Abstract
The ketogenic diet is a valuable therapeutic approach for epilepsy, one in which most clinical experience has been with children. Although the mechanism by which the diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influence the dynamics of the major inhibitory and excitatory neurotransmitter systems in brain. The pattern of protection of the ketogenic diet in animal models of seizures is distinct from that of other anticonvulsants, suggesting that it has a unique mechanism of action. During consumption of the ketogenic diet, marked alterations in brain energy metabolism occur, with ketone bodies partly replacing glucose as fuel. Whether these metabolic changes contribute to acute seizure protection is unclear; however, the ketone body acetone has anticonvulsant activity and could play a role in the seizure protection afforded by the diet. In addition to acute seizure protection, the ketogenic diet provides protection against the development of spontaneous recurrent seizures in models of chronic epilepsy, and it has neuroprotective properties in diverse models of neurodegenerative disease.
Introduction
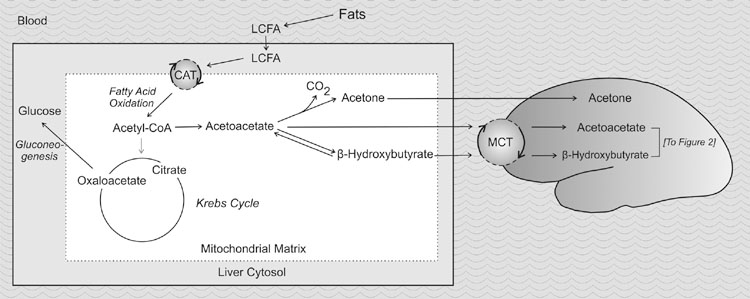
Since the early 1920s, the ketogenic diet has been used successfully to treat patients with intractable epilepsy. The diet is high in fat and low in carbohydrate and protein, providing sufficient protein for growth but insufficient amounts of carbohydrates for all the metabolic needs of the body [1]. Energy is derived largely from fatty acid oxidation in mitochondria. During high rates of fatty acid oxidation, large amounts of acetyl-CoA are generated, leading to the synthesis, primarily in the liver, of the three ketone bodies β-hydroxybutyrate, acetoacetate, and acetone (Fig. 1). The metabolic efficiency of the Krebs cycle is reduced, and excess acetyl-CoA is shunted to the production of ketone bodies. Ketone bodies spill into the circulation, causing serum levels to rise severalfold, and then are utilized as an energy source in extrahepatic tissues, including the brain.
Figure 1.
Alterations in intermediary metabolism during the high-fat, low-carbohydrate ketogenic diet that lead to the formation of ketone bodies. The ketogenic diet provides high levels of long chain fatty acids and is deficient in carbohydrates so that glucose availability is severely limited. The demand to maintain serum glucose causes oxaloacetate to be shunted from the Krebs cycle to the pathway for gluconeogenesis, a multistep process that is the reverse of glycolysis. As a result of diminished oxaloacetate, the Krebs cycle has reduced capacity to handle the high levels of acetyl-CoA generated from fat. Instead, acetyl-CoA is converted to the ketone body acetoacetate which spontaneously degrades to acetone. Acetoacetate also is converted enzymatically to β-hydroxybutyrate in a reversible reaction catalyzed by the NADH-dependent mitochondrial enzyme β-hydroxybutyrate dehydrogenase. Ketone bodies represent alternative energy substrates for the brain. Not all Krebs cycle intermediates are shown in the schematic. Abbreviations: CAT, carnitine-acylcarnitine translocase; LCFA, long chain fatty acids; MCT, monocarboxylic acid transporter.
Glucose is ordinarily the sole fuel for the human brain; fatty acids cannot be used because they do not cross the blood-brain barrier. Ketone bodies do enter the brain, in proportion to the degree of ketosis. Ordinarily, utilization of ketones by the brain is minimal. During the ketogenic diet, however, ketone bodies partly replace glucose as fuel for the brain. The ketone bodies are converted to acetyl-CoA by D-β-hydroxybutyrate dehydrogenase, acetoacetate-succinyl-CoA transferase, and acetoacetyl-CoA-thiolase and then enter the Krebs cycle within brain mitochondria, leading to the production of adenosine triphosphate (ATP) (Fig. 2).
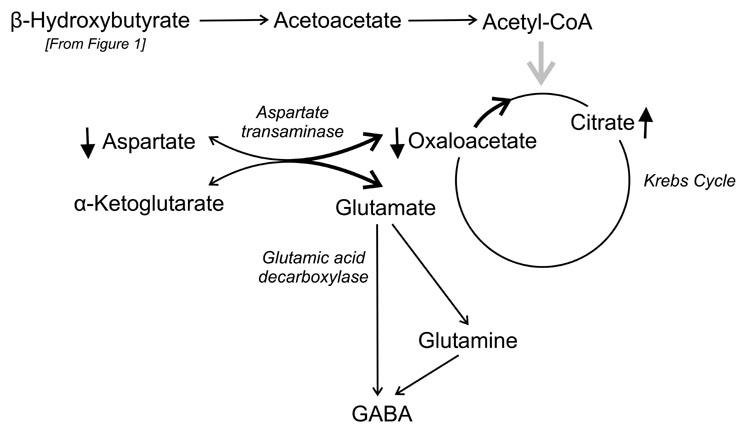
Figure 2.
Alterations in the metabolism of excitatory amino acids and γ-aminobutyric acid (GABA) during the high-fat, low-carbohydrate ketogenic diet. Metabolism of acetyl-CoA generated from fats leads to high consumption of oxaloacetate (see Fig. 1). L-Aspartate, a nonessential amino acid, is formed by the transamination of oxaloacetate with an amino group from glutamate. Reduced availability of oxaloacetate along with robust availability of α-ketoglutarate from high activity of the first part of the Krebs cycle leads to low aspartate levels. It has been hypothesized that more glutamate is thus accessible to glutamic acid decarboxylase for production of GABA [33]. Not all Krebs cycle intermediates are shown in the schematic.
It has been known since the time of Hippocrates that fasting is an effective treatment for seizures, and the ketogenic diet was designed to mimic the fasting state [2]. However, despite intensive research in recent years, the mechanism by which the diet protects against seizures remains obscure. The diet is associated with a wide range of neurochemical changes, some of which may contribute to its therapeutic actions and others that are epiphenomenal. In a sense, the state of knowledge of mechanisms for the ketogenic diet is similar to that for many anticonvulsant medications; that is, a diversity of pharmacological actions have been described, but it is often a challenge to create a definitive link between any specific action and seizure protection [3]. Ultimately, the link is made by a consideration of the overall profile of the cellular actions of the drug as well as the results of testing in diverse animal seizure models [4].
Here, we examine the known metabolic and physiological actions of the ketogenic diet in the brain that could be relevant to its protective activity against seizures and we summarize the available information on the actions of the diet in animal seizure models. We then compare the profile of the diet with the profiles of the major antiepileptic drugs, to assess whether the underlying mechanism of action of the diet is likely to be similar to or different from that of any of the drugs.
Although far from proven, there is emerging evidence that the diet may also have disease-modifying actions in epilepsy and may confer neuroprotection in animal models and clinical states in which there is death of neurons [5]. We therefore also consider actions of the diet that could be relevant to these additional, and potentially important, actions of the diet.
