Abstract
Nursing sickness, the largest cause of death in female adult mink, is a metabolic disorder characterized by hyperglycemia. The impacts of body condition, dietary supplements, and reproductive status on the blood glucose concentration in female mink during the reproductive cycle were investigated. Mink dams on 3 farms were assigned to receive either herring oil (HerO) or chromium picolinate (CrPic) or to be in a control group, receiving only the basal diet, for 6 wk at the onset of lactation. Hyperglycemia was observed throughout the reproductive cycle. Significant differences in blood glucose levels were observed between farms, emphasizing the importance of herd genetics and of animal management and feeding practices in glycemic regulation. Female mink exhibiting hyperglycemia early in the reproductive cycle tended to remain hyperglycemic and to have poorer health and fewer kits. Glucose levels > 7 mmol/L can be considered critical in this regard. Supplementing the diet with CrPic reduced the blood glucose concentration. Results from this study suggest that a diet containing high-quality n-3 polyunsaturated fatty acids, high levels of carbohydrate, and CrPic supplementation may help the nursing mink dam maintain a normal blood glucose concentration during lactation.
Résumé
Chez le vison, la maladie de l’allaitement est un désordre métabolique caractérisé par une hyperglycémie et est la cause la plus importante de mortalité des visons femelles adultes. Les impacts de l’état de chair, des suppléments alimentaires et du statut reproducteur sur la glycémie chez le vison femelle durant le cycle reproducteur ont été étudiés. Des visons femelles sur 3 fermes ont été assignées à recevoir soit de l’huile de hareng (HerO), soit du picolinate de chrome (CrPic) ou d’être dans un groupe témoin, ne recevant alors que la diète de base, pour une durée de 6 semaines à partir du début de la lactation. Une hyperglycémie a été observée durant toute la durée du cycle reproducteur. Des différences significatives dans les niveaux de glucose sanguin étaient notées entre les fermes, démontrant l’importance de la génétique du troupeau de même que des pratiques de régie des animaux et d’alimentation sur la régulation de la glycémie. Les visons femelles démontrant de l’hyperglycémie tôt dans le cycle reproducteur avaient une tendance à demeurer hyperglycémiques et à avoir une moins bonne santé et moins de rejetons. Des niveaux de glucose > 7 mmol/L peuvent être considérés comme critique à cet égard. Une réduction de la concentration du glucose sanguin a été obtenue en supplémentant la diète avec du CrPic. Les résultats de la présente étude suggèrent qu’une diète contenant des acides gras n-3 polyinsaturés de haute qualité, de hauts niveaux de carbohydrates et un supplément de CrPic peut aider les visons femelles allaitantes à maintenir une glycémie normale durant la lactation.
(Traduit par Docteur Serge Messier)
Introduction
Nursing sickness is a disorder that develops from a complex of metabolic, nutritional, and environmental factors that influence the ability of the mink dam to meet the extreme demands of lactation (1). The disorder appears to be linked to a disruption in glucose homeostasis (2), and it has been proposed that the pathogenesis of the disease exhibits striking similarity to that of acquired insulin resistance (3). Glucose is a primary nutrient for conceptus growth and milk synthesis, and its provision is a metabolic priority for the pregnant or lactating mammal (4). Owing to low dietary carbohydrate intake, nursing mink dams rely heavily on gluconeogenesis from dietary amino acids to meet the demands for glucose (2).
In most mammals, there is an adaptive reduction of insulin sensitivity in glucose-metabolizing tissues during late gestation and lactation, accompanied by a compensatory increase in pancreatic β-cell mass and insulin secretion (4–6). Predisposing genetic and environmental factors may result in an exaggerated response and, consequently, the development of hyperglycemia, acquired insulin resistance, or both (5). Abnormally high levels of plasma glucose have been observed in the blood of dams affected by nursing sickness (7). It has recently been proposed (3) that acquired insulin resistance in mink females may develop in response to obesity or lipodystrophy (deficiency of adipose tissue), deficiency of n-3 polyunsaturated fatty acids (PUFA), oxidative stress, or a combination of these factors.
