Abstract
Background
High resolution ultrasonography (HR-US) can monitor the molecular changes and biochemical interactions between proteins in real-time. The aim of this study was to use HR-US to characterize the real-time interactions between plasminogen coated beads and PrPSc and to determine if this approach could be applied to the identification of animals affected by prion diseases. Plasminogen, immobilized to beads, was used as a capturing tool for PrPSc in brain homogenates from scrapie affected sheep and the binding reaction was monitored in real-time in an ultrasonic cell.
Results
Changes in the ultrasonic parameters suggested that three processes occurred during the incubation: binding, protein-protein network formation and precipitation and that these processes occurred in a concentration dependent manner. Conversely, when homogenates from normal sheep were similarly examined, no evidence for the occurrence of these processes was found indicating the specificity of the interaction between the plasminogen coated beads and PrPSc.
Conclusion
These results indicate firstly, that the plasminogen coated beads binded selectively to PrPSc and secondly, that a HR-US system can discriminate between scrapie affected and non-affected samples and thus has potential as a tool for the rapid diagnosis for prion diseases. This approach has the significant advantage of not requiring a proteinase K pre-digestion step, which is routinely used in current PrPSc detection assays.
Background
Prion diseases such as CJD in humans, BSE in cattle and scrapie in sheep are a group of neurodegenerative disorders, which are characterised by the accumulation in the central nervous system of the protease resistant form (PrPSc) of a host-coded membrane glycoprotein (PrPc) [1]. The transformation of PrPc into PrPSc implies a conformational change from a mainly alpha helical form into a beta sheet rich structure [2,3]. This conformational difference is responsible for the distinct physicochemical properties of both isoforms. While PrPc exist as a monomer and it is rapidly degraded by proteinase K (PK), the infectious isoform PrPSc, forms detergent-insoluble aggregates and displays a higher resistance to degradation with PK [4,5].
Most currently used diagnostic techniques are based on the immunological detection of PrPSc using antibodies specific to the prion protein. As antibodies used on current validated assays are not able to differentiate between PrPSc and PrPc, these diagnostic procedures rely on the elimination of PrPc by PK digestion, PrPSc remaining due to its PK resistance. However, the use of PK sample pre-treatments may compromise the sensitivity of an assay as some PrPSc conformations are known to be relatively protease-sensitive.
Many recent investigations are focused on increasing the sensitivity of current diagnosis assays and much effort has been directed toward the development of novel reagents that could be used as sensitive tools for prion detection. Several such reagents, aimed at the specific detection of PrPSc, include nucleic acid-aptamers [6], anti-DNA antibodies [7], PrPSc specific monoclonal antibodies antibodies [8,9] and plasminogen protein [10].
Plasminogen, a plasma proenzyme involved in the process of fibrinolysis [11], has shown potential for the detection and differentiation of PrPSc without the necessity for a PK pre-treatment. The specific binding of plasminogen, covalently bound to magnetic beads, to PrPSc aggregates in tissues from several species has been demonstrated [10,12]. While binding of PrPc may occur, PrPSc is preferentially bound under experimental conditions that enhance the formation of fibrils [13].
High-resolution ultrasonic spectroscopy is a novel real-time analytical non-destructive technique for material analysis, in which changes in the behaviour of ultrasonic waves, as they pass through a test material, may provide information regarding the structure and composition of the material. Ultrasonic spectroscopy allows simultaneous measurements of two independent parameters: velocity and attenuation, both of which are very sensitive to changes in intermolecular interactions and molecular organization. Attenuation is a measure of the energy loss in the compressions and decompressions produced as an ultrasonic wave passes through a material and gives information on the microscopic structural organisation of the material. Ultrasonic velocity is a measurement of the density and elastic response of the material to the oscillating pressure and provides information regarding its molecular organization. The ability of an ultrasonic system to monitor both structural and chemical processes in real time has been demonstrated. For example, it has been shown to be well suited to a wide range of applications such as the study of aggregation and gelation in food colloids, conformational transitions in polymers and biopolymers, and phase transitions and formation of micelles [14-18]. It has also been recently applied to the analyses of chemical reactions [19,20]. Advantages of this technique include the ability of the ultrasonic wave to propagate through a broad range of samples, including opaque materials (in contrast to optical spectroscopy), and the opportunity to carry out on line analysis because ultrasonic detection can be done without pre-treatment of the material. Another distinct advantage is the high resolution in measurements of velocity, allowing detection of concentrations down to ppm level.