Clinical Aspects of the Ketogenic Diet Relevant to its Mechanism
Today, several types of ketogenic diets are used for epilepsy treatment. The most frequently used is the traditional ketogenic diet originally described by Wilder in 1921, which is based on long-chain saturated fats and incorporates a low percentage of protein and carbohydrate [6]. The protocol, as applied at the Johns Hopkins Hospital, consists of fat in a 4:1 ratio with respect to protein and carbohydrate combined. In the 1950s, a medium-chain triglyceride diet was introduced, which was thought to be more palatable [7]. Although this diet is easier to prepare and produces greater ketosis (because the fats used, decanoic and octanoic acids, yield more ketones per calorie), it has not been widely accepted because it is associated with bloating and abdominal discomfort. A third variation on the diet, developed at the John Radcliffe Hospital in Oxford, England, represents a combination of the traditional and medium-chain triglyceride diets [8]. Despite some differences in their ability to generate ketones, all three diets have similar efficacy; the Hopkins protocol has been the most widely studied.
In the Johns Hopkins protocol, patients are admitted to the hospital and fasted for about 24 hours before beginning the diet [9]. On occasion, there is immediate improvement in seizure frequency or severity after the initial fast, and some patients do not show an improvement until a few weeks later [10]. In addition to the acute beneficial effects on seizures, many patients experience a long-term decrease in seizure frequency even after the ketogenic diet has been stopped [5,10,11]. Factors such as seizure duration, electroencephalographic characteristics, and demographic factors do not appear to predict clinical response [10,12].
Do Ketone Bodies Mediate the Effects of the Ketogenic Diet?
Early in the study of the ketogenic diet, it was noted that levels of the ketone bodies β-hydroxybutyrate, acetoacetate, and acetone are elevated in the peripheral blood and in the urine. Levels of β-hydroxybutyrate are easily assayed spectrophotometrically or using commercially available test strips, and urine levels are used to monitor the degree of ketosis during therapy. In neocortical neuron slices, the ketone bodies β-hydroxybutyrate and acetoacetate prevent cell injury induced by the oxidative stressors hydrogen peroxide and the thiol oxidant diamide [13]. These ketone bodies also inhibit glutamate-induced mitochondrial reactive oxygen species generation [14]. Oxidative stressors may contribute to the development of epilepsy (reviewed in [15]). Therefore, β-hydroxybutyrate and acetoacetate may prevent neuronal damage from free radicals and confer neuroprotective (and possibly antiepileptogenic) effects. However, the link between ketone bodies and the anticonvulsant efficacy of the ketogenic diet is not as clear. Studies of the effects of the ketogenic diet on pentylenetetrazol threshold in rats have failed to show an association between plasma β-hydroxybutyrate levels and seizure threshold [16]. Moreover, exogenously administered β-hydroxybutyrate is not anticonvulsant in animal models such as the Frings audiogenic seizure-susceptible mouse [17].
In contrast, acetoacetate and acetone do have anticonvulsant properties in animal models [18]. In rats, these metabolites are protective against seizures induced by pentylenetetrazol and AY-9944 (an inhibitor of the biosynthesis of cholesterol that induces slow spike-and-wave discharges and is proposed as a model of chronic atypical absence seizures). Acetone is also protective in the maximal electroshock test [19] and the amygdala kindling model [19]. In mice, acetone protects against clonic seizures induced by pentylenetetrazol, as is the case in rats, and it also is protective against tonic seizures induced by 4-aminopyridine [20]. It is also active against audiogenic seizures in the Frings mouse [17].
It has been proposed that acetone is directly responsible for the anticonvulsant activity of the ketogenic diet [18]. However, the overall profile of acetone in animal seizure models is distinct from that of the ketogenic diet (Table 1). In particular, unlike acetone, the diet does not protect against pentylenetetrazol-induced seizures in mice, does not have robust activity against tonic seizures induced by maximal electroshock or 4-aminopyridine, and does not have sustained efficacy in the rat kindling model [21,22]. These differences raise doubts about the hypothesis that acetone is responsible for the anticonvulsant activity of the diet, although it could be contributory.
Table 1. Protective activity of the ketogenic diet in rodent seizure models.
| Seizure Model | Mouse | Rat | References |
|---|---|---|---|
| Maximal electroshock | +/− | − | [26,29,89,90] |
| Electroconvulsive threshold | +/− | + | [26,42,73,89,91] |
| Pentylenetetrazol | − | + | [26,27,29,92] |
| Bicuculline | + | + | [26,31] |
| Picrotoxin | + | [31] | |
| 6 Hz | + | A.L.H., M. Lyle, M.G., and M.A.R, unpublished data | |
| Kainic acid (i.v.) | + | LST | [31,93] |
| Amygdala kindling | +/− | [22,24] | |
| Flurothyl | + | − | [58,63,94] |
| Genetic absence epilepsy (GAERS rats) | − | [57] | |
| EL mice | + | [95] | |
| Frings audiogenic seizure susceptible mice | + | [17] | |
| Spontaneous recurrent seizures after kainic acid status epilepticus | + | [82] | |
| Spontaneous recurrent seizures after lithium-pilocarpine status epilepticus | − | [94] |
Abbreviations:
LST = Lowers seizure threshold
+, −, and +/− = Effective, ineffective, and conflicting results, respectively
Studies in brain slices have shown that acetone can suppress epileptiform discharges, albeit at high concentrations (W.D. Yonekawa, unpublished data). The molecular anticonvulsant mechanism of action of acetone in vivo or in vitro is not known. Because acetone is not easily assayed, there is little information on the levels present in animals or humans consuming the ketogenic diet. Measurements of cerebral acetone using 1H-magnetic resonance spectroscopy in children on the ketogenic diet have indicated that levels in some subjects may be in the range of 0.7 mmol/L and that seizures are well controlled when acetone is detected [23].
Breath acetone correlates with plasma ketone body measurements in children consuming a ketogenic diet [24] and, although it is assumed that acetone is eliminated mainly via pulmonary ventilation, some of it is metabolized further into other products, including acetol, 1,2-propanediol, methylglyoxal, and pyruvic acid. These metabolites are unlikely to contribute to the anticonvulsant effects of acetone in vivo [20].
Comparisons of the levels of acetone in brain during the ketogenic diet with those achieved by anticonvulsant doses of exogenously administered acetone will be necessary to assess whether the acetone hypothesis is plausible. In one study of rats consuming a ketogenic diet, blood levels of acetone were more than 10-fold lower than concentrations previously shown to be anticonvulsant [21].
GABA Systems
GABA (γ-aminobutyric acid) is the major inhibitory neurotransmitter in mammals. Many anticonvulsant medications are believed to act through effects on GABA systems, ultimately leading to an enhancement in GABA-mediated inhibition [3]. Antiepileptic actions are obtained by drugs that target GABA systems in a diversity of ways, including positive modulation of synaptic or extrasynaptic GABAA receptors, inhibition of GABA transporters, inhibition of GABA transaminase, and possibly by effects on intermediary metabolism which lead to enhanced intracellular levels of GABA. High intracellular GABA may lead to increases in extracellular GABA as a result of reversal of GABA transporters, resulting in enhanced tonic inhibition mediated by extrasynaptic GABAA receptors [25].
Are Results in Animal Seizure Models Consistent with a Mechanism That Involves GABA Systems?
Anticonvulsant agents that target GABA systems invariably protect against clonic seizures induced by the GABAA receptor antagonist pentylenetetrazol in rodents. In 1972, Uhlemann and Neims [26] reported that a ketogenic diet did not confer protection against pentylenetetrazol-induced clonic seizures in mice, a result we have recently confirmed in our laboratory (A.L.H., M. Lyle, M.G., and M.A.R., unpublished data) (Table 1). In the study of Uhlemann and Neims [26], the diet provided partial protection in the maximal electroshock test in mice, a model of tonic seizures that has a distinct profile from that of the pentylenetetrazol test. Anticonvulsant agents that target the GABA system are usually only weakly effective in the maximal electroshock test and some are ineffective. In contrast, the ketogenic diet was found to be protective in the pentylenetetrazol test in rats [25–28]. The diet also was effective against seizures induced by the GABAA receptor antagonists bicuculline and picrotoxin in rats [31], but did not confer sustained protection against fully kindled seizures in the rat amygdala kindling model [21,22]. Anticonvulsant treatments that act through effects on GABA mechanisms are generally active in these models.