Variable glucose levels have been observed in lactating mink (7), but little research has been performed to explore possible connections between blood glucose levels and nursing sickness. We investigated glycemic control in the mink female throughout the reproductive cycle with the aim of determining the impact of body condition, dietary antidiabetic supplements, and reproductive status on glycemic regulation during the reproductive cycle on commercial farms.
Materials and methods
Animals
This research was carried out on 3 collaborating mink farms in Nova Scotia. Standard Black yearling mink females were used, each farm supplying a minimum of 30 females (total n = 107). The animals were housed in individual cages with free access to water. The experiment was performed from March to July 2003. The experimental procedures and husbandry conditions were approved by the Animal Care and Use Committee of the Nova Scotia Agricultural College and in accordance with the guidelines of the Canadian Council on Animal Care (8).
Sample collection and analysis
Blood sampling took place at breeding, mid-gestation, and late lactation, at the end of the 6-wk experimental feeding period. Approximately 90 min postprandially, blood samples were taken from the toenail for glucose analysis with the Accu-Chek Compact blood glucose monitor (Roche Diagnostics, Laval, Quebec), as previously described (9). At the time of sample collection, the mink were weighed (to the nearest 0.1 g), and body condition score (BCS) was determined according to a 5-point scale, as previously described (9). The BCS system involved palpating the shoulders, rib cage, and rump area. At lactation, whelping date and kit number, as well as the total litter weight, were recorded.
Experiment
The experiment had a randomized block design, with a minimum of 10 animals allocated for each treatment per farm. For ease of ranch-level administration, groups of 5 animals, rather than individual animals, were randomly assigned to treatment.
Each mink received 1 of 3 dietary treatments administered daily for 6 wk, beginning near the onset of lactation (May 6 to 8): a non-supplemented wet mink-lactation ration, which also served as the basal diet (control diet); addition of dietary herring oil (HerO), at a 1% to 3% inclusion level per day; or supplementation with chromium picolinate (CrPic), 200 μg/d.
The overall ingredient composition of the basal diet fed throughout the production year differed markedly among the participating farms. Farm A fed higher amounts of herring meal and salmon racks (24.7%) than farms B and C (2.9% and 6.8%, respectively), whereas farms B and C fed high levels of cod (48.9% and 45.5%, respectively). Farm A also fed higher amounts of cereal (28.0%) than farms B and C (15.0% and 15.9%, respectively). Owing to the nature of the research, it was not possible to carry out nutrient analyses on the diets.
Statistical analysis
Potential sources of variation between farms, such as air temperature, distance from human disturbance, and sizes of cage and nest box, were identified and measured; no differences were observed in variant data between farms. Statistical analyses were performed with SAS, version 8 (SAS Institute, Cary, North Carolina, USA); the MIXED procedure was performed with the use of a model with farm (block) and treatment as fixed variables. No significant interaction effects were observed between farm and treatment; therefore, the term was removed from the model. Blood glucose, body weight, and litter weight at lactation sampling were examined as response variables. Blood glucose levels and dam weights at breeding and gestation, along with kit age and litter weight, were analyzed as covariates. Covariates not influencing the response (P > 0.15) were excluded from the model. The CORR procedure was used to calculate correlations between blood glucose level and litter size and weight as well as correlations between blood glucose level and BCS and body weight. Results are reported as least-squares means (with standard error in parenthesis). Categorical data were evaluated with the Fisher’s exact test option of procedure FREQ. A statistical significance level of P < 0.05 was used unless otherwise stated.
Results
Farm-level differences
Blood glucose concentration
Significant differences were observed in blood glucose values between the 3 mink farms at the different sampling periods. As shown in Table I, the values for mink dams on farm A were lower than those observed on farms B and C at breeding and gestation. The values for the dams on farms B and C did not differ significantly. The dams on farm A had relatively constant blood glucose values, around 5 mmol/L, whereas the dams on farms B and C showed greater variation throughout the reproductive cycle, values ranging from 5.9 to 7.5 mmol/L, and a decrease in values after gestation. The overall mean value for the dams was significantly lower (P < 0.001) on farm A, at 5.1 (0.2) mmol/L, than on farms B and C, at 6.7 (0.2) and 6.8 (0.2) mmol/L, respectively. The dams on farm B had a significantly larger change in blood glucose concentration from breeding to gestation than those on farms A and C (P = 0.002 and 0.001, respectively), as well as a significantly larger change from gestation to lactation (Table II) than those on farm A (P = 0.008).
