that a higher RT dose delivered over a shorter period of time to a larger treatment volume would result in both increased toxicity and improved cure rates20. Because of differences in eligibility especially related to surgical staging, we could not completely evaluate the impact of the higher RT dose and shortened treatment time associated with GOG 165 compared to GOG 120. However, there was a suggestion of more toxicity, predominantly to the rectum among patients treated on the more dose intense GOG 165. Unfortunately, the GOG does not evaluate the timing of these treatment-related toxicities making it difficult to estimate the timeline of this adverse event.
Although there appears to be a complex interaction between clinical/pathologic factors and RT duration, the later variable may influence prognosis in some subsets of patients. Women on GOG 165 who were to be treated in under eight weeks according to the protocol demonstrated a worse PFS (Figure 3) with prolonged (delayed for any reason) RT (≥ 8 weeks) as well as a shorter OS (data not shown). However, the poor prognostic significance of prolonged RT was not evident in GOG 120 (Figure 2) where the treatment duration goal was 10 weeks. One might speculate that subtle variations in treatment time such as those due to bad-weather, holidays or unfavorable tumor geometry might impact prognosis very little if every reasonable effort is made to treat patients according to “protocol” but that more prolonged delays in RT may be clinically important and lead to a worse outcome6,12,14. Indeed, similar to other investigations21,22, prolonged RT duration in GOG 165 appeared to be highly associated with advanced (FIGO IIIB) stage, which probably relates to a poorer response to RT 22,23 as well as tumor extent and/or size. Based on these observations, prolonged RT may be frequently confounded by adverse tumor characteristics, and overall treatment time may be a proxy variable for other predictors of poor prognosis24.
RT delays may become more common with the addition of increasingly aggressive chemotherapy to pelvic RT. Increased myelosuppression associated with chemotherapy doublets may lead to “breaks” in the radiation schedule, thus prolonging treatment time. Importantly, recent retrospective analyses of other cooperative group trials using methods similar to the current report but involving other tumor types (e.g. lung cancer) have led to parallel conclusions suggesting that, in the new era of combined chemotherapy and radiation, prolonged radiation treatment time may be associated with decreased survival 25.
Prognostic factors commonly associated with survival in cervical cancer remain evident in the era of combination cisplatin-based chemotherapy and RT. Surgical staging in the current era of FDG-PET remains controversial, but clinical staging by CT or MRI may not detect aortic metastases (especially in stage III/IV patients) as evidenced by the improved survival associated with GOG 120 (surgical staging required) compared to 165 (surgical staging optional). Moreover, RT prolongation in patients with clinical staging (as in GOG 165) may be detrimental. These observations are potentially important, but require confirmation.
ARTICLE PRÉCIS
Prognostic clinical and pathologic factors are examined among patients with locally advanced cervical cancer treated with chemotherapy and radiation on two Gynecologic Oncology Group Trials.
Acknowledgments
This study was supported by National Cancer Institute grants to Dr. Monk (K23 CA87558), and to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469) and the GOG Statistical and Data Center (CA 37517). The following GOG member institutions participated in this study:
University of Mississippi Medical Center, University of Oklahoma, Indiana University School of Medicine, Duke University Medical Center, Wayne State University, SUNY-Downstate Medical Center, University of California at Irvine, Ellis Fischel Cancer Center, Walter Reed Army Medical Center, University of Southern California at Los Angeles, Tampa Bay/H. Lee Moffitt Cancer Center, University of Iowa Hospitals and Clinics, University of Kentucky, Medical University of South Carolina, Colorado Gynecologic Oncology Group, PC, M.S. Hershey Medical Center, University of Texas Southwestern Medical Center at Dallas, Columbus Cancer Council, Case Western Reserve University, University of Alabama at Birmingham, University of North Carolina School of Medicine, Wake Forest University School of Medicine, Stanford University Medical Center, Tacoma General Hospital, University of California at Los Angeles, University of Washington/Puget Sound Oncology Consortium, Tufts-New England Medical Center, Abington Memorial Hospital, University of Miami School of Medicine, University of Cincinnati, Cleveland Clinic Foundation, SUNY-Stony Brook, Cooper Hospital/University Medical Center, Fox Chase Cancer Center, Women’s Cancer Center, Oregon Health Sciences University, Rush-Presbyterian-St. Luke’s Medical Center, Eastern Pennsylvania Gynecology/Oncology Center, University of Massachusetts Medical Center, University of Chicago, University of Rochester Medical Center, University of Minnesota Medical School, Emory University Clinic, Georgetown University Hospital, Community Cancer Oncology Program, University of Virginia, and Mayo Clinic.
The authors wish to acknowledge the editorial assistance of Caron Modeas and Anne Reardon.