A combination of specific reagents for the disease associated prion protein and this novel ultrasonic technique make this approach extremely promising for the analysis and diagnosis of prion disease. Thus, the aim of this study was to investigate the interactions between plasminogen coated beads and PrPSc in scrapie affected brain tissue, particularly in the absence of a proteinase K pre-digestion step, with a view to exploring its potential for use as a sensitive detection system for PrPSc.
Results
The interaction of PrPSc to plasminogen was analyzed in real time using the ultrasonic spectroscopy measurements of velocity and attenuation. In each experiment, the evolution of ultrasonic profiles was investigated over a period of approximately four hours after the addition of a plasminogen coated bead solution (10 μl added giving a final concentration of 2 × 107) or alternatively in the absence of beads. Specificity was demonstrated using uncoated beads. In a first series of experiments, binding kinetics were analyzed by titration of PK undigested homogenates at three concentrations 10, 5 and 2%. As the 2% homogenate gave the clearest results, all further experimentation focused on this concentration and only these results are reported here. Similarly, velocity measurements gave the most valuable information; attenuation measurements failed clearly to differentiate different homogenate variables. Thus, only the results of the velocity measurements are presented here.
PK untreated homogenates
In scrapie affected, PK untreated homogenates, velocity profiles demonstrated three distinct stages during the incubation period (see Figure 1). The first stage is characterized by an increase in the velocity values, reaching a peak after about 40 minutes. The second stage was characterized by an extended plateau period, lasting approximately 90 minutes and finally there was a sharp decrease, taking about 10 to 20 minutes to reach T0 levels. This pattern of binding was found consistently in repeat experiments.
Figure 1.
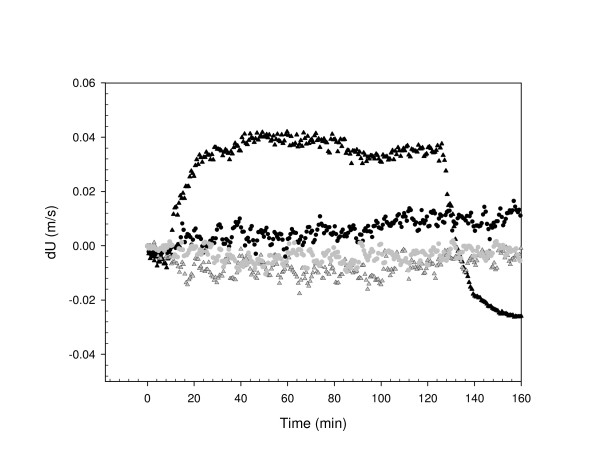
Illustration of ultrasonic velocity (dU) profiles of PK-untreated scrapie infected and healthy brain homogenates prepared at 2% in the presence and absence of plasminogen coated beads. Binding reaction was performed in the reaction buffer at 25°C. A 10 ul-buffer solution with coated beads (2 × 107) or without (0) was added to each sample (t = 0). Ultrasonic profiles of scrapie infected homogenate in the presence (black triangle) and absence (grey triangle) of beads and healthy brain homogenates in the presence (black circle) and absence of beads (grey circle) are shown over approximately 3-hour of the reaction.
By contrast, scrapie unaffected homogenates gave profiles (Figure 1) which were quantitatively and qualitatively distinct from those of affected samples (P < 0.05). They showed a gradual but small increase over the four hour period of study and were very similar to those of the control samples, both scrapie affected and unaffected, which were performed in the absence of beads (Figure 1).
Thus, it was only in the scrapie affected tissues that there evidence of a significant reaction and these results argue for an interaction between the plasminogen coated beads and PrPSc but not between the plasminogen coated beads and PrPc or any non-PrP component of the sample.
Effect of PK
Further studies to investigate the specificity of the velocity profiles were performed using PK-treated homogenates. By contrast to the three-phase velocity profile seen with PK untreated scrapie positive tissues, PK appears to have the effect of eliminating the plateau phase giving instead, a two-phase reaction. This comprised firstly a gradual increase over a period of ~70 minutes and followed by a sudden decrease, taking ~10 minutes to reach T0. The amplitude of the Phase-1 increase in these PK treated tissues was always less than that seen in the PK untreated.