Table I.
Blood glucose levels at breeding and gestation of mink dams on 3 Nova Scotia farms
| Farm; least-squares mean of blood glucose level (and standard error), mmol/L
|
|||
|---|---|---|---|
| Variable | A | B | C |
| Breeding, n | 40 | 33 | 34 |
| 5.2 (0.1)a | 6.5 (0.3)b | 6.9 (0.3)b | |
| Gestation, n | 40 | 30 | 32 |
| 5.3 (0.2)a | 7.4 (0.3)b | 6.8 (0.3)b | |
| Change between sampling periods | −0.1(0.3)a | 1.2 (0.3)b | 0.2 (0.3)a |
Values that have different superscripts in a row are significantly different, at P≤ 0.001.
Table II.
Data for the dams and their litters 6 wk post partum (at the end of treatment, in late lactation) and changes over the treatment period
| Farm; least-squares mean (and standard error)
|
||||
|---|---|---|---|---|
| Variable | A (n =40) | B (n =29) | C (n = 29) | P |
| Blood glucose level, mmol/L | ||||
| 6 wk post partum | 5.3 (0.2)a | 5.9 (0.2)b | 6.1 (0.2)b | 0.03 |
| Change | −0.2 (0.3)a | −1.3 (0.3)b | −0.7 (0.3)ab | 0.03 |
| Dam weight, g | 1086.5 (18.5)a | 990.8 (23.1)b | 997.0 (23.4)b | 0.001 |
| Change, g | −122.5 (18.8)a | −230.8 (24.0)b | −231.7 (24.0)b | <0.001 |
| Change, % | −8.8 (2.1)a | −17.0 (2.3)b | −14.4 (2.2)b | 0.005 |
| Litter size | 4.5 (0.4)a | 4.3 (0.4)a | 5.5 (0.4)b | 0.04 |
| Litter weight, g | 2430.4 (171.3)a | 1754.4 (201.2)b | 2168.3 (193.3)ab | 0.07 |
| Barren females, no | 3 | 3 | 2 | |
| Dam deaths, no. | 0 | 4 | 5 | |
Values that have different superscripts in a row are significantly different.
Body weight and BCS
The dams on farms B and C lost significantly more weight over the treatment period than those on farm A (Table II), in terms of both grams (P < 0.001) and percentage of body weight (P < 0.001 and = 0.002, respectively). As shown in Table III, the BCS varied among the dams on individual farms between sampling periods. Throughout the trial, the dams on farm A were categorized as 2 (thin) through 4 (heavy), with approximately 83% of ideal condition. At breeding, all the studied dams on farms B and C were classified as ideal in condition, but at lactation they had more prominent losses and gains in body condition, only 52% and 69%, respectively, scoring as ideal.
Table III.
Body condition scores of the dams during the study period
| Sampling period and farm | Score;a % of dams
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Pb | |
| Breeding | ||||||
| A | — | 10 | 82 | 8 | — | |
| B | — | — | 100 | — | — | 0.007 |
| C | — | — | 100 | — | — | |
| Gestation | ||||||
| A | — | 10 | 78 | 12 | — | |
| B | — | — | 73 | 17 | 10 | 0.61 |
| C | — | 7 | 78 | 16 | — | |
| Lactation | ||||||
| A | — | 5 | 88 | 8 | — | |
| B | — | 21 | 52 | 24 | 3 | 0.002 |
| C | 10 | 17 | 69 | 3 | — | |
On a 5-point scale, previously described (9), 3 being ideal.
Table probability: the P-values refer to the frequency distribution between farms within each sampling period.
Reproductive parameters
As Table II shows, farm C had a significantly higher litter size than farms A and B (P = 0.05 and 0.02, respectively). However, the litter weights did not differ significantly on farms A and C, indicating that farm A weaned larger kits than did farm C. Although 32% of the dams on farm C weaned litters of 7 to 9 kits, a much higher proportion than on farms A and B (Table IV), 15% (5/34) of the studied dams on farm C had died by the end of the study, compared with no deaths on farm A.
Table IV.
