Abstract
The purpose of this study was to determine if a cannabinoid CB1 receptor antagonist would selectively decrease consumption of highly palatable food in non-human primates. The CB1 receptor antagonist SR141716 (rimonabant; 0.12 - 1.0 mg/kg, i.m.) and the stimulant anorectic drug d-amphetamine (0.12 - 1.0 mg/kg, i.m.) were administered to non-food deprived baboons for the purpose of measuring the effect of each drug on consumption of the normal diet, and a large single meal of a high-carbohydrate candy. Four male and four female baboons had access to food 24 hr each day, but they had to complete a two phase operant procedure in order to eat. Responding on one lever during a 30-min appetitive phase was required before animals could start a consumption phase, where responding on another lever led to food delivery, i.e., a meal. Three days a week baboons received a jelly sugar-coated candy (Skittles®) during the first meal and then pellets were available in subsequent meals. All baboons ate as many individual candies in one meal as they did pellets throughout the entire day. Acute d-amphetamine and, to a lesser extent, SR141716 decreased both candy intake in a single meal and pellet intake in a single meal and over 24 hr. d-Amphetamine, but not SR141716 increased latency to the candy meal and the first pellet meal indicating that the two drugs differentially altered feeding topography. Although males ate more food pellets than females, few other sex differences were observed. Thus, although effective in decreasing food intake, there was no evidence of a specific effect of CB1 receptor antagonism on consumption of a large meal or a palatable food.
Keywords: Food Intake, Baboon, Motivation, Amphetamine, SR141716, Cannabinoid Antagonist, rimonabant, Binge
1. Introduction
For decades, if not centuries, smoked marijuana and oral Δ9-tetrahydrocannabinol (THC) have been associated with increases in appetite and food intake (Abel, 1975). With the relatively recent identification of endogenous cannabinoids and the development of cannabinoid receptor antagonists specific for type 1 (CB1) receptors, located primarily in the brain, much work has focused on the potential therapeutic effects of cannabinoid antagonists for the treatment of obesity (Kirkham, 2005). The endogenous cannabinoids interact in multiple ways with systems believed to control eating behavior (Antel, 2006; Matias et al., 2006). For example, activation of presynaptic CB1 receptors decreases the release of glutamate, GABA, dopamine and norepinephrine. During food deprivation, ghrelin levels as well as endocannabinoid levels increase and administration of a CB1 antagonist decreases ghrelin levels. In a complementary manner, satiety is associated with increased leptin levels and decreased endocannabinoid levels (Kirkham et al., 2002). Thus, endocannabinoid levels vary in a logical way with levels of hormones known to vary during feeding episodes. Finally, long-term treatment with CB1 antagonists reduces fat mass by increasing energy metabolism in mice (Jbilo et al., 2005), and decreases cardiovascular risk associated with obesity by improving the lipid profile in obese humans (Van Gaal et al., 2005).
Indeed, CB1 receptor antagonists reliably decrease intake of standard chow diets in laboratory rodents across a range of experimental conditions. CB1 receptor antagonists decrease operant responding for food pellets in both body-weight restricted (De Vry et al., 2004; Freedland et al., 2000; Thornton-Jones et al., 2005) and non-deprived rodents (De Vry et al., 2004; Solinas & Goldberg, 2005), and decrease freely-available food consumption in non-deprived rodents (Gardner & Mallet, 2006). An important point is that doses of CB1 receptor antagonists that decrease food intake do not affect locomotor activity, suggesting that alterations in feeding behavior are not due to non-specific behavioral disruptions (De Vry et al., 2004; Gardner & Mallet, 2006; Verty et al., 2004).
An interesting study outcome, was reported by Arnone et al. (1997). In that study, the CB1 receptor antagonist rimonabant (SR141716) specifically decreased consumption of sucrose pellets, but not standard “bland” chow-based pellets in rats. A specific effect of cannabinoids on sweet or palatable food consumption aligns with the desire for sweets (the “munchies”) reported by marijuana users (Abel, 1975). A controlled laboratory study in human marijuana smokers (Foltin et al., 1988) also reported that smoked marijuana specifically increased consumption of high-sugar, high-fat sweet snack foods. Finally, Arnone et al. (1997) also reported that SR141716 decreased ethanol drinking. Thus, endocannabinoids may have a role in modulating intake of palatable, preferred foods, and perhaps have a role in modulating other reinforcing behaviors (e.g., Maldonado et al., 2006). Additional studies have confirmed that CB1 receptor antagonists decrease sucrose (Higgs et al., 2003; Perio et al., 2001) and ethanol consumption (Freedland et al., 2001; Poncelet et al., 2003; but see Ginsburg & Lamb, 2006, for an exception).