Velocity profiles in scrapie unaffected tissues showed no evidence of any reaction and no significant difference was seen between those which were PK treated and those untreated (P > 0.05).
Discussion
The ability of plasminogen coated beads to selectively bind PrPSc was confirmed in experiments involving PK-treated healthy and PK-treated infected brain samples. The results of the PK-treated healthy samples, which showed no evidence of any binding, indicated that no non-PrP molecule is bound by the plasminogen coated beads. Thus, in PK treated homogenates, only PrPSc is bound to plasminogen. In experiments involving PK untreated homogenates, we have demonstrated that no binding occurs in healthy samples, indicating that PrPc does not bind in these reaction conditions. By contrast, in PK untreated scrapie affected samples, there is good evidence that PrPSc is bound to plasminogen. These results corroborate previous investigations, which indicated a selective binding response of plasminogen to PrPSc [10,12] demonstrating the feasibility of plasminogen and the potential of this technique to be used as a diagnostic tool to differentiate between PrP isoforms.
Controversial to these results are the studies of Ellis et al. [21], Ryou et al. [22], Kornblatt et al. [23], and Cuccioloni et al. [24]. These investigations, performed with recombinant prion protein as well as purified full-length PrPc, suggested that PrPc could also interact with plasminogen. The apparently contradictory evidence might be explained by the different binding properties of plasminogen kringle domains to PrP in varying detergent conditions. It has been suggested that the preferential binding of plasminogen to one PrP isoform or another depends of the physical state of PrP in the particular detergent solution [12,22]. Fischer et al. [10] and Maissen et al. [12] pointed out that the high amyloid content of PrPSc and/or its aggregated state could have favored the binding of plasminogen to the PrPSc isoform over the soluble PrPc form. Thus, the binding of prion isoforms to plasminogen is affected by the detergent extraction conditions. Shaked et al. [13] reported that the selective binding of plasminogen to PrPSc occurs under detergent conditions that cause raft disruption and enhance the aggregate state of PrPSc. These authors reported that PrPc, extracted from natural sources, was able to interact to plasminogen only in detergent conditions in which rafts remained intact. In the present study, homogenates were prepared in Sarkosyl, an anionic detergent which promotes aggregation, and most likely, solubilises rafts [25,26]. It is therefore likely that the reaction conditions used in this study promoted the interaction of plasminogen and the aggregated PrPSc isoform. There was no evidence that endogenous palsminogen would interfere with this assay in this study as all five scrapie positive tissues interacted with the plasminogen coated beads.
Although velocity profiles of both PK-untreated and treated scrapie infected samples revealed binding, complex formation and precipitation, the magnitude of velocity changes, rate of binding and time intervals at which precipitation occurs varied between samples. Conclusions could not be drawn in accordance with the quantitative kinetics of rate of binding in those curves as the accurate concentration of protein and therefore the number of binding sites on the surface is unknown. However, comparative qualitatively analyses of velocity profiles of PK-treated and untreated samples revealed that the rate of binding and magnitude of change in velocity was higher and faster in PK-untreated samples than PK-treated samples. In contrast, the precipitation of protein complexes seems to occur more rapidly in PK-treated than in PK-untreated samples. These results suggest a differential binding response of plasminogen to PK treated and untreated PrP aggregates. Variations in velocity parameters observed could be explained by the formation of two different types of aggregates possibly very similar in size but different in the structure and the amount of accessible groups at the surface of the aggregates. Plasminogen is a blood serum protein, which has been shown to bind to different molecular surfacesthat contain exposed carboxy-terminal lysines [11,27]. This interaction is kringle mediated. The mechanisms of binding of PrPSc to plasminogen are unclear and investigations in this process have been curtailed by the lack of structural information on the PrPSc prion protein. However, it has been demonstrated that the interaction of plasminogen to PrPSc aggregates is lysine mediated [10]. Sheep prion protein contains several clusters of lysine residues in the core (104, 107, 109, 113, 188, 197, and 207) and also in the flexible tail (25, 26, and 29). The N-terminal core of the protein is a flexible extended tail, thus it is very sensitive and susceptible to the degradation by PK regardless the source of PrPSc [28]. It is possible that the treatment of PrPSc aggregates with proteinase K not only may remove important lysine binding sites but induce a conformational change leading to molecular structural rearrangement that might masks binding sites and other parts of the PrP molecule. New structural rearrangement of aggregates could induce a decrease in the ligand-binding efficiency of plasminogen kringles. Proteins with carboxy-terminal lysyl residues appear to function as plasminogen binding sites [27]. However, it is known that other structural characteristics of peptides and proteins, such the presence of additional lysyl residues proximal to the aminus terminus, can increase their affinity for plasminogen [27]. Several studies performed with recombinant prion protein have demonstrated the importance of n-terminal sequences in enhancing plasminogen binding activity [22,29].