The basis for the differential effectiveness of the ketogenic diet against pentylenetetrazol seizures in rats and mice is not known. The lack of activity in mice raises doubt that effects on GABA mechanisms contribute in a substantial way to the seizure protection conferred by the diet. Recently, we found that the ketogenic diet is highly protective against 6 Hz electroshock seizures in mice, a model of limbic seizures that shows a similar sensitivity to anticonvulsant agents that act on GABA systems as does the pentylenetetrazol test [32,33] (A.L.H., M. Lyle, M.G., and M.A.R., unpublished data). In particular, anticonvulsant agents that target GABA systems are highly effective and relatively potent in the 6 Hz model.
The 6 Hz model is attractive for studying the ketogenic diet in rats and mice for other reasons. Animals fed a ketogenic diet may gain weight less well than those fed a control diet, and it has been suggested that the mismatch in weights between the diet and control groups can artefactually skew results obtained in the pentylenetetrazol infusion test because threshold doses are calculated on a per body mass basis [30]. One advantage of the 6 Hz model is thus the elimination of the confounding effects of weight differences.
Table 1 compares the activity profile of the ketogenic diet in mouse and rat seizure models. Some important differences between the two species are revealed. For example, the diet protects against pentylenetetrazol-induced seizures in rats but not mice, whereas the opposite pattern is observed for flurothyl seizures. The significance of these species differences is not clear, especially given that a divergence between the efficacy of anticonvulsant drugs in mice and rats is rarely observed. (One exception is provided by gabapentin, which protects against pentylenetetrazol seizures in mice but not in rats [34]).
Effects of the Ketogenic Diet on GABA Synthesis
GABA is synthesized from glutamate by glutamic acid decarboxylase, an enzyme present only in GABAergic neurons. Cheng et al. [35] reported that rats fed a calorie-restricted diet for 7 days show increased expression of various glutamic acid decarboxylase isoforms in selected brain regions, including the superior colliculus and cerebellar cortex. However, the changes in expression were similar whether the animals received an ordinary calorie restricted diet or a high-fat, ketogenic diet, indicating that high fat and ketosis do not have specific effects of glutamic acid decarboxylase expression. Moreover, the brain regions with elevated glutamic acid decarboxylase expression are not those generally considered relevant to seizure protection.
Effects on intermediary metabolism represent an alternative way in which the ketogenic diet could increase GABA levels. Thus, it has been proposed that reduced availability of oxaloacetate drives the reversible aspartate transamination reaction that converts oxaloacetate to aspartate (utilizing an amino group from glutamate) in the direction of oxaloacetate (Fig. 2). This leads to reduced availability of aspartate, and indeed aspartate levels have been found to be reduced in the forebrain and cerebellum of mice that consumed a ketogenic diet for 3 days [36].
In the presence of plentiful acetyl-CoA from fat metabolism, the first segments of the Krebs cycle are highly active, producing an abundance of α-ketoglutarate, the levels of which have been observed to be increased in an adult rat ketogenic diet model [37]. The equilibrium of the transamination reaction toward oxaloacetate in the presence of increased α-ketoglutarate implies that availability of glutamate will be enhanced. Total brain glutamate is not increased by the ketogenic diet [38,39], although there may be small regional increases [40]. Radiotracer study has demonstrated increased synthesis of glutamate in ketotic mice consuming a ketogenic diet [38]. Thus, the flux through glutamate synthesis is enhanced, but because glutamate levels are only slightly elevated if at all, glutamate metabolism must also be increased.
Glutamate is the precursor of GABA via the GABA shunt, and it has been hypothesized that the increased flux through glutamate reflects increased GABA production [38,40]. However, in various ketogenic diet models, GABA levels were unchanged in mouse forebrain and cerebellum [36], mouse whole brain [38], mouse neocortex [41], and rat whole brain [42,43]. Although these data indicate that widespread increases in brain GABA levels do not occur in the ketogenic diet, they do not rule out the possibility of more specific local or regional changes in GABA content [40]. Moreover, when ketotic animals were loaded with alanine (a potential amino group donor in the conversion of α-ketoglutarate to glutamate in GABA neurons [44]) or leucine (which may play a similar role in GABA synthesis [38]), they did exhibit higher total brain GABA levels than did control mice. Because glutamate is the precursor of GABA, enhanced glutamate availability provided by the amino acids could potentially be responsible for the increased GABA.
Although animal studies have failed to demonstrate affects of the ketogenic diet on brain GABA levels in the absence of amino acid loading, a recent study on cerebro-spinal fluid amino acid levels before and during the ketogenic diet found evidence of increased cerebrospinal fluid GABA [45]. GABA levels were higher in responders than in nonresponders, and, in the best responders, GABA levels were significantly higher at baseline as well as during the diet. In that study, children under 5.5 years of age had higher cerebrospinal fluid GABA levels during the diet than did older children.
The fact that some of the key enzymes that could be relevant to the response to ketosis are expressed maximally early in life—including the monocarboxylic acid transporter MCT-1, which transports the ketones β-hydroxybutyrate and acetoacetate across the blood-brain barrier [46]—could explain the higher cerebrospinal fluid GABA levels seen in younger children in this study. Magnetic resonance spectroscopy can be used in human subjects for noninvasive determination of brain GABA levels. At present, only limited and inconclusive data are available [47], but the approach has promise.
Effects of Ketone Bodies on the Physiology of GABA Inhibition
Early in the study of the ketogenic diet, a structural similarity was noted at the carboxy-terminus between GABA and the ketone bodies acetoacetate and β-hydroxy-butyrate [40]. It was proposed that these ketone bodies might act as GABA receptor agonists. However, studies in cultured rat hippocampal neurons [48] and rodent neocortical neurons [49] failed to demonstrate an effect of the ketone bodies on GABA receptor currents, making this proposed mechanism unlikely.
On the other hand, in vivo field recordings in rats fed a ketogenic diet demonstrated greater angular bundle-evoked paired-pulse inhibition in the dentate gyrus than in animals fed a normal diet [50]. In addition, the threshold for activation of electrographic seizures was elevated in the animals receiving the ketogenic diet. Moreover, both phenomena also occurred in rats on a calorie-restricted diet. This study is important in that it is the most direct demonstration available to date that the ketogenic diet is associated with enhanced fast (GABA-mediated) synaptic inhibition. However, the fact that inhibition was also enhanced with calorie restriction alone suggests that it may be the calorie restriction in the ketogenic diet, and not ketosis per se, that is the critical factor in the effect on inhibitory synaptic function. This conclusion is compatible with the studies, already noted, in which ketone bodies did not influence GABA receptor responses [48,49].
Although the available evidence suggests that ketone bodies are not a factor in the effect of the ketogenic diet on inhibitory synaptic function, ketone bodies have been found to increase brain synaptosomal GABA content [51], as occurs with the anticonvulsant vigabatrin [52], raising the possibility that ketone bodies might have functional effects similar to those of vigabatrin. In these two studies, however, the effect on synaptosomal GABA levels did not result from inhibition of GABA-transaminase.
Do Neurosteroids Play a Role in the Anticonvulsant Activity of the Ketogenic Diet?
Endogenous neurosteroids derived from steroid hormone precursors, including allopregnanolone and tetrahydrodeoxycorticosterone, have been implicated as endogenous regulators of seizure susceptibility [53]. These neurosteroids act as powerful positive allosteric modulators of GABAA receptors, and they have anticonvulsant activity in diverse animal seizure models that are sensitive to agents that act on GABA systems. Perhaps elevated steroid synthesis during consumption of a ketogenic diet high in saturated fats [54] could lead to increased neurosteroids, accounting for the effects the diet has on seizures. This intriguing hypothesis has received only scant experimental support to date.