With the exception of the data provided by Arnone et al. (1997), it has been difficult to demonstrate a specific effect of CB1 receptor antagonists on consumption of palatable foods rather than a non-specific antagonist induced decrease in consummatory behavior (e.g., McLaughlin et al., 2003; Rowland et al., 2002; Verty et al., 2004; Ward & Dykstra, 2005). In, to the best of our knowledge, the only other study using non-human primates, Simiand et al. (1998) reported that SR141716 decreased consumption of a sugar-cane mash without affecting consumption of the standard diet in marmosets. More recently, Gessa et al. (2006) reported that SR141716 decreased consumption of a chocolate-flavored low-caloric beverage to a much greater extent than normal chow in rats. Furthermore, the small decrease in chow intake disappeared within several days while the decrease in the chocolate beverage was more robust with repeated SR141716 dosing. One factor that may have contributed to the specificity of effects in the above studies was the greater baseline intake of the palatable food than the standard diet: animals consumed 3 to 5 times more of the palatable food. Thus, the specificity may have been related to differences in baseline rates of intake.
The variability in the study outcomes suggests that palatability and physical properties of food items play a role in determining the effects of CB1 receptor antagonists on eating behavior. Further evidence on the complex effects of ingestants was provided by Ward and Dykstra (2006), who studied the reinforcing effects of a sweet fluid (Ensure®, 30% fat) and corn oil in CB1 knockout mice. The progressive ratio breakpoints (a measure of reinforcing effects) for corn oil were not different between wild-type and knockout mice, but knockout mice had significantly smaller breakpoints for Ensure® than wild-type mice, suggesting that endocannabinoids modulate the reinforcing effects of sweet foods.
We (Foltin, 2006b) have recently developed a feeding regime that generates large single meals of preferred foods in non-human primates based on procedures developed by Corwin and colleagues to generate excessive eating of single food items in rodents (e.g., Corwin & Buda-Levin, 2004): rats given access to fat for 2 hr/day (2 hr before the dark cycle) on only 3 days per week develop a binge-type eating pattern of fat intake during those 2 hrs (Corwin et al., 1998; Dimitriou et al., 2000). When access to a sweet, high-sugar preferred food (Skittles® candy) was limited to a single meal in the morning 3 days a week, baboons consumed a quantity of candy in one meal equivalent to the quantity of food pellets eaten in multiple meals over an entire day (Foltin, 2006b).
Given the paucity of data in non-human primates, and the variability in previous study outcomes, the first purpose of this study was to evaluate the effects of the CB1 receptor antagonist SR141716 on consumption of a large single meal of preferred sweet food, i.e., a “binge” meal, compared to consumption of the standard maintenance food pellet in baboons over 24 hr. The effects of SR141716 were compared to the effects of the prototypic stimulant anorectic drug, amphetamine (AMPH; Foltin, 2004). Given that only one of the studies on the effects of CB1 receptor antagonists on feeding behavior mentioned above used female animals, the second purpose of this study was to evaluate possible effects of sex on response to SR141716 by testing male and female baboons.