If two different structural types of aggregates exist, with the PK-untreated type having a higher affinity for plasminogen than the PK-treated type, then, differences in velocity changes between PK-treated and untreated samples may be expected. Contributions to velocity changes depend on the elastic properties of the newly protein-protein network and therefore on the number of involved interactions between aggregate particles. In the case of PK-untreated samples the protein network is rapidly formed and stabilized by lysine interacting plasminogen kringles. In contrast, PK-treated aggregates which are not able to form additional bonds interact slowly with plasminogen forming protein-protein complex of reduced stability. The faster rate of binding on PK-untreated samples compared to PK-treated could be explained in terms of binding cooperativity. Ryou et al. [22], showed a positive cooperative binding process between kringle domains and recombinant PrP.
Changes in ultrasonic velocity and attenuation observed during the course of the experiments suggest that considerable physicochemical alterations were occurring in the solution. Ultrasonic velocity is determined by the density and elastic response of the medium to the oscillating pressure and this parameter can be expressed in terms of compressibility [30]. An increase on ultrasonic velocity at initial stages of the reaction suggests that both conformational changes and protein-protein complex formation processes took place during the binding event. Both, chemical interactions, involved in the binding process of plasminogen to PrPSc, along with structural characteristics of the newly formed protein network, could have contributed to the positive velocity changes observed. Conformational changes in the plasminogen protein molecule upon binding possibly contributed to the increment observed in the velocity values by decreasing the compressibility of the system. Significant conformational changes involving changes from a compact structure to a less compact in binding to small molecules such as aminocarboxylic acids or proteins such as fibrin have been frequently reported [31-33]. A change from a closed to an open conformation has been reported to occur, upon binding of plasminogen to a ligand. It has been suggested that this conformational change is able to produce a decrease in the system volume possibly due to the increase in exposure of surface area to the solvent, disruption of electrostatic interactions and decrease of size voids [34]. The increase in exposure of the protein to water molecules (i.e. hydration) has a significant effect on the ultrasonic velocity. An increase of water in the hydration shell of the solute molecules results in a rise in ultrasonic velocity in solutions due to lowering in the compressibility of the overall system. In the present study, similar factors contributing to a decrease in the compressibility of the system were expected to occur upon this type of conformational change. The increase in velocity is consistent with the protein changing from a compact to a more loosening structure. It was interesting that the most consistent profiles were detected at the most dilute concentration of homogenate (2%). As proteins change in conformation, cavities in the interior of the molecule are created and a rearrangement of atomic groups take place. Groups that were initially buried within the molecule may become exposed during the conformational transformation inducing variations in the hydrogen bonding and hydrophobic interactions of the protein. Positive velocity changes could also be related to the changes on elasticity in the solution due to the formation of the protein-protein network. Elasticity can been used to characterize protein network formation. Formation of a protein network introduces resistance to deformation and elasticity. As the protein-protein network is formed, the medium become more rigid and compact, so the velocity of the sound will be expected to increase in this new less elastic medium. Eventually, this protein interacting network precipitates restoring the initial medium properties. Precipitation was indicated by a decrease in velocity values. It is probable that the reaction conditions in the 2% homogenate was most favourable to detecting these molecular interactions.
Conclusion
this study highlights the potential of the HR-US system to detect plasminogen-PrPsc aggregates and to discriminate between PrP isoforms. The specificity of the interaction of plasminogen to PrPSc was demonstrated on scrapie PK-untreated and PK-treated samples and confirmed on healthy brain samples. This test is significant as it does not require a proteinase-K pretreatment step, which considerably improves the specificity of the assay.