Reproductive status of the dams 6 wk post partum
| No. of kits weaned (% of dams)
|
||||||
|---|---|---|---|---|---|---|
| Farm | Dam death (% of dams) | None | 1–3 | 4–6 | 7–9 | Pa |
| A | 0 | 8 | 8 | 82 | 2 | |
| B | 12 | 9 | 12 | 64 | 3 | <0.001 |
| C | 15 | 6 | 12 | 35 | 32 | |
Table probability.
Blood glucose regulation
Glucose history
Blood glucose values observed at breeding marginally affected those observed at gestation (P = 0.06) but significantly affected those observed at lactation, with a modest positive correlation (r = 0.53, P < 0.001). Changes observed in blood glucose values from gestation to lactation were significantly influenced by the changes between the 1st and 2nd samplings (r = −0.72, P < 0.001). Dams showing little initial change (close to zero) from breeding to gestation tended to show little change later on, whereas those showing large fluctuations initially tended to continue the trend.
Body weight and BCS
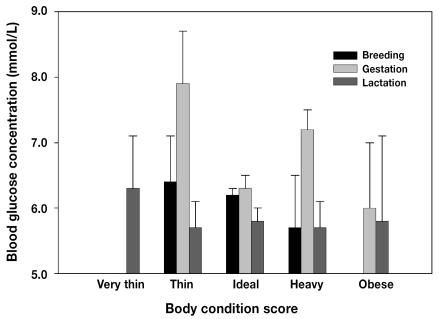
Body weight did not have a significant effect on blood glucose levels at any of the time points. Although no differences were observed in blood glucose values between categories of BCS at breeding or lactation, interesting trends were observed at the gestation sampling (Figure 1): dams scoring as ideal had lower blood glucose values than those scoring as thin (P = 0.06) or as heavy (P = 0.02), at 6.3 (0.2) versus 7.9 (0.8) and 7.2 (0.3), respectively. Although fewer dams were scored as nonideal, they had greater variation in blood glucose values throughout the reproductive cycle than did the dams scored as ideal.
Figure 1.
Mean blood glucose concentrations in mink females at breeding, gestation, and lactation according to body condition score: very thin (n = 0, 0, 3), thin (n = 4, 6, 13), ideal (n = 100, 78, 70), heavy (n = 3, 15, 11), and obese (n = 0, 3, 1).
When the individual females were followed longitudinally, dams that were thin during gestation showed an increase in blood glucose concentration of 0.6 (0.7) mmol/L during lactation, whereas those in better condition during gestation showed a decrease during lactation (results not shown). Those scored as obese during gestation experienced a larger decrease over the treatment period (P = 0.04) than those scored as ideal, 2.5 (0.9) versus 0.6 (0.1) mmol/L.
Reproductive parameters
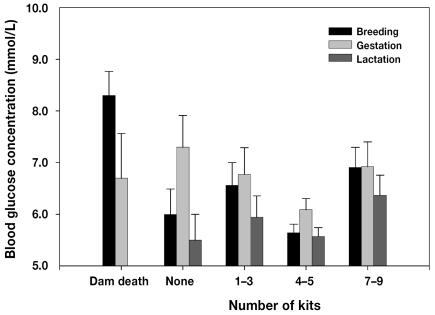
Litter weight and kit age did not have a significant effect on the blood glucose concentration of the dam at the lactation sampling. However, several dams with high blood glucose levels at 1 or more stages became sick, died, or did not whelp. As shown in Figure 2, dams that died during gestation or lactation had significantly higher blood glucose levels at breeding (8.3 [0.5] mmol/L; P < 0.05) than all the dams that whelped and nursed litters. Females carrying 1 to 3 kits had higher blood glucose levels at breeding than did those pregnant with 4 to 6 kits (6.6 [0.5] versus 5.6 [0.2] mmol/L; P = 0.05). The blood glucose levels at breeding were also significantly higher in the dams with 7 to 9 kits (7.0 [0.4] mmol/L; P = 0.002) than in those with 4 to 6 kits. Barren females had marginally higher blood glucose concentrations at the gestation sampling time than did females carrying 4 to 6 kits (7.3 [0.6] versus 6.1 [0.2] mmol/L; P = 0.06). At the lactation sampling, a marginal increase in glucose levels was observed in the dams nursing 7 to 9 kits compared with those nursing 4 to 6 kits (6.4 [0.4] versus 5.6 [0.2] mmol/L; P = 0.06).