2. Methods
2.1 Animals
Four male baboons (Papio cynocephalus anubis), weighing 18.3 to 23.1 kg, and four female baboons, weighing 10.7 to 15.8 kg, were individually housed in standard non-human primate cages (0.94 × 1.21 × 1.52 m high) at The New York State Psychiatric Institute. The room was illuminated with fluorescent lighting from 7:00 AM to 7:00 PM daily. In addition to food and candy earned during experimental sessions, two chewable vitamins, two pieces of fresh fruit, and a dog biscuit were also given daily. Water was available ad libitum from a spout located at the back of each cage. All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
2.2 Schedule of Reinforcement
Responding under each phase of a two-phase chain schedule of reinforcement was on a separate response manipulandum. The session began with the illumination of a single light above the appetitive lever. Completion of the first 10 responses (Fixed ratio; FR 10) on the appetitive lever began a 30-min timer and illuminated a second light over the appetitive lever, i.e., the 30-min appetitive phase was indicated by the illumination of two lights above the appetitive lever. The appetitive phase was a fixed-interval (FI) 30 min schedule, with a FR 10 second-order phase [FI 30′ (FR 10:S)]. Thus, after every 10th response during the FI phase, the stimuli associated with reinforcer delivery during the second phase were presented. There was a 10 min limited hold for the appetitive phase, such that after the expiry of the 30 min FI, the next FR 10 had to be completed within 10 min. Failure to complete a FR 10 within 10 min cancelled that appetitive phase, and extinguished one light over the appetitive lever such that only a single light was illuminated over the appetitive lever. The baboon received no indication that the 30-min interval had elapsed. The first FR 10 completed after 30 min resulted in the two lights above the left lever being extinguished and a single light above the right lever being illuminated, signaling the availability of food under the FR consumption phase of the chain schedule. The consumption phase of the chain schedule was reinforced using a FR 10 schedule of pellet delivery. After a 10-min interval in which no responses occurred, the consumption phase terminated, i.e., meal size was determined by each baboon. The single light above the right consumption lever was then extinguished, and the single light above the left appetitive lever was again illuminated. In order to initiate another meal, the baboon was required to start another 30-min appetitive phase by pulling on the left lever 10 times. This schedule was in effect 24 hr/day beginning at 9:00 AM, with the exception of a brief period during which the data were backed up and printed (∼5 min), which occurred at 8:55 AM each morning.
During each regular-diet meal, baboons received 1 food pellet (banana-flavored 1-g food pellets containing 3.3 kcal/g: 0.55 g carbohydrate, 0.03g fat, 0.2 g protein; Bio-Serv, Frenchtown, NJ). Pellet delivery was accompanied by the illumination of all 4 stimulus lights above the 2 levers for 8 s. The illumination of the 4 lights for 8 s also occurred upon completion of each FR10 during appetitive phases preceding food consumption phases. During the candy meal, baboons received 1 Skittle® (Mars Corp., Hackettstown, NJ; 4.3 Kcal: 0.9 g carbohydrate, 0.04 g fat, 0 g protein); 10% Kcal derived from fat. Candy delivery was accompanied by the flashing (1 s on:1 s off) of 2 white stimulus lights located above the food hopper for 8 s. The flashing of the 2 white lights for 8 s also occurred upon completion of each FR10 during the appetitive phase preceding the candy consumption phase.
2.3 Procedure and Drugs
Four days a week (Tuesday, Thursday, Saturday, and Sunday), only food pellets were available. On the other three days each week (Monday, Wednesday, and Friday), daily sessions began with a single candy meal. Baboons were free to start responding for pellets or candy beginning at 9:00 AM. Completion of the first 10 responses on the appetitive lever started the appetitive phase, which lasted a minimum of 30 min and a maximum of 40 min. After completion of the appetitive phase, baboons earned 1 pellet or 1 piece of candy after every 10 responses, with the “meal” ending when the baboon stopped pulling the lever for 10 min. On pellet days baboons could have multiple pellet meals over 24 hr, but they had to complete a 30 min appetitive phase before each consumption phase. On candy days, after the end of the candy meal, the baboons then could work for pellet meals until 9:00 AM the following morning. There were no stimuli indicating if the first meal of the day would be candy or pellets until the first stimulus presentation during the appetitive phase, i.e, light flashes indicated a candy meal would occur after completion of the appetitive phase, and prolonged illumination of a different set of lights indicated a pellet meal would occur after completion of the appetitive phase.
The effects of AMPH-sulfate (0.12-1.0 mg/kg, Sigma Chemical Corp., St. Louis, MO) were determined over a 6-wk period and 4 months later the effects of rimonabant (0.12-1.0 mg/kg, provided as SR141716 by the National Institute on Drug Abuse) were determined over a 6-wk period. Sequential drug doses varied by 0.30 log units. Drug doses are expressed as total weight of the salt or base.