Methods
Principles of plasminogen capture ultrasonic assay
In this study, the ultrasonic system was used to continuously monitor the binding reaction of plasminogen to sheep PrPSc with the aim of differentiating scrapie affected from non-affected brain tissues. Various preparations from normal or scrapie-affected brains were prepared, each at three different homogenate concentrations (2, 5, and 10%) and incubated with plasminogen coated beads in an HR-US cell. Incubation conditions were optimised to promote preferential binding of PrPSc to plasminogen (J. Grassi, personal communication). Both PK digested and undigested homogenates were tested in order firstly to investigate the specificity of PrPSc binding in the presence of PrPc and secondly, to investigate the possibility that a digestion step might be capable of being omitted – a great advantage should a TSE diagnostic assay be developed.
Experimental procedures
Covalent coupling of plasminogen to magnetic beads
Coupling of plasminogen to magnetic beads was performed following manufacturer's instructions. Briefly, 100 μg of human plasminogen (Sigma) was covalently bound to paramagnetic Tosyl-activated beads (M-280 Dynabeads, 2 ml) purchased from Dynal (Oslo) in 1 ml of coupling buffer (0.1 M borate pH 9.5). After incubation for 48 hours at 4°C, the beads were washed twice with phosphate buffered saline (PBS), containing 0.1% (w/v) BSA, for 5 min. Blocking of free tosyl-groups was accomplished by incubating the beads with 0.2 M Tris pH 8.5 containing 0.1% (w/v) BSA for 24 h at 20°C. Finally, beads were washed once in PBS pH 7.4 containing 0.1% (w/v) BSA for 5 min at 4°C and made up to a final concentration 2 × 109/ml in PBS/BSA buffer containing 0.02% sodium azide. The coated bead suspension was stored at 4°C in.
Brain tissues and homogenate sample preparation
Brain tissues (cerebellum) were kindly supplied by the CVRL (Abbotstown). Scrapie affected tissues were from naturally infected sheep. Animals (two male and 3 female) were aged between 2–4 years of age Samples were collected at post-mortem and scrapie diagnosed by histopathology and confirmed by immunohistochemistry. Homogenates (20% w/v in 5% sucrose) of brains from five normal and five scrapie infected sheep were prepared and aliquots were stored at -20°C. Duplicate aliquots were brought to 2, 5 or 10% by dilution with ice-cold capture buffer (0.1 M phosphate buffer pH 7.4, 0.5% M NaCl, 0.1% Sarkosyl) with or without PK (Sigma). Samples digested with PK were incubated with 5 μg/ml at 37°C for 20 min. The digestion was terminated by addition of Pefabloc (protease inhibitor purchased from Sigma) to a final concentration of 4 mM. Pefabloc at the same final concentration was also added to the undigested brain samples.
High-resolution ultrasonic spectroscopy analyses
Ultrasonic velocity and attenuation were measured using a HR-US 102 ultrasonic spectrometer from Ultrasonic Scientific Ltd.; cell temperature (25°C) was controlled with a Haake F8 water circulator bath (Haake C5) (Karlsruhe, Germany). Measurements were taken at different frequencies, 5000, 8000 and 11400 MHz, the margin of error being 0.2 mm/s and 0.1% for velocity and attenuation respectively. All measurements were carried out in a differential mode with two identical acoustical resonator cells: a measuring and a reference cell. Solutions were degassed prior to loading into the resonance cells. A volume of 900 μl of brain homogenate, prepared in capture buffer, was added to the measuring cell and the reference cell was filled with the same volume of capture buffer only. Prior to ultrasonic measurements the samples were allowed to equilibrate to the desired temperature (25°C) for approximately 20 minutes. Each reaction was initiated by addition of a solution of plasminogen coated beads in a single 10-μl aliquot (2 × 107 beads) to both measuring and reference cells, the solution being delivered with a Hamilton syringe by injection through a small hole in the lid of each cell. Reaction mixtures were kept constantly stirred at 700 rpm. Changes in velocity and attenuation in both cells were continuously monitored for 3–4 hours and the difference between measuring and reference cells was automatically calculated and recorded. Control experiments without beads were performed under the same conditions as described above, a 10-μl aliquot of capture buffer rather than beads being injected into the measuring and reference cell. All experiments were performed in duplicate or triplicate. Data were analysed using a general linear model repeated measures analysis of variance.
Authors' contributions
CN generated and analysed all of the experimental data and contributed to writing the manuscript. EM assisted with the prion protein handling and methodologies and contributed to writing the manuscript. TS had overall responsibility for supervising the project, interpretation of the data and contributed to writing the manuscript.
Figure 2.