The neurosteroid synthesis inhibitor finasteride appeared to reverse the ability of the ketogenic diet to raise the threshold for clonic seizures induced by intravenous kainic acid in mice (A.L.H., M.G., and M.A.R., unpublished data). In experiments with the 6 Hz model, however, which is highly sensitive to neurosteroids [55], the protective effects of the ketogenic diet were not influenced by finasteride pretreatment (A.L.H., M.G., and M.A.R., unpublished data). Moreover, Rhodes et al. [56] reported that plasma levels of the neurosteroids allopregnanolone and androstanediol actually decreased in female rats fed a ketogenic diet. (The blood samples used for these measurements were collected after the animals had received pentylenetetrazol for seizure testing; confirmation of this result in animals not exposed to pentylenetetrazol is necessary.)
Overall, the available evidence does not indicate that the neurosteroid hypothesis should be given serious consideration.
Excitatory Amino Acid Systems
Decreases in glutamate, the major excitatory neuro-transmitter in brain, could theoretically contribute to the anticonvulsant activity of the ketogenic diet. A recent study of 5-month-old rats of the GAERS (genetic absence epilepsy rats of Strasbourg) strain receiving the ketogenic diet for 3 weeks did reveal small decreases in cortical glutamate levels by 13C nuclear magnetic resonance spectroscopy [57]. (Note that the diet did not provide seizure protection in this model of primary generalized epilepsy, despite human data indicating that it is highly effective for generalized epilepsies without an obvious environmental precipitant [1].)
As already noted, however, most studies have not observed decreases in glutamate levels. Rather, metabolic flux through glutamate may be enhanced by the ketogenic diet as a result in the shift in the equilibrium of aspartate transamination toward glutamate because of reduced availability of the reactant oxaloacetate (Fig. 2). The shift in this transamination reaction would be expected to reduce aspartate, and substantial (23%) decreases in whole brain aspartate levels have been observed in mice receiving a ketogenic diet for 3 days [38], although this is not a consistent finding [39]. Anticonvulsant effects of the diet are not seen until after 7 to 10 days in rodents [42,58]. Whether there would be larger changes in aspartate or glutamate at later times is not known.
Yudkoff et al. [59] reported that the ketone body acetoacetate markedly reduces transamination of glutamate to aspartate by astrocytes, providing an alternative way in which the ketogenic diet could reduce aspartate levels. Although aspartate selectively activates N-methyl-D-aspartate-type glutamate receptors, the status of aspartate as an excitatory neurotransmitter is unresolved.
Aspartate is not a substrate for the vesicular glutamate transporters, and some studies have indicated that aspartate in not released in a calcium-dependent fashion by depolarizing stimuli. Recently, however, evidence has been presented that aspartate is contained in synaptic vesicles in the hippocampus, and possibly other brain regions, where it can be released in a calcium-dependent manner through mechanisms that are regulated differently from that of glutamate [60].
Release of aspartate has been proposed to occur mainly outside of synaptic active zones, where it may serve as an agonist for extrasynaptic N-methyl-D-aspartate receptors [61]. Regulating the tonic activation of neurons by extrasynaptic N-methyl-D-aspartate receptors is an attractive mechanism by which level of seizure susceptibility could be set. If this mechanism is shown to influence vulnerability to seizures, it is apparent that reducing aspartate levels, as occurs with the ketogenic diet, would bias toward reduced seizures.
A recent study of cerebrospinal fluid amino acids in 26 children (ages 1.3 to 15.8 years) on the ketogenic diet provides partial confirmation that the diet induces alterations in the metabolism of excitatory amino acids, with greater effects on aspartate than on glutamate [45]. In these children, there were nonsignificant trends toward reduced aspartate (72% of baseline) and glutamate (75% of baseline) levels in cerebrospinal fluid samples obtained during the diet, compared with a prediet baseline value. In 13 younger subjects (age <5.5 years), the reductions were statistically significant, with aspartate levels reduced to 50% of baseline and glutamate reduced to 79% of baseline.
Do Ketone Bodies Influence Ionotropic Glutamate Receptors?
Agents that block ionotropic glutamate receptors exert anticonvulsant activity in diverse animal seizure models, including the maximal electroshock test [62]. The ketogenic diet may have activity in the maximal electroshock test in mice, although not in rats (Table 1), raising the possibility that some aspect of the diet, perhaps ketone bodies themselves, could block ionotropic glutamate receptors. However, studies in cell culture have failed to observe effects of β-hydroxybutyrate or acetoacetate on N-methyl-D-aspartate or AMPA receptor-mediated synaptic currents [48] or on responses to exogenously applied N-methyl-D-aspartate or AMPA [49]. Whether acetone is similarly inactive on ionotropic glutamate receptors remains to be determined.
Role of Norepinephrine and Peptidergic Neurotransmitter Systems
Alterations in the activity of regionally specific neuro-transmitters such as the monoamines norepinephrine, serotonin, and histamine and the neuropeptides galanin and neuropeptide Y can influence seizure susceptibility. Little is known about the effects of the ketogenic diet on these neurotransmitter systems. However, seizure protection by the ketogenic diet, at least in the flurothyl seizure model, appears to be dependent upon the integrity of norepinephrine signaling. Thus, mice lacking norepinephrine due to knockout of the gene for dopamine β-hydroxylase, which encodes the enzyme that catalyzes the conversion of dopamine to norepinephrine, failed to show an elevation in flurothyl threshold when fed a ketogenic diet [63].
In this regard, the ketogenic diet is similar to commonly used anticonvulsant medications that also require an intact norepinephrine system, such as phenobarbital [64]. In a separate study, effects of the diet on maximal electroshock seizures were unaltered in mice lacking the norepinephrine transporter, which would be expected to elevate synaptic norepinephrine levels [65]. Whether alterations in norepinephrine signaling would affect the activity of the ketogenic diet in other seizure models remains to be determined.
It has been suggested that anticonvulsant neuropeptides involved in fat metabolism could have a role in the efficacy of the ketogenic diet [66], but the diet does not change galanin or neuropeptide Y levels [67].
Effects on Ion Channels and Proteins Associated with Synaptic Transmission
Alterations in the expression of ion channels or in the machinery of synaptic transmission would be expected to influence seizure susceptibility. Indeed, a recent microarray study of gene expression in the hippocampus of rats fed a ketogenic diet for three weeks revealed changes in the transcripts of a total of 39 genes that encode such proteins [39]. Most transcripts were reduced in animals receiving the diet, including the voltage-dependent calcium channel subunits γ4 and α1D, the ClCN1 chloride channel, the KCNH3 and KCNE1-like potassium channels, P2X3 and P2X7 purinergic receptors, and synapto-tagmins 6 and XI. Other transcripts were upregulated, including subunits of the ionotropic glutamate receptors GluR2 and KA1, the KCNN2 potassium channel, the SCN1a type Iα sodium channel subunit, and the glutamate transporter EAAC1.
No changes were observed in GABA receptors subunits, although in another study expression of the GABAA receptor α4 subunit was downregulated in the hippocampus of mice fed a ketogenic diet [68]. That study also found reduced expression of the G-protein-coupled inward rectifier potassium channel Kir3.3. The functional significance of these changes in ion channel expression remains to be determined.