Drugs were given intramuscularly (i.m.) in a thigh muscle (location varying among sessions) on Monday before a candy session and Thursday before a food pellet session of each week at 0900, with placebo injections given on Tuesday and/or Friday of each week. The 2 smaller doses of each drug were tested before the 2 larger doses of each drug: within each dose pair, dose was counterbalanced such that 2 females and 2 males received the smallest dose of each dose pair first, and 2 females and 2 males received the largest dose of each dose pair first. Because 3 of the 4 females did not eat any candy after the 0.50 mg/kg AMPH dose, the 1.0 mg/kg AMPH dose was not tested during candy sessions.
2.4 Data Analysis
Measures included total number of reinforcers earned during appetitive and consummatory components, latency to the first consummatory component (including the time required to complete the first appetitive component), number of pellet meals (there was only 1 candy meal), and the running rate of responding during the first appetitive and consummatory components. Separate analyses were conducted for: 1) candy (days when candy was available as a first meal of the day), 2) food pellets (days when only food pellets were available), 3) food pellets after candy (days when food meals followed candy meal), and 4) the first food pellet meal of the session on days when only food pellets were available.
Data for each drug were summarized using analyses of variance (ANOVA) with Sex as a between group factor and Drug (placebo vs. active; there was one placebo session for each active dose session), and Dose (4 doses) as 2-within group factors. Data were considered significantly different at P < 0.05, using Huynh-Feldt corrections where appropriate.
3. Results
Figure 1 compares the effects of SR141716 and AMPH on candy and pellet intake on pellet-only days in males and females. Under placebo conditions, males ate about 250 food pellets and females ate about 130 pellets in 24 hr on pellet-only days. During the period when AMPH was evaluated, males ate about 160 candies and females ate about 130 candies in a single meal on placebo days. There was a significant main effect of AMPH [F(1,6) = 98.7, P < 0.0001] on candy intake within a single meal with baboons eating fewer candies following AMPH (73 ± 12; significant main effects refer to the mean effect of all 4 doses) than on placebo days (144 ± 9): the effect of AMPH on candy intake was dose-dependent [F(2,12) = 15.9, P < 0.0004]. There was a significant interaction between sex and AMPH dose [F(2,12) = 6.5, P < 0.01]: males were more affected by the 0.25 mg/kg dose than females and the opposite was true for the 0.50 mg/kg dose. There was a significant main effect of AMPH [F(1,6) = 199.3, P < 0.0001] on pellet intake with baboons eating fewer pellets (108 ± 15) following AMPH than on placebo days (189 ± 14): the effect of AMPH on pellet intake was dose-dependent, i.e., there was a significant drug condition × AMPH dose interaction [F(3,18) = 152.8, P < 0.0001]. Males ate more pellets than females [F(1,6) = 15.9, P < 0.007], but there were no significant sex × AMPH dose interactions. The largest AMPH dose nearly eliminated all candy intake in a single meal and pellet intake over 24 hr.
Figure 1.
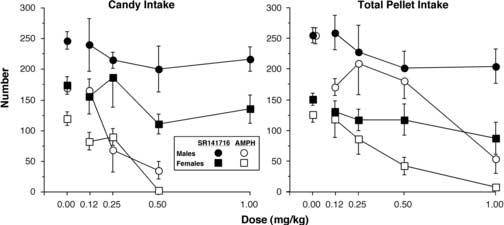
Total number of candies earned during the single candy meal of each candy session and total daily number of food pellets earned on pellet-only days for males and females as a function of drug and dose. Error bars represent ±1 SEM. Error bars for the placebo values are based on all 4 placebo days.
SR141716 was evaluated about 4 months after AMPH. Candy intake increased over that time such that males ate about 250 candies and females ate about 175 candies in a single meal on placebo days when SR141716 was evaluated. Total daily pellet intake under placebo conditions was similar when AMPH and SR141716 were evaluated. As shown in the left panel of Figure 1, there was a significant main effect of SR141716 [F(1,6) = 14.1, P < 0.009] on candy intake within a single meal with baboons eating fewer candies following SR141716 (182 ± 11) than on placebo days (210 ± 11): the effect of SR141716 on candy intake was dose-dependent [F(3,18) = 4.3, P < 0.02]. Although males ate more candy than females, this difference was only borderline significant (P < 0.07), and there were no significant sex × SR141716 dose interactions. As shown in the right panel of Figure 1, there was a significant main effect of SR141716 [F(1,6) = 23.2, P < 0.003] on 24-hr pellet intake on pellet-only days with baboons eating fewer pellets (168 ± 13) following SR141716 than on placebo days (203 ± 12): the Dose effect of SR141716 on pellet intake was only borderline significant (P < 0.09). Males ate more pellets than females [F(1,6) = 16.1, P < 0.007], but there were no significant sex × SR141716 dose interactions. SR141716 produced similar maximal decreases in both intake of candy in a single meal and food pellets available over 24 hr.