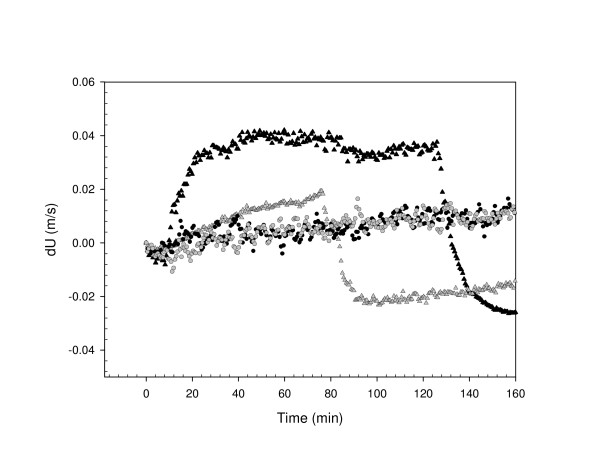
Illustration of ultrasonic velocity (dU) profiles of PK-untreated and PK-treated scrapie infected and healthy brain homogenates prepared at 2%. Binding reaction was performed in the reaction buffer at 25°C. A 10 ul-buffer solution containing plasminogen coated beads (2 × 107) was added to each sample (t = 0). Scrapie PK-untreated (black triangle), PK-treated (grey triangle) and healthy PK-untreated (black circle) and PK-treated (grey circle) are shown over approximately 3-hour of the reaction.
Acknowledgments
Acknowledgements
The financial support of the Department of Agriculture and Food (Food Institutional Research Measure, project reference number01/R&D/D/167) is gratefully acknowledged. We would like to thank our colleagues at the Central Veterinary Research Laboratory for supply of tissues.
Contributor Information
Carmen Negredo, Email: carmen.negredo@ucd.ie.
Eoin Monks, Email: Monkse@eircom.net.
Torres Sweeney, Email: torres.sweeney@ucd.ie.
References
- Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, Prusiner SB. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. A Protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Sayer NM, Cubin M, Rhie A, Bullock M, Tahiri-Alaoui A, James W. Structural determinants of conformationally selective, prion-binding aptamers. J Biol Chem. 2004;279:13102–13109. doi: 10.1074/jbc.M310928200. [DOI] [PubMed] [Google Scholar]
- Zou WQ, Zheng J, Gray DM, Gambetti P, Chen SG. Antibody to DNA detects scrapie but not normal prion protein. Proc Natl Acad Sci USA. 2004;101:1380–1385. doi: 10.1073/pnas.0307825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wüthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou WQ, Estey LA, Lamontagne J, Lehto MT, Kondejewski LH, Francoeur GP, Papadopoulos M, Haghighat A, Spatz SJ, Head M, Will R, Ironside J, O'Rourke K, Tonelli Q, Ledebur HC, Chakrabartty A, Cashman NR. A prion protein epitope selective for the pathologically misfolded conformation. Nat Med. 2003;9:893–899. doi: 10.1038/nm883. [DOI] [PubMed] [Google Scholar]
- Fischer MB, Roeckl C, Parizek P, Schwarz HP, Aguzzi A. Binding of disease-associated prion protein to plasminogen. Nature. 2000;408:479–483. doi: 10.1038/35044100. [DOI] [PubMed] [Google Scholar]
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- Maissen M, Roeckl C, Glatzel M, Goldmann W, Aguzzi A. Plasminogen binds to disease-associated prion protein of multiple species. Lancet. 2001;357:2026–2028. doi: 10.1016/S0140-6736(00)05110-2. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Engelstein R, Gabizon R. The binding of prion proteins to serum components is affected by detergent extraction conditions. J Neurochem. 2002;82:1–5. doi: 10.1046/j.1471-4159.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- Kudryashov E, Kapustana T, Morrissey S, Buckin VA, Dawson K. The Compressibility of Alkyltrimethylammonium Bromide Micelles. J Colloid Interface Sci. 1998;203:59–68. doi: 10.1006/jcis.1998.5333. [DOI] [Google Scholar]
- Smyth C, Dawson K, Buckin VA. Ultrasonic analysis of heat-induced coagulation in calcium fortified milk. Progr Colloid Polym Sci. 1999;112:221–226. [Google Scholar]
- Buckin VA, Smyth C. High-resolution ultrasonic resonator measurements for analysis of liquids. Seminars of Food Analysis. 1999;4:89–105. [Google Scholar]
- Buckin VA, Kudryashov E, O'Driscoll B. An alternative spectroscopy technique for biopharmaceutical applications. Pharmaceutical Technology Europe. 2002;14:33–37. [Google Scholar]