Figure 2.
Mean blood glucose concentrations in mink females at breeding, gestation, and lactation according to number of kits in the litter at late lactation: none (n = 8), 1–3 (n = 11), 4–6 (n = 66), and 7–9 (n = 13). A total of 9 females died during the experiment, 5 between breeding and gestation and 4 between gestation and the end of the study.
Treatment effect
With numerous factors contributing to the development of hyperglycemia, easily applicable ranch-level treatments to improve glycemic control in the mink dams were investigated. Those receiving a daily CrPic supplement showed significantly lower blood glucose levels during late lactation (P = 0.03) compared with the other 2 treatment groups and had a greater decrease over the treatment period (P = 0.01), 1.2 (0.2) mmol/L (Table V). The effect of HerO was no different from that of the control diet in late lactation. No significant differences were observed among the effects of the experimental treatments on final dam weight, change in dam body weight, or litter weight over the treatment period.
Table V.
Data for the dams and their litters 6 wk post partum, at the end of treatment with the control diet or with supplementary herring oil (HerO) or chromium picolinate (CrPic)
| Dietary group; least-squares mean (and standard error)
|
||||
|---|---|---|---|---|
| Variable | Control | HerO | CrPic | P |
| Blood glucose level, mmol/L | ||||
| 6 wk post partum | 6.0 (0.2)a | 6.0 (0.2)a | 5.3 (0.2)b | 0.03 |
| Change | −0.3 (0.2)a | −0.6 (0.2)a | −1.2 (0.2)b | 0.01 |
| Dam weight, g | 1059.3 (19.5) | 1005.3 (20.6) | 1009.7 (20.6) | 0.11 |
| Change, g | −162.2 (20.2) | −219.7 (21.2) | −203.2 (21.3) | 0.14 |
| Change, % | −11.0 (1.9) | −15.7 (2.1) | −13.5 (2.1) | 0.17 |
| Litter weight, g | 2180.5 (157.9) | 2000.1 (167.5) | 2172.4 (167.4) | 0.68 |
Values that have different superscripts in a row are significantly different.
Discussion
Our findings indicate that there was a significant farm effect on glycemic control, maintenance of body weight and condition, litter size and weight, and overall health in mink females during the reproductive cycle. The blood glucose concentrations determined for farm A were similar to previously reported values in healthy lactating females: 5.3 (0.3) mmol/L (7). The variation in glucose values observed between farms demonstrates the importance of ranch-level factors in glycemic regulation. These may include combined genetic effects of the herd and animal management and feeding practices (3,10). It is known that the mink on farms B and C may be genetically similar because of frequent exchange of breeding stock.
With regard to feeding practices, large differences were observed between the farms in the dietary constituents fed throughout the production year. Farm A, which showed lower and more consistent blood glucose values throughout the reproductive cycle, fed significantly larger amounts of herring meal and salmon racks than farms B and C throughout the year. Fish oil is known to be high in the long-chain n-3 PUFA (11,12); however, some fish are better sources than others. Farms B and C fed high levels of cod racks, a low-oil fish that is a poorer source of n-3 PUFA than salmon and herring (13). When substituted for other types of lipids in the diet, fish oils high in n-3 PUFA have beneficial effects on insulin-stimulated glucose transport and metabolism in peripheral tissues (14–16). Facilitative glucose transporter 4 (GLUT-4) uptakes glucose in response to insulin (17–19), and increased cellular GLUT-4 content has been identified in the muscle and adipose tissue of rodents fed diets high in fish oil (14,20). The larger amounts of herring and salmon fed throughout the production year on farm A may have contributed to the lower and more stable blood glucose concentrations observed throughout the reproductive cycle.
Similarly, large dietary differences were observed in the amount of cereal fed, farm A feeding significantly larger amounts than farms B and C. When fed large amounts of carbohydrate, the mink has the ability to store excess glucose as glycogen (2). Fink and Børsting (21) suggested lower de novo synthesis of glucose in dams with a high carbohydrate supply, possibly owing to decreased activity of the gluconeogenic enzymes. Mink were able to utilize high levels of dietary digestible carbohydrates without critically elevating plasma glucose concentrations (22). In the current study, significantly lower blood glucose levels were observed in the farm feeding greater levels of carbohydrates.