When AMPH was evaluated the latency to the candy meal under placebo conditions was about 50 min for both males and females, and the latency to the first pellet meal on pellet-only days under placebo conditions was longer for females (180 min) than for males [42 min; F(1,6) = 6.3, P < 0.05]. There was a significant main effect of AMPH [F(1,6) = 11.8, P < 0.01] on latency to the candy meal with a longer latency following AMPH (127 ± 20 min) than placebo (73 ± 15 min): the effect of AMPH on latency was dose-dependent [F(2,12) = 13.3, P < 0.002]. There was a significant interaction between sex and AMPH dose [F(2,12) = 4.2, P < 0.05]: AMPH increased the latency to first candy meal to a greater extent in females than males. There was also a significant main effect of AMPH [F(1,6) = 193.7, P < 0.0001] on latency to the first pellet meal with a longer latency following AMPH (518 ± 68 min) than on placebo days (110 ± 22 min), and the effect was dose-dependent [F(3,18) = 21.1, P < 0.0001]. In contrast to the effects of AMPH on latency to the candy meal, there were no differences between males and females as a function of AMPH dose.
When SR141716 was evaluated the latency to the candy meal under placebo conditions was also about 50 min for both males and females and the latency to the first pellet meal under placebo conditions was longer for females (110 min) than for males (50 min; P < 0.06). As shown in Figure 2, SR141716 did not affect the latency to the candy meal or first pellet meal on pellet-only days.
Figure 2.
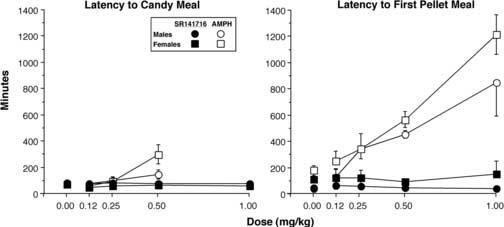
Latency to the candy meal and first food pellet meal as a function of drug and dose. Error bars represent ±1 SEM. Error bars for the placebo values are based on all 4 placebo days.
Neither SR141716 nor AMPH altered any other parameters of responding, i.e., response rate, during candy sessions. Both drugs did alter other parameters of responding during pellet-only days. There was a significant main effect of AMPH [F(1,6) = 8.5, P < 0.027] on the number of pellets consumed during the first pellet meal on pellet-only days with baboons eating fewer pellets following AMPH (29 ± 5) than on placebo days (54 ± 6). Similarly, there was a significant main effect of SR141716 [F(1,6) = 12.3, P < 0.013] on the number of pellets consumed during the first pellet meal on pellet-only days with baboons eating fewer pellets following SR141716 (42 ± 3) than on placebo days (73 ± 7). AMPH did not alter the total number of appetitive reinforcers during pellet sessions, but SR141716 increased the number of appetitive reinforcers in males from 89 ± 6 following placebo to 101 ± 9, and decreased the number of appetitive reinforcers in females from 73 ± 11 following placebo to 43 ± 6 [F(1,6) = 7.5, P < 0.033]. Males generally had one more pellet meal than females [F(1,6) = 10.4, P < 0.018]. The largest AMPH dose decreased the number of pellet meals in males and females [F(3,18) = 4.0, P < 0.023] by about 1.5 meals.
Although there was a significant main effect of AMPH on pellet intake after the candy meal [F(1,6) = 7.4, P < 0.034], there was also a significant Sex by AMPH Dose interaction [F(2,12) = 4.1, P < 0.045]. AMPH increased pellet intake on candy days in males without affecting pellet intake on candy days in females. SR141716 did not alter pellet intake after a candy session.