In addition to effects on ion channel expression, metabolic changes produced by the diet could cause functional alterations in ion channel activity. ATP-sensitive potassium channels (KATP), which are inhibited by high intra-cellular ATP levels, are of particular relevance [1,69]. A role for KATP channels in the ketogenic diet was suggested by data showing that the ketone bodies β-hydroxybutyrate and acetoacetate decreased the firing rate of rodent substantia nigra pars reticulata neurons in vitro; blockade of these channels abolished the effect, indicating that the decrease in neuronal firing is due to opening of KATP channels induced by the ketone bodies [70]. To date, however, it has been difficult to show a role for KATP channels in the regulation of seizure susceptibility except in conditions of metabolic stress [71,72]. Moreover, the ketogenic diet [73], and β-hydroxybutyrate [74,75] in particular, has been reported to elevate ATP levels, which would be expected to inhibit KATP channels and, if anything, would promote (rather than depress) brain excitability. Not all studies have shown an increase in ATP levels, however [43].
Effects on Energy Metabolism
During consumption of the ketogenic diet, ketone bodies replace glucose as a source of energy for the brain. These ketone bodies may be a more efficient source of energy per unit oxygen than glucose [76]. In addition, the ketogenic diet causes a coordinated upregulation of mitochondrial genes and genes involved in energy metabolism, and appear to stimulate the biogenesis of mitochondria as assessed by electron microscopy [39]. Together, the availability of a more efficient fuel and an increase in the number of mitochondria provide an increase in cellular energy production capacity and reserves. It seems plausible that the greater energy reserve would enhance the capacity of neurons to withstand metabolic challenges and could account for the ability of the diet to confer neuroprotection in models of neurodegenerative diseases or stroke [5]. It also has been proposed that effects of the ketogenic diet on brain energetics contribute to the seizure protection conferred by the diet [37,42], although there is little experimental support for this concept.
Changes in Cerebral pH and Ion Concentrations in the Ketogenic Diet
One of the first hypotheses proposed to explain the anticonvulsant action of the ketogenic diet was that the diet causes a drop in cerebral pH. However, changes in cerebral pH have not been observed in rats consuming a ketogenic diet, nor have changes been noted in blood pH in humans consuming the ketogenic diet [7,43]. Similarly, there is no evidence that the diet causes changes in ion concentrations that could account for its anticonvulsant activity. There were no differences in blood or brain potassium or calcium concentrations in rats consuming a ketogenic diet, although rats on the diet did have lower brain (but not blood) sodium levels than controls [42]. Serum levels of sodium, potassium, and calcium were similarly unaltered in humans consuming a ketogenic diet [77].
Comparison between the Profiles of the Ketogenic Diet and Anticonvulsant Drugs in Animal Seizure Models
Table 2 summarizes the profile of the ketogenic diet in mouse models commonly used to identify the anticonvulsant activity of test substances. The table also gives the profiles of the major clinical antiepileptic drugs in the same models. Notably, the ketogenic diet has a different pattern of activity from all of the medicines listed in the table. This pattern suggests the ketogenic diet exerts its anticonvulsant actions via a distinct set of mechanisms from that of the major antiepileptic drugs.
Table 2. Comparison of the ketogenic diet with antiepileptic drugs in selected acute seizure tests in mice.
| Treatment | PTZ | MES | 6 Hz | BIC | KA | NMDA | AMPA | ATPA | AMN | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Ketogenic diet | − | +/− | + | + | + | − | LST | − | − | [26,93] and A.L.H., M. Lyle, M.G., and M.A.R., unpublished data |
| Phenytoin | − | + | − | − | +/− | +/− | + | + | − | [34,96–99] |
| Carbamazepine | − | + | + | − | +/− | +/− | + | +/− | [34,96–98,100,101] | |
| Oxcarbazepine | + | + | + | nt | nt | nt | nt | nt | nt | [102–104] |
| Lamotrigine | − | + | − | − | + | + | + | + | nt | [34,96,98,105] |
| Zonisamide | − | + | + | − | + | nt | nt | − | nt | [34,103] and R.M. Kaminski, unpublished data |
| Ethosuximide | + | − | + | + | +/− | −/LST | + | + | − | [34,96–99,100] |
| Clonazepam | + | + | + | + | + | +/− | + | + | + | [34,96,98,100,106] |
| Vigabatrin | +/− | − | nt | + | + | − | − | − | [98,105–107] | |
| Tiagabine | + | − | + | − | nt | nt | nt | nt | nt | [34,96,105] |
| Phenobarbital | + | + | + | + | + | + | + | + | + | [34,96–98] |
| Valproate | + | + | + | + | +/− | + | + | + | + | [34,96,98,108] |
| Felbamate | + | + | + | − | nt | + | nt | nt | nt | [34,96,109] |
| Topiramate | − | + | − | − | + | − | − | + | nt | [34,96,100] |
| Gabapentin | + | +/− | + | − | + | + | + | + | nt | [34,98,103,105] |
| Levetiractam | − | − | + | + | − | − | − | − | nt | [34,96,98] |
Abbreviations:
AMN = Aminophylline
AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
ATPA = 2-amino-3-(5-tert-butyl-3-hydroxy-4-isothiazolyl)propionic acid
BIC = Bicuculline
GABA = γ-aminobutyric acid
KA = Kainic acid
LST = Lowers seizure threshold
MES = Maximal electroshock
NMDA = N-methyl-d-aspartate
nt = Not tested
PTZ = Pentylenetetrazol
+, −, and +/− = Effective, ineffective, and conflicting results, respectively
The profile of the diet is distinct from classical sodium channel blocking drugs because, unlike these agents, it has variable activity in the maximal electroshock test and is highly active in the 6 Hz model. Similarly, the profile of the diet is distinct from that of drugs that are believed to act predominantly through GABA systems (benzodiaz-epines, vigabatrin, tiagabine) in that it is not active in the PTZ model in mice. The profile of the ketogenic diet also differs from agents with complex actions including topiramate and felbamate.
Although limited data are available, the profile of zonisamide may be most similar to the ketogenic diet; however, the ketogenic diet protects against seizures induced by bicuculline, whereas zonisamide does not [78]. Zonisamide probably acts through a variety of mechanisms, including via effects on sodium channels and possibly T-type calcium channels, although a complete understanding of its cellular actions is probably still not at hand [4]. In any case, it seems likely that the ketogenic diet also acts through several different mechanisms, and may even exert its effects through different mechanisms in different patients [1].
The ketogenic diet, with its high fat content, has been compared also to valproate, a fatty acid derivative [79]. The anticonvulsant mechanism of action of valproate is likewise not well understood. However, Table 2 reveals many differences in the profiles of valproate and the ketogenic diet. Note that many patients who do well on the ketogenic diet have failed valproate therapy, supporting the concept that valproate and the diet do not have overlapping mechanisms.
Anecdotal evidence suggests that the ketogenic diet may be effective in patients who have failed many of the antiepileptic drugs listed in Table 2, indicating that it is indeed unique in its actions from antiepileptic drugs.
Antiepileptogenic Activity of the Ketogenic Diet
Many conventional anticonvulsant medications do not interfere with epileptogenesis in animal epilepsy models, such as the amygdala kindling model. However, there are some anticonvulsant medications (most notably valproate, levetiracetam, and drugs such as benzodiazepines that potentiate GABAA receptors) that do suppress the evolution of kindling in rodents [80,81]. Nevertheless, to date, no anticonvulsant medication has been demonstrated to protect against the development of epilepsy in clinical trials.
As is the case for the aforementioned medications, there is evidence that the ketogenic diet can protect against the development of epileptic seizure susceptibility, an effect that is distinct from its ability to inhibit seizures (Table 1). This was first demonstrated in 1999 by Muller-Schwarze et al. [82], who found that rats fed a ketogenic diet had fewer and briefer spontaneous recurrent seizures after kainic acid-induced status epilepticus than did control animals. In a subsequent study it was found that early initiation of the diet is necessary to obtain this antiepileptogenic effect [83].