Although females on farm A lost less body weight on a percentage basis during the lactation period than those on farms B and C, the latter percentages were in agreement with previous findings: Hansen and Berg (23) found that apparently healthy mink dams lost approximately 15% of their body weight during the 1st 6 wk of lactation, with 10% lost over the last 2 wk. Similar differences were shown in the females’ ability to maintain body condition. Previous studies found that readily available carbohydrates helped mink females maintain their body condition during the preweaning period (24,25). By ingesting more carbohydrate, females may be better able to meet the increasing energy demands of lactation (2) and, in turn, be less prone to the mobilization of body reserves that often leads to the development of nursing sickness (1,7). The higher levels of dietary carbohydrates fed on farm A may have contributed to the dams’ ability to conserve body weight and condition during lactation.
Differences were also observed between farms in both litter size and litter weight at late lactation. Although females on farm C nursed larger litters than those on farms A and B, farm A weaned larger kits. Genetic background and nutritional management influence milk yield in many species. The source of dietary fat influences the fatty acid composition of milk and kit tissue in the mink (26,27). Variations in milk composition may influence the efficiency of nutrient utilization (28); however, the influence of the maternal supply of dietary n-3 PUFA on kit growth has not been clarified. Conversely, previous findings indicate that dam and kit health may be directly affected by the level of dietary carbohydrate (25,29): in a recent study (25), mink dams fed a high carbohydrate diet exhibited increased milk production, lower percent weight loss, lower total heat production, and lower protein oxidation than dams fed a diet low in carbohydrates. Feeding a diet high in carbohydrates may allow nursing females to redirect carbohydrates towards milk production by increasing glycogen synthesis and inhibiting gluconeogenesis (2). The combination of larger amounts of high-quality dietary n-3 PUFA and dietary carbohydrate fed on farm A may have helped the dams to better regulate the blood glucose concentration and to conserve body weight and condition during lactation, thus contributing to faster kit growth and better dam health. Further studies on a larger number of farms would clarify the relative importance of diet and genetics in the performance of mink dams.
With regard to the influence of elevated blood glucose levels early in the nursing period on those observed as lactation progresses, modest but significant correlations were observed. Additionally, dams experiencing large fluctuations in blood glucose concentration between breeding and gestation showed similar changes during the nursing period. Blood glucose values > 8.0 mmol/L during breeding were associated with increased risk of death among the dams, whereas values > 7.0 mmol/L during gestation resulted in reduced litter size. Irregularities in glucose homeostasis were previously identified in lactating mink (7). Our observations suggest that hyper-glycemia in mink females before whelping may have significant implications for glycemic control during lactation. Similarly, humans that experience gestational and postpartum insulin resistance are believed to have a degree of insulin resistance before their pregnancy (30–33). The implication is that certain females may be predisposed to disruptions in glucose homeostasis during lactation, likely as a consequence of both genetic and environmental factors. Inheritance of glucose intolerance (34–36) has been identified as a risk factor for the development of insulin resistance in rats. Obesity is also a key determinant in the development of gestational insulin resistance (37); increased basal hepatic glucose production and decreased hepatic glucose uptake have been observed in obese rats before the development of hyperglycemia (38). We suggest that hyperglycemia is not a transient condition in female mink occurring solely from the demands of lactation but may be, in part, predicted by elevated blood glucose levels early in the reproductive cycle. Glucose concentrations > 7 mmol/L can be considered indicative of a disruption in glucose homeostasis and critical in predicting potential problems with dam health and reproduction.