4. Discussion
Providing baboons access to a single meal of a highly palatable candy 3 days per week engendered a large intake of mostly sugar. The number of candies consumed in a single meal was comparable to the number of food pellets consumed across an entire day. Administration of the CB1 receptor antagonist SR141716 decreased single-meal candy intake and pellet intake in the first meal and over 24 hr. Similarly, administration of the stimulant anorectic drug AMPH also decreased single-meal candy and pellet intake in the first meal and over 24 hr. Although males ate more pellets than females, few sex differences in drug effects were observed.
The finding that SR141716 decreases feeding behavior in a large non-human primate replicates numerous studies conducted in rodents (e.g., De Vry et al., 2004; Freedland et al., 2000; Thornton-Jones et al., 2005) and one study conducted in a small non-human primate (Simiand et al., 1998). SR141716 did not alter the latency to the candy meal or first meal on pellet-only days, but reliably decreased the size of the candy meal, decreased the size of the first pellet meal on pellet-only days, and decreased total pellet intake. Several previous studies have reported that CB1 receptor antagonists produce specific or greater effects on consumption of a highly-palatable food than the standard diet (Arnone et al, 1997; Gessa et al., 2006; Simiand et al., 1998). The present findings offer scant support for a specific effect of CB1 receptor antagonists on preferred palatable food intake. Given that the candy meal was comprised mostly of sugar, the results also provide scant support for a specific effect of CB1 receptor antagonists on sweet food or high-carbohydrate food intake. Failure to find a specific effect of CB1 receptor antagonists on high-carbohydrate food intake replicates findings by McLaughlin et al. (2003) and Verty et al. (2004).
Specific effects of CB1 receptor antagonists on palatable food intake are most noticeable when intake of the palatable food is greater than intake of the regular diet. (e.g., Gessa et al., 2006: Simiand et al., 1998). In the present study, intake during a single candy meal was much greater than intake during a single pellet meal, but the size of the single candy meal was similar to the total daily intake of food pellets. Thus, the minimal effect of food type may be related to the similar total intake of candy and pellets. The progressive-ratio breakpoint for a single candy was about 3 times greater in these animals than the progressive ratio breakpoint for a single food pellet (Foltin, 2006b) indicating that candy is a more efficacious reinforcer than pellets. Thus, candy is a preferred highly palatable food.
We examined the effects of the anorectic drug d-amphetamine as a positive control (Foltin, 2004). AMPH produced large dose-dependent decreases in both candy and pellet intake during both the first pellet meal and over 24 hr. The anorectic effects of AMPH did not vary as a function of food type. In contrast to SR141716, AMPH produced significant dose-dependent increases in latency to both the candy meal and the first pellet meal, and increased pellet intake after the candy session in males. AMPH produced greater effects on feeding behavior, but we did not have enough SR141716 to test larger doses so we can’t conclude that SR141716 is less effective than AMPH in decreasing food intake. The shallow dose-response function for SR141716 suggests that the effects of CB1 receptor antagonists on food intake are more subtle, and perhaps more naturalistic, than the effects of AMPH.
The more shallow dose-response function with less anorectic efficacy for SR141716 in non-human primates compared to rats, parallels other findings from this laboratory using sibutramine (Foltin, 2006a), another medication used for weight loss by humans (Luque & Rey, 2002). Neither sibutramine nor SR141716 have robust effects on weight loss in humans. Thus, the more subtle effects observed in baboons compared to rats, approximates the subtle effects of both medications when used clinically (Luque & Rey, 2002; Van Gaal et al., 2005).
Although the terminal half-lives of i.m. AMPH and SR141716 in the baboon are not published, following oral dosing in humans, the half-life of AMPH is about 15 hr (Mas et al., 1999), and the half-life for SR141716 is about 8 days in normal weight individuals (Padwal & Majumder, 2007). In spite of the large difference in estimated half-lives, there were no behavioral effects on the day after dosing, suggesting that neither medication had long-lasting behavioral effects.