The ketogenic diet was also found to have antiepileptogenic-like activity in an in vivo rat model of progressive hippocampal hyperexcitability that resembles kindling [50]. In this model, repetitive electrical stimulation of the angular bundle causes the progressive prolongation of afterdischarges in the dentate gyrus. Animals fed a ketogenic calorie-restricted diet exhibited a reduced rate in the evolution of this kindling-like phenomenon, compared with rats receiving either a normal calorie-restricted diet or a normal ad libitum diet. A recent study provides a hypothesis to explain the antiepileptogenic activity of the switch to fat metabolism from glycolysis in the ketogenic diet [84]. In that study, the nonmetabolizable glucose analog 2-deoxy-D-glucose was found to inhibit the development of kindling and to suppress seizure-induced increases in the transcription of brain-derived neurotrophic factor and its receptor TrkB. These results are intriguing in view of the evidence implicating brain-derived neurotrophic factor signaling in the development of temporal lobe epilepsy [85]. However, it remains to be demonstrated that reduced glycolysis acting through brain-derived neurotrophic factor signaling is the specific mechanism for antiepileptogenesis in the ketogenic diet.
Conclusions
Although the ketogenic diet has been applied clinically to the treatment of epilepsy for more than 85 years and now is often used as a last resort therapeutic modality for the most seriously affected patients, the mechanisms underlying seizure protection conferred by the ketogenic diet are still poorly defined.
A consideration of the spectrum of activity of the diet in acute animal seizure models suggests that the diet acts in a mechanistically distinct way from clinically used antiepileptic drugs. It has been proposed that GABAergic mechanisms could play a role, and this has been confirmed in at least one physiological model, but understanding of how the diet could influence GABAergic function is elusive. There is no compelling evidence that the ketogenic diet alters brain metabolism to induce global changes in GABA levels, although the possibility of regional changes or effects on the dynamics of synaptic GABA have not been studied adequately. In addition, there is no experimental support for effects of the diet or ketone bodies on GABA-mediated synaptic transmission or on the activity of GABA neurons. Inparticular, ketone bodies do not appear to influence postsynaptic GABAA receptors, which represent a key target for some traditional antiepileptic medications. The situation is comparable to that of valproate, which does not affect GABAergic inhibition through known mechanisms at therapeutic concentrations but is effective in many of the same animal models as drugs that act through GABA systems.
The ketogenic diet and valproate have different profiles ofactivityinanimalmodels, and the diet may be effective for some patients who have failed to respond adequately to valproate (which suggests that their mechanisms are distinct). The conclusion that the ketogenic diet acts in a mechanistically novel way is supported by anecdotal clinical evidence that it confers seizure protection for some patients with pharmacoresistant epilepsies. Therefore, it is unlikely that the mechanism of the diet overlaps completely with drugs currently available.
The potential role of acetone in the mechanism of action of the ketogenic diet is intriguing. Studies to determine whether brain levels of acetone during consumption of the diet are within the range of those known to confer seizure protection have not yet been conducted because of the difficulty of measuring acetone, but are feasible. In addition, it will be of interest to determine whether acetone protects against epileptiform activity in in vitro models through traditional mechanisms, or whether it acts on novel targets, such as gap junctions, which appear to mediate epilepti-form activity in some circumstances and are influenced by small molecules that are structurally similar to ketone bodies [86].
A better understanding of the mechanism of action of the ketogenic diet will refine its clinical use. It is already clear that different dietary regimens, such as the Atkins diet [87] or the low glycemic index diet [88], confer seizure protection, but there may be differences from the classical ketogenic diet, if only in palatability and side effects. An insight into the underlying mechanisms should allow dietary therapy to be optimized, maximizing efficacy while minimizing side effects.
The ketogenic diet is now being considered for diverse neurological indications other than epilepsy, largely because of its putative neuroprotective properties [5]. A mechanistic understanding will allow a more rational assessment of the potential utilities of the diet (or variants) in a broad range of conditions associated with neurodegeneration and neural injury including Alzheimer disease, Parkinson disease, traumatic brain injury, and stroke.
Acknowledgments
The authors gratefully acknowledge the contributions of Megan Lyle to the collection of unpublished data presented in Tables 1 and 2.
Footnotes
This work was supported by the Intramural Research Program of the U.S. National Institutes of Health, National Institute of Neurological Disorders and Stroke; by the University of California, Davis School of Medicine; and by the Epilepsy Foundation through the generous support of Pfizer, Inc. (A.L.H.).
References
- 1.Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 2006;68:145–80. doi: 10.1016/j.eplepsyres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bailey EE, Pfeifer HH, Thiele EA. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005;6:4–8. doi: 10.1016/j.yebeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Rogawski MA. Principles of antiepileptic drug action. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 3–22. [Google Scholar]
- 4.Macdonald RL, Rogawski MA. Cellular effects of antiepileptic drugs. In: Engel J Jr, Pedley TA, Aicardi J, Dichter MA, Moshé S, Perucca E, et al., editors. Epilepsy: a comprehensive textbook. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. in press. [Google Scholar]
- 5.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–9. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Proc. 1921;2:307–8. [Google Scholar]
- 7.Huttenlocher PR, Wilbourn AJ, Signore JM. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology. 1971;21:1097–103. doi: 10.1212/wnl.21.11.1097. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz RM, Boyes S, Aynsley-Green A. Metabolic effects of three ketogenic diets in the treatment of severe epilepsy. Dev Med Child Neurol. 1989;31:152–60. doi: 10.1111/j.1469-8749.1989.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 9.Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55:1433–7. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 10.Vining EP. Clinical efficacy of the ketogenic diet. Epilepsy Res. 1999;37:181–90. doi: 10.1016/s0920-1211(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108:898–905. doi: 10.1542/peds.108.4.898. [DOI] [PubMed] [Google Scholar]
- 12.Than KD, Kossoff EH, Rubenstein JE, Pyzik PL, McGrogan JR, Vining EP. Can you predict an immediate, complete, and sustained response to the ketogenic diet? Epilepsia. 2005;46:580–2. doi: 10.1111/j.0013-9580.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, Brederode JV, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007 Mar 30; doi: 10.1111/j.1471-4159.2007.04483.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–64. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37:1951–62. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Bough KJ, Chen RS, Eagles DA. Path analysis shows that increasing ketogenic ratio, but not β-hydroxybutyrate, elevates seizure threshold in the rat. Dev Neurosci. 1999;21:400–6. doi: 10.1159/000017390. [DOI] [PubMed] [Google Scholar]
- 17.Rho JM, Anderson GD, Donevan SD, White HS. Acetoacetate, acetone, and dibenzylamine (a contaminant in L-(+)-β-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 2002;43:358–61. doi: 10.1046/j.1528-1157.2002.47901.x. [DOI] [PubMed] [Google Scholar]
- 18.Likhodii SS, Burnham WM. Ketogenic diet: does acetone stop seizures? Med Sci Monit. 2002;8:HY19–24. [PubMed] [Google Scholar]