Footnotes
Contact for Reprints: Denise Mackey, GOG Administrative Office, Four Penn Center, 1600 JFK Blvd., Suite 1020, Philadelphia, PA 19103, Phone: 215-854-0770, Email: dmackey@gog.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Sem Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- 2.Lanciano RM, Won M, Coia LR, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. Int J Rad Oncol Biol Phys. 1991;20:667–76. doi: 10.1016/0360-3016(91)90007-q. [DOI] [PubMed] [Google Scholar]
- 3.Eifel PJ, Moughan J, Owen J, et al. Patterns of radiotherapy practice for patients with squamous carcinoma of the uterine cervix: patterns of care study. Int J Radiat Oncol Biol Phys. 1999;43:351–8. doi: 10.1016/s0360-3016(98)00401-5. [DOI] [PubMed] [Google Scholar]
- 4.Eifel PJ, Moughan J, Erickson B, et al. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: a pattern of care study. Int J Radiat Oncol Biol Phys. 2004;60:1144–53. doi: 10.1016/j.ijrobp.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 5.J Natl Cancer Inst Monogr; National Institutes of Health Consensus Conference on Cervical Cancer; Bethesda, MD. April 1–3, 1996; 1996. pp. 1–148. [PubMed] [Google Scholar]
- 6.Rose PG. Chemoradiotherapy: the new standard care for invasive cervical cancer. Drugs. 2000;60:1239–44. doi: 10.2165/00003495-200060060-00001. [DOI] [PubMed] [Google Scholar]
- 7.The National Cancer Institute Clinical Announcement on Cervical Cancer. 1999 February 22; http://www.nci.nih.gov/clinicaltrials/digestpage/cervical-cancer-announcement.
- 8.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 9.Lanciano R, Calkins A, Bundy B, et al. A randomized comparison of radiation plus weekly cisplatin versus protracted venous infusion 5-FU in combination with concurrent radiation in advanced cervix cancer: a GOG study. J Clin Oncol. 2005 Nov 20;23(33):8289–95. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ. Controversies in the radiotherapeutic management of cervical cancer. J Clin Oncol. 2003;21(suppl 10):218–23. doi: 10.1200/JCO.2003.01.224. [DOI] [PubMed] [Google Scholar]
- 11.Stehman FB, Bundy BN, DiSaia PJ, et al. Carcinoma of the cervix treated with radiation therapy. A multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–85. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Cervical Cancer - v.1.2006. http://www.nccn.com/professionals/physician_gls/PDF/cervical.pdf. [PubMed]
- 13.Scheidler J, Hricak H, Yu KK, Subak L, Segal MR. Radiological evaluation of lymph node metastases in patients with cervical cancer. A meta-analysis. JAMA. 1997 Oct 1;278(13):1096–101. [PubMed] [Google Scholar]
- 14.DiSaia PJ, Creasman WT, editors. Invasive Cervical Cancer. Clinical Gynecologic Oncology. 6. 2002. pp. 35–112. [Google Scholar]
- 15.Havrilesky LJ, Kulasingam SL, Matachar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97(1):183–91. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Arif S. Histopathologic parameters of prognosis in cervical cancer-a review. Int J Gynecol Cancer. 2004;14(5):741–50. doi: 10.1111/j.1048-891X.2004.014504.x. [DOI] [PubMed] [Google Scholar]
- 17.Monk BJ, Im S, Wang J, et al. Rethinking the use of chemotherapy and radiotherapy after radical hysterectomy: a clinico-pathologic analysis of SWOG 8797/GOG 109 by the Gynecologic Oncology Group. Gynecol Oncol. 2005;96(3):721–8. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 19.Farley JH, Hines JF, Taylor RR, et al. Equal care ensures equal survival for African-American women with cervical carcinoma. Cancer. 2001;91(4):869–73. [PubMed] [Google Scholar]
- 20.Greer BE, Koh WJ, Stelzer KJ, et al. Expanded pelvic radiotherapy fields for treatment of local-regionally advanced carcinoma of the cervix: outcome and complications. Am J Obstet Gynecol. 1996;174(4):1141–9. doi: 10.1016/s0002-9378(96)70656-7. [DOI] [PubMed] [Google Scholar]
- 21.Eifel PJ, Thames HD. Has the influence of treatment duration on local control of carcinoma of the cervix been defined? Int J Radiat Oncol Biol Phys. 1995;32(5):1527–9. doi: 10.1016/0360-3016(95)00264-Y. [DOI] [PubMed] [Google Scholar]
- 22.Petereit DG, Sarkaria JN, Chappell R, et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995;32(5):1301–7. doi: 10.1016/0360-3016(94)00635-X. [DOI] [PubMed] [Google Scholar]
- 23.Fyles AW, Pintilie M, Kirkbride P, Levin W, Manchul LA, Rawlings GA. Prognostic factors in patients with cervix cancer treated by radiation therapy: results of a multiple regression analysis. Radiother Oncol. 1995;35:107–17. doi: 10.1016/0167-8140(95)01535-o. [DOI] [PubMed] [Google Scholar]
- 24.Lanciano R. Optimizing radiation parameters for cervical cancer. Semin Radiat Oncol. 2000;10:36–43. doi: 10.1016/s1053-4296(00)80019-3. [DOI] [PubMed] [Google Scholar]
- 25.Machtay M, Hsu C, Komaki R, Sause WT, Swann RS, Langer CJ, Byhardt RW, Curran WJ. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005 Nov 1;63(3):667–71. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]