The present procedure provided measures of both appetitive and consummatory aspects of candy and food pellet consumption. Responding during the 30-min appetitive component before each meal, i.e., the single candy meal during candy sessions and multiple meals during pellet sessions, was the main measure of appetitive behavior. The second measure of appetitive behavior was the latency to begin the first meal of the sessions. SR141716 had inconsistent effects on appetitive behavior: SR141716 increased responding during the appetitive component of pellet-only sessions in females, but decreased responding during the appetitive component of pellet-only sessions in males, and did not affect the latency to begin either a candy or pellet meal. Thornton-Jones et al. (2005) also used a 2-component chain schedule of reinforcement to assess the appetitive (first part of the chain leading up to access to food) and consummatory effects of SR141716 in male rats. In contrast to the current results, SR141716 significantly decreased both appetitive and consummatory behavior. An earlier study by Freedland et al. (2001) assessed appetitive behavior by having rats respond on a lever before getting 20-min access to a sipper tube containing sucrose. Again, in contrast to the present results, SR141716 increased the latency to initiate lever pressing, i.e., appetitive behavior, and decreased licks on the sucrose tube, i.e., consummatory behavior.
Other than changes in consummatory behavior, there were no differences in behavior as a function of SR141716 dose. In contrast, baboons were more sensitive to external stimuli and occasionally displayed blank stares following the largest AMPH dose. It is possible that larger SR141716 doses may have produced more behavioral effects. Intramuscular SR141716 doses between 0.1 and 1.0 mg/kg block the behavioral effects of THC in rhesus monkeys and slightly decrease the rate of responding in rhesus monkeys (Wiley et al., 1995; Winsauer et al., 1999). Intramuscular SR141716 doses larger than 1.0 mg/kg disrupt learning and performance of complex tasks in rhesus monkeys for up to 24 hr (Winsauer et al., 1999). Thus, the i.m. dose-range tested here appears appropriate for assessing the effects of SR141716 on feeding behavior in non-human primates.
Previous studies from this laboratory (e.g., Foltin, 2005) and elsewhere (e.g., Collier, 1983, 1985; Doucet et al., 2003) have shown that the appetitive and consummatory phases of eating can be behaviorally and pharmacologically differentiated. AMPH has been shown to both decrease appetitive behavior by increasing the latency to the first meal and increase appetitive behavior by increasing responding during appetitive components when only food pellets were available (Foltin, 2004). In the present study AMPH only increased the latency to the candy meal and the first food pellet meal. The absence of an effect of AMPH on responding that results in presentation of the stimuli paired with reinforcement in the present study suggests that the present procedure which pairs different types of lights with candy and food pellets is not sensitive to drug effects on appetitive responding.
Males, who were about 50 percent larger than females consumed about 50 percent more pellets: males had a shorter latency to the first food pellet meal and had more pellet meals than the females. In contrast, males ate slightly, though not significantly (P <0.07), more candy than females. There were only 3 instances where a drug effect was influenced by sex: females were more sensitive to the latency-increasing effect of AMPH during candy sessions, AMPH increased pellet intake after candy sessions in males only, and SR141716 increased the number of appetitive reinforcers in males, but decreased the number of appetitive reinforcers in females on pellet-only days. Tseng and colleagues reported that 1) female rats were more sensitive to the antinociceptive and cataleptic effects of THC than male rats (Tseng & Craft, 2001); 2) the behavioral effects were mediated via CB1 receptors (Tseng & Craft, 2004); and 3) female rats produced greater levels of active metabolites than male rats (Tseng et al., 2004). The absence of sex differences in response to SR141716 in the present study may reflect a species difference, an insufficient sample size, an insensitive behavioral measure, the testing of a limited dose range, or a genuine absence of sex differences in non-human primates.
In humans, menstrual cycle phase influences the response to stimulants such that the positive subjective effects of amphetamine and cocaine are greater during the follicular phase than the luteal phase (Justice et al., 1999; Mendelson et al., 1999; Evans et al., 2002). The pattern of results observed in humans highlights a weakness of the present study in that, although the female baboons had menstrual cycles, dose-response functions were obtained irrespective of cycle phase.