- Buckin VA, Kudryashov E. Supersonic – high-resolution ultrasonic spectroscopy. The Biochemist. 2002;24:25–27. [Google Scholar]
- Kudryashov E, Smyth C, O'Driscoll B, Buckin VA. High-Resolution Ultrasonic Spectroscopy for analysis of chemical reactions in real time. Spectroscopy. 2003;18 [Google Scholar]
- O'Driscoll B, Kudryashov E, Buckin VA. Future determination of biochemical monitoring using high-resolution ultrasonic spectroscopy. American Biotechnology Laboratory. 2004;1:26–27. [Google Scholar]
- Ellis V, Daniels M, Misra R, Brown DR. Plasminogen activation is stimulated by prion protein and regulated in a copper-dependent manner. Biochemistry. 2002;41:6891–6896. doi: 10.1021/bi025676g. [DOI] [PubMed] [Google Scholar]
- Ryou C, Prusiner SB, Legname G. Cooperative binding of dominant-negative prion protein to kringle domains. J Mol Biol. 2003;329:323–333. doi: 10.1016/S0022-2836(03)00342-5. [DOI] [PubMed] [Google Scholar]
- Kornblatt JA, Marchal S, Rezaei H, Kornblatt MJ, Balny C, Lange R, Debey MP, Hui Bon Hoa G, Marden MC, Grosclaude J. The fate of the prion protein in the prion/plasminogen complex. Biochem Biophys Res Comm. 2003;305:518–522. doi: 10.1016/S0006-291X(03)00804-0. [DOI] [PubMed] [Google Scholar]
- Cuccioloni M, Amici M, Eleuteri AM, Biagetti M, Barocci S, Angeletti M. Binding of recombinant PrPc to human plasminogen: kinetic and thermodynamic study using a resonant mirror biosensor. Proteins. 2005;58:728–734. doi: 10.1002/prot.20346. [DOI] [PubMed] [Google Scholar]
- McKinley MP, Meyer RK, Kenaga L, Rahbar F, Cotter R, Serban A, Prusiner SB. Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J Virol. 1991;65:1340–1351. doi: 10.1128/jvi.65.3.1340-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem. 1997;10:6324–31. doi: 10.1074/jbc.272.10.6324. [DOI] [PubMed] [Google Scholar]
- Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: identification of α-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- Hubbard SJ. The structural aspects of limited proteolysis of native proteins. Biochim et Biophys Acta. 1998;1382:191–206. doi: 10.1016/s0167-4838(97)00175-1. [DOI] [PubMed] [Google Scholar]
- Epple G, Langfeld K, Baier M, Holzhütter HG, Schleuning WD, Köttgen E, Geßner R, Praus M. Both lysine-clusters of the NH2-terminal prion-protein fragment PrP23–110 are essential for t-PA mediated plasminogen activation. Thromb Haemost. 2004;91:465–472. doi: 10.1160/TH03-06-0382. [DOI] [PubMed] [Google Scholar]
- Sarvazyan AP. Ultrasonic velocimetry of biological compounds. Mol Biol (Mosk) 1983;17:916–927. [PubMed] [Google Scholar]
- Lucas MA, Fretto LJ, McKee PA. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1982;258:4249–4256. [PubMed] [Google Scholar]
- Marshall JM, Brown AJ, Ponting CP. Conformational studies of Human plasminogen and plasminogen fragments: Evidence for a novel third conformation of plasminogen. Biochemistry. 1994;33:3599–3606. doi: 10.1021/bi00178a017. [DOI] [PubMed] [Google Scholar]
- Markus G. Conformational changes in plasminogen, their effect on activation and the agents that modulate activation rates – a review. Fibrinolysis. 1996;10:75–85. doi: 10.1016/S0268-9499(96)80082-8. [DOI] [Google Scholar]
- Kornblatt JA, Kornblatt MJ, Clery C, Balny C. The effects of hydrostatic pressure on the conformation of plasminogen. Eur J Biochem. 1999;265:120–126. doi: 10.1046/j.1432-1327.1999.00695.x. [DOI] [PubMed] [Google Scholar]