- 19.Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC, 3rd, Burnham WM. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann Neurol. 2003;54:219–26. doi: 10.1002/ana.10634. [DOI] [PubMed] [Google Scholar]
- 20.Gasior M, French A, Joy M, Tang R, Hartman AL, Rogawski MA. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-pro-panediol, methylglyoxal or pyruvic acid. Epilepsia. 2007;48:793–800. doi: 10.1111/j.1528-1167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 21.Nylen K, Likhodii SS, Hum KM, Burnham WM. A ketogenic diet and diallyl sulfide do not elevate afterdischarge thresholds in adult kindled rats. Epilepsy Res. 2006;71:23–31. doi: 10.1016/j.eplepsyres.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–8. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 23.Seymour KJ, Bluml S, Sutherling J, Sutherling W, Ross BD. Identification of cerebral acetone by 1H-MRS in patients with epilepsy controlled by ketogenic diet. MAGMA. 1999;8:33–42. doi: 10.1007/BF02590633. [DOI] [PubMed] [Google Scholar]
- 24.Musa-Veloso K, Rarama E, Comeau F, Curtis R, Cunnane S. Epilepsy and the ketogenic diet: assessment of ketosis in children using breath acetone. Pediatr Res. 2002;52:443–8. doi: 10.1203/00006450-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Richerson GB. Looking for GABA in all the wrong places: the relevance of extrasynaptic GABAA receptors to epilepsy. Epilepsy Curr. 2004;4:239–42. doi: 10.1111/j.1535-7597.2004.46008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhlemann ER, Neims AH. Anticonvulsant properties of the ketogenic diet in mice. J Pharmacol Exp Ther. 1972;180:231–8. [PubMed] [Google Scholar]
- 27.Bough KJ, Eagles DA. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999;40:138–43. doi: 10.1111/j.1528-1157.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 28.Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35:21–8. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- 29.Likhodii SS, Musa K, Mendonca A, Dell C, Burnham WM, Cunnane SC. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia. 2000;41:1400–10. doi: 10.1111/j.1528-1157.2000.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 30.Nylen K, Likhodii S, Abdelmalik PA, Clarke J, Burnham WM. A comparison of the ability of a 4:1 ketogenic diet and a 6.3:1 ketogenic diet to elevate seizure thresholds in adult and young rats. Epilepsia. 2005;46:1198–204. doi: 10.1111/j.1528-1167.2005.71204.x. [DOI] [PubMed] [Google Scholar]
- 31.Bough KJ, Gudi K, Han FT, Rathod AH, Eagles DA. An anticonvulsant profile of the ketogenic diet in the rat. Epilepsy Res. 2002;50:313–25. doi: 10.1016/s0920-1211(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 32.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–27. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–7. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- 34.White HS, Woodhead JH, Wilcox KS, Stables JP, Kupferberg HJ, Wolf HH. Discovery and preclinical development of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 36–48. [Google Scholar]
- 35.Cheng CM, Hicks K, Wang J, Eagles DA, Bondy CA. Caloric restriction augments brain glutamic acid decarboxylase-65 and -67 expression. J Neurosci Res. 2004;77:270–6. doi: 10.1002/jnr.20144. [DOI] [PubMed] [Google Scholar]
- 36.Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Brain amino acid metabolism and ketosis. J Neurosci Res. 2001;66:272–81. doi: 10.1002/jnr.1221. [DOI] [PubMed] [Google Scholar]
- 37.De Vivo DC, Leckie MP, Ferrendelli JS, McDougal DBJ. Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–7. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 38.Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, et al. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47:119–28. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 40.Nordli DR, Jr, De Vivo DC. The ketogenic diet revisited: back to the future. Epilepsia. 1997;38:743–9. doi: 10.1111/j.1528-1157.1997.tb01460.x. [DOI] [PubMed] [Google Scholar]
- 41.Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 42.Appleton DB, De Vivo DC. An animal model for the ketogenic diet. Epilepsia. 1974;15:211–27. doi: 10.1111/j.1528-1157.1974.tb04943.x. [DOI] [PubMed] [Google Scholar]
- 43.Al-Mudallal AS, LaManna JC, Lust WD, Harik SI. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia. 1996;37:258–61. doi: 10.1111/j.1528-1157.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 44.Schousboe A, Sonnewald U, Waagepetersen HS. Differential roles of alanine in GABAergic and glutamatergic neurons. Neurochem Int. 2003;43:311–5. doi: 10.1016/s0197-0186(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 45.Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–25. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes L. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38:519–27. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, et al. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy: exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–9. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- 48.Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–31. doi: 10.1212/wnl.54.2.325. [DOI] [PubMed] [Google Scholar]
- 49.Donevan SD, White HS, Anderson GD, Rho JM. Voltage-dependent block of N-methyl-D-aspartate receptors by the novel anticonvulsant dibenzylamine, a bioactive constituent of L-(+)-β-hydroxybutyrate. Epilepsia. 2003;44:1274–9. doi: 10.1046/j.1528-1157.2003.07203.x. [DOI] [PubMed] [Google Scholar]
- 50.Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–60. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 51.Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem. 1996;67:2325–34. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- 52.Abdul-Ghani AS, Norris PJ, Smith CC, Bradford HF. Effects of γ-acetylenic GABA and γ-vinyl GABA on synaptosomal release and uptake of GABA. Biochem Pharmacol. 1981;30:1203–9. doi: 10.1016/0006-2952(81)90298-7. [DOI] [PubMed] [Google Scholar]
- 53.Rogawski MA, Reddy DS. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho JM, Sankar R, Cavazos JE, editors. Epilepsy: scientific foundations of clinical practice. New York: Marcel Dekker; 2004. pp. 319–55. [Google Scholar]
- 54.Mazier MJP, Jones PJH. Diet fat saturation and feeding state modulate rates of cholesterol synthesis in normolipidemic men. J Nutr. 1997;127:332–40. doi: 10.1093/jn/127.2.332. [DOI] [PubMed] [Google Scholar]
- 55.Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–27. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhodes ME, Talluri J, Harney JP, Frye CA. Ketogenic diet decreases circulating concentrations of neuroactive steroids of female rats. Epilepsy Behav. 2005;7:231–9. doi: 10.1016/j.yebeh.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melo TM, Sonnewald U, Touret M, Nehlig A. Cortical glutamate metabolism is enhanced in a genetic model of absence epilepsy. J Cereb Blood Flow Metab. 2006;26:1496–506. doi: 10.1038/sj.jcbfm.9600300. [DOI] [PubMed] [Google Scholar]
- 58.Rho JM, Kim DW, Robbins CA, Anderson GD, Schwartzkroin PA. Age-dependent differences in flurothyl seizure sensitivity in mice treated with a ketogenic diet. Epilepsy Res. 1999;37:233–40. doi: 10.1016/s0920-1211(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 59.Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot Essent Fatty Acids. 2004;70:277–85. doi: 10.1016/j.plefa.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Zhou M, Peterson CL, Lu YB, Nadler JV. Release of glutamate and aspartate from CA1 synaptosomes: selective modulation of aspartate release by ionotropic glutamate receptor ligands. J Neurochem. 1995;64:1556–66. doi: 10.1046/j.1471-4159.1995.64041556.x. [DOI] [PubMed] [Google Scholar]
- 61.Bradford SE, Nadler JV. Aspartate release from rat hippocampal synaptosomes. Neuroscience. 2004;128:751–65. doi: 10.1016/j.neuroscience.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 62.Löscher W, Rogawski MA. Epilepsy. In: Lodge D, Danysz W, Parsons CG, editors. Ionotropic glutamate receptors as therapeutic targets. Johnson City, TN: F.P. Graham Publishing Co; 2002. pp. 91–132. [Google Scholar]
- 63.Szot P, Weinshenker D, Rho JM, Storey TW, Schwartzkroin PA. Norepinephrine is required for the anticonvulsant effect of the ketogenic diet. Brain Res Dev Brain Res. 2001;129:211–4. doi: 10.1016/s0165-3806(01)00213-9. [DOI] [PubMed] [Google Scholar]