The large candy meals engendered by periodic access to candy may provide a model for excessive food intake in a single meal by humans. In addition, the current procedure provides two types of eating behavior baselines in the same animals which can be used to assess the specific effects of pharmacological manipulations on multiple types of eating behavior, e.g., large meal, normal meals, balanced diet, diet high in refined sugar. Based on the varied results of earlier studies, we had hypothesized that antagonism of CB1 receptors would be more effective in reducing intake of palatable foods than the normal diet. Evidence for this hypothesis was scant: both SR141716 and the prototypical stimulant anorectic drug AMPH reduced consumption of palatable food and pellets. These results do not support the hypothesis that antagonism of CB1 receptors would be effective in specifically decreasing palatable food intake in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research was supported by DA-04130 from The National Institute on Drug Abuse, and MH-65024 from the National Institute on Mental Health, and approved by the New York State Psychiatric Institute Animal Care and Use Committee. The assistance of Jean Willi, April Modrzakowski, Angel Ramirez, Elysia Michaels, Catalina Saldaña and Drs. Suzette Evans and Mohamed Osman is gratefully acknowledged.
References
- Abel EL. Cannabis: Effects on hunger and thirst. Behav Biol. 1975;15:255–281. doi: 10.1016/s0091-6773(75)91684-3. [DOI] [PubMed] [Google Scholar]
- Antel J, Gregory PC, Nordheim U. CB1 cannabinoid receptor antagonists for treatment of obesity and prevention of comorbid metabolic disorders. J Med Chem. 2006;49:4008–4016. doi: 10.1021/jm058238r. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Collier GH. Life in a closed economy: The ecology of learning and motivation. John Wiley and Sons, Ltd.; 1983. [Google Scholar]
- Collier GH. Satiety: An ecological perspective. Brain Research Bulletin. 1985;14:693–700. doi: 10.1016/0361-9230(85)90120-0. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Doucet E, St-Pierre S, Almeras N, Tremblay A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite. 2003;40:137–143. doi: 10.1016/s0195-6663(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of amphetamine, dexfenfluramine, diazepam, and dietary manipulations on responding reinforced by stimuli paired with food in nonhuman primates. Pharmacol Biochem Behav. 2004;77:471–479. doi: 10.1016/j.pbb.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of dietary and pharmacological manipulations on appetitive and consummatory aspects of feeding in non-human primates. Appetite. 2005;45:110–120. doi: 10.1016/j.appet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of sibutramine on the appetitive and consummatory aspects of feeding in non-human primates. Physiol Behav. 2006a;87:280–286. doi: 10.1016/j.physbeh.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Foltin RW. “Tasting and wasting” Behavior in non-human primates: Aberrant behavior or normal behavior in “Times of plenty”. Physiol Behav. 2006;89(b):587–597. doi: 10.1016/j.physbeh.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11:1–14. doi: 10.1016/s0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ′silent antagonist′. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Orru A, Lai P, Maccioni P, Lecca R, Lobina C, Carai MA, Colombo G. Lack of tolerance to the suppressing effect of rimonabant on chocolate intake in rats. Psychopharmacology (Berl) 2006;185:248–254. doi: 10.1007/s00213-006-0327-1. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Cannabinoid effects on behaviors maintained by ethanol or food: A within-subjects comparison. Behav Pharmacol. 2006;17:249–257. doi: 10.1097/00008877-200605000-00006. [DOI] [PubMed] [Google Scholar]
- Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: Microstructural analysis of sucrose drinking after delta(9) tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl) 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. Faseb J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque CA, Rey JA. The discovery and status of sibutramine as an antiobesity drug. Eur J Pharmacol. 2002;440:119–28. doi: 10.1016/s0014-2999(02)01423-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR141716A and AM251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Mas M, Farre M, De La Torre R, Roset PN, Ortuno J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4- methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1990;290:136–145. [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Matias I, Bisogno T, Di Marzo V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int J Obes (Lond) 2006;30(Suppl 1):S7–S12. doi: 10.1038/sj.ijo.0803271. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, subutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- Perio A, Barnouin MC, Poncelet M, Soubrie P. Activity of SR141716 on post-reinforcement pauses in operant responding for sucrose reward in rats. Behav Pharmacol. 2001;12:641–645. doi: 10.1097/00008877-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. CB1(1) receptor mediation of cannabinoid behavioral effects in male and female rats. Psychopharmacology (Berl) 2004;172:25–30. doi: 10.1007/s00213-003-1620-x. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Verty AN, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: Effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]