- 64.Fischer W, Muller M. Pharmacological modulation of central monoaminergic systems and influence on the anticonvulsant effectiveness of standard antiepileptics in maximal electroshock seizure. Biomed Biochim Acta. 1988;47:631–45. [PubMed] [Google Scholar]
- 65.Martillotti J, Weinshenker D, Liles LC, Eagles DA. A ketogenic diet and knockout of the norepinephrine transporter both reduce seizure severity in mice. Epilepsy Res. 2006;68:207–11. doi: 10.1016/j.eplepsyres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Schwartzkroin PA. Mechanisms underlying the antiepileptic efficacy of the ketogenic diet. Epilepsy Res. 1999;37:171–80. doi: 10.1016/s0920-1211(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 67.Tabb K, Szot P, White SS, Liles LC, Weinshenker D. The ketogenic diet does not alter brain expression of orexigenic neuropeptides. Epilepsy Res. 2004;62:35–9. doi: 10.1016/j.eplepsyres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM, et al. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res Mol Brain Res. 2004;129:80–7. doi: 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Vamecq J, Vallee L, Lesage F, Gressens P, Stables JP. Antiepileptic popular ketogenic diet: emerging twists in an ancient story. Prog Neurobiol. 2005;75:1–28. doi: 10.1016/j.pneurobio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci. 2007;27:3618–25. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogawski MA. Epilepsy. In: Pullan L, Patel J, editors. Neurotherapeutics: emerging strategies. Totowa, NJ: Humana Press; 1996. pp. 193–273. [Google Scholar]
- 72.Cosford NDP, Meinke PT, Stauderman KA, Hess SD. Recent advances in the modulation of voltage-gated ion channels for the treatment of epilepsy. Curr Drug Targets CNS Neurol Disord. 2002;1:81–104. doi: 10.2174/1568007023339463. [DOI] [PubMed] [Google Scholar]
- 73.Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–80. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- 74.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–7. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- 77.Chesney D, Brouhard BH, Wyllie E, Powaski K. Biochemical abnormalities of the ketogenic diet in children. Clin Pediatr (Phila) 1999;38:107–9. doi: 10.1177/000992289903800207. [DOI] [PubMed] [Google Scholar]
- 78.Kitano Y, Komiyama C, Makino M, Takasuna K, Takazawa A, Sakurada S. Anticonvulsant properties of the novel nootropic agent nefiracetam in seizure models of mice and rats. Epilepsia. 2005;46:811–8. doi: 10.1111/j.1528-1167.2005.66504.x. [DOI] [PubMed] [Google Scholar]
- 79.Cullingford TE. The ketogenic diet: fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;70:253–64. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Schmutz M, Klebs K, Baltzer V. Inhibition or enhancement of kindling evolution by antiepileptics. J Neural Transm. 1988;72:245–57. doi: 10.1007/BF01243423. [DOI] [PubMed] [Google Scholar]
- 81.Löscher W, Honack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284:474–9. [PubMed] [Google Scholar]
- 82.Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999;10:1517–22. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- 83.Su SW, Cilio MR, Sogawa Y, Silveira DC, Holmes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Brain Res Dev Brain Res. 2000;125:131–8. doi: 10.1016/s0165-3806(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 84.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–7. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 85.Scharfman HE. Brain-derived neurotrophic factor and epilepsy: a missing link? Epilepsy Curr. 2005;5:83–8. doi: 10.1111/j.1535-7511.2005.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gajda Z, Szupera Z, Blazso G, Szente M. Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia. 2005;46:1581–91. doi: 10.1111/j.1528-1167.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 87.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47:421–4. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 88.Pfeifer HH, Thiele EA. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65:1810–2. doi: 10.1212/01.wnl.0000187071.24292.9e. [DOI] [PubMed] [Google Scholar]
- 89.Thavendiranathan P, Chow C, Cunnane S, McIntyre Burnham W. The effect of the ’classic’ ketogenic diet on animal seizure models. Brain Res. 2003;959:206–13. doi: 10.1016/s0006-8993(02)03744-7. [DOI] [PubMed] [Google Scholar]
- 90.Otani K, Yamatodani A, Wada H, Mimaki T, Yabuuchi H. Effect of ketogenic diet on the convulsive threshold and brain amino acid and monoamine levels in young mice. No To Hattatsu. 1984;16:196–204. In Japanese. [PubMed] [Google Scholar]
- 91.Millichap JG, Jones JD, Rudis BP. Mechanism of anticonvulsant action of ketogenic diet: studies in animals with experimental seizures and in children with petit mal epilepsy. Am J Dis Child. 1964;107:593–604. [PubMed] [Google Scholar]
- 92.Bough KJ, Matthews PJ, Eagles DA. A ketogenic diet has different effects upon seizures induced by maximal electroshock and by pentylenetetrazole infusion. Epilepsy Res. 2000;38:105–14. doi: 10.1016/s0920-1211(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 93.Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, et al. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–28. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]
- 95.Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–40. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 96.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–27. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 97.Czuczwar SJ, Janusz W, Wamil A, Kleinrok Z. Inhibition of aminophylline-induced convulsions in mice by antiepileptic drugs and other agents. Eur J Pharmacol. 1987;144:309–15. doi: 10.1016/0014-2999(87)90383-9. [DOI] [PubMed] [Google Scholar]
- 98.Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 99.Steppuhn KG, Turski L. Modulation of the seizure threshold for excitatory amino acids in mice by antiepileptic drugs and chemoconvulsants. J Pharmacol Exp Ther. 1993;265:1063–70. [PubMed] [Google Scholar]
- 100.Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Turski L, Niemann W, Stephens DN. Differential effects of antiepileptic drugs and beta-carbolines on seizures induced by excitatory amino acids. Neuroscience. 1990;39:799–807. doi: 10.1016/0306-4522(90)90262-3. [DOI] [PubMed] [Google Scholar]
- 102.Luszczki JJ, Czuczwar SJ. Isobolographic characterisation of interactions among selected newer antiepileptic drugs in the mouse pentylenetetrazole-induced seizure model. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:41–54. doi: 10.1007/s00210-005-1088-9. [DOI] [PubMed] [Google Scholar]
- 103.Shannon HE, Eberle EL, Peters SC. Comparison of the effects of anticonvulsant drugs with diverse mechanisms of action in the formalin test in rats. Neuropharmacology. 2005;48:1012–20. doi: 10.1016/j.neuropharm.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 104.Wamil AW, Schmutz M, Portet C, Feldmann KF, McLean MJ. Effects of oxcarbazepine and 10-hydroxycarbamazepine on action potential firing and generalized seizures. Eur J Pharmacol. 1994;271:301–8. doi: 10.1016/0014-2999(94)90787-0. [DOI] [PubMed] [Google Scholar]
- 105.Dalby NO, Nielsen EB. Comparison of the preclinical anticonvulsant profiles of tiagabine, lamotrigine, gabapentin and vigabatrin. Epilepsy Res. 1997;28:63–72. doi: 10.1016/s0920-1211(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 106.Holland KD, McKeon AC, Canney DJ, Covey DF, Ferrendelli JA. Relative anticonvulsant effects of GABAmimetic and GABA modulatory agents. Epilepsia. 1992;33:981–6. doi: 10.1111/j.1528-1157.1992.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 107.Bernasconi R, Klein M, Martin P, Christen P, Hafner T, Portet C, et al. Gamma-vinyl GABA: comparison of neurochemical and anticonvulsant effects in mice. J Neural Transm. 1988;72:213–33. doi: 10.1007/BF01243421. [DOI] [PubMed] [Google Scholar]
- 108.Borowicz KK, Zadrozniak M, Swiader M, Kowalska A, Kleinrok Z, Czuczwar SJ. Interaction of the neurosteroid alphaxalone with conventional antiepileptic drugs in different types of experimental seizures. Eur J Pharmacol. 2002;449:85–90. doi: 10.1016/s0014-2999(02)01975-1. [DOI] [PubMed] [Google Scholar]
- 109.White HS, Wolf HH, Swinyard EA, Skeen GA, Sofia RD. A neuropharmacological evaluation of felbamate as a novel anticonvulsant. Epilepsia. 1992;33:564–72. doi: 10.1111/j.1528-1157.1992.tb01711.x. [DOI] [PubMed] [Google Scholar]