Our data support the concept that body condition is linked to the ability of the mink dam to regulate blood glucose during the reproductive cycle, particularly during gestation. Few studies have demonstrated the relationship between body condition and glycemic control in the mink female (9,39). However, obesity has been identified as a major risk factor in the development of insulin resistance in both humans (40–42) and companion animals (6,43,44). Lipodystrophy, a deficiency of adipose tissue, is also associated with impaired responsiveness to insulin (45), and pronounced insulin resistance and reduced β-cell insulin secretion have been observed in lean women during gestation (46). In agreement with previous findings (9), evidence of impaired glycemic control was observed in mink females scored as having a nonideal body condition during the reproductive cycle. Frayn (45) proposed that when the storage capacity of adipose tissue is overwhelmed, or when there is insufficient adipose tissue to absorb dietary fatty acids, other glucose-metabolizing tissues (i.e., skeletal muscle, liver, and pancreatic β-cells) are exposed to triacylglycerols, ultimately leading to insulin resistance. The normal adaptive reduction of insulin sensitivity that occurs during pregnancy and lactation (5) may be exaggerated in mink females with a nonideal body condition, leading to a reduced ability to regulate blood glucose levels during lactation.
Our results suggest that abnormalities in glucose homeostasis early in the breeding season may be an indicator for reproductive failure and dam illness. In humans, increases in maternal blood glucose concentration are linked to an increased risk of fetal and neonatal disease (5,47,48). Elevated blood glucose values were observed early in the reproductive cycle of both barren females and those with smaller litters. Although increased levels were observed in dams experiencing decreased reproductive success, they were also detected in those with larger litters (7 to 9 kits) during breeding and lactation. Increasing litter size has been identified as a major risk factor for nursing sickness (1,10).
In a study by Børsting and Damgaard (49), the increased demand for milk production found in females nursing 8 kits in comparison with dams nursing only 4 kits resulted in higher glucose production in the former. One would expect a similar increase in glucose demand to be associated with carrying larger litters. During pregnancy and lactation, a condition of insulin resistance develops, a mechanism favoring glucose supply to the growing fetus and milk production (5). The increased demand for glucose associated with larger litters may aggravate underlying insulin resistance in mink females. It is apparent that abnormalities in glucose homeostasis occur throughout the reproductive cycle in mink females; the consequences may include reproductive failure and dam illness or death.
In studies of humans and animals, offspring of mothers with gestational diabetes have been found to be at increased risk of obesity, glucose intolerance, and insulin resistance (5,36,50,51). Boloker et al (36) found that the altered metabolism in a diabetic pregnancy causes permanent defects in glucose homeostasis in the offspring that may lead to diabetes later in life. It is plausible that mink kits born to dams with impaired gestational glucose regulation may be prone to the development of a similar condition. Genetic selection for mink females with large litters may inadvertently result in the selection of females predisposed to poor glycemic regulation inherited from their mothers. Future studies should explore this relationship.
Conditions that increase circulating insulin and glucose concentrations, including pregnancy (52) and obesity, increase urinary chromium output (53–55), and chromium deficiency may be an aggravating factor in the progression of insulin resistance (56,57). Improved glucose tolerance and decreased insulin resistance have been observed in rats (58) and cats (6) receiving chromium supplementation. Chromium acts to increase insulin binding to cells through an increased number of insulin receptors and an increase in insulin receptor phosphorylation (59,60). In the current study, CrPic supplementation decreased blood glucose levels in mink dams during lactation when compared with the values in the other 2 treatment groups.
Dietary supplementation with herring oil, at the level given in this study, did not affect blood glucose levels in the mink dams during late lactation. This was unexpected, as previous studies have shown dietary n-3 PUFA to improve glycemic regulation (14–16,61), and in the current study the mink dams fed higher levels of fish known to be high in n-3 PUFA had lower and more consistent blood glucose values. Substitution of saturated fat with high-quality n-3 PUFA within the diet may be required to significantly improve insulin sensitivity in the mink dam during lactation. This practice may be most beneficial during the fall, when body fat reserves are accumulated in preparation for the winter (62), as mink body fat composition has been shown to be highly responsive to dietary fatty acid profiles (63).
Conclusions
Our findings indicate that in mink dams the inability to maintain glucose homeostasis is not a problem occurring solely from the demands of lactation. Poor glucose regulation occurs throughout the reproductive cycle and may predispose to decreased reproductive success and poor dam health. Glucose concentrations > 7 mmol/L can be considered critical in this regard. Overall, females demonstrating large changes in glucose levels from breeding to gestation had increased glycemic variability while nursing. Significant differences were also observed in blood glucose levels between farms, emphasizing the importance of ranch-level factors (herd genetics and animal management and feeding practices) in glycemic regulation.
Another important factor may be proper conditioning of the females to avoid thinness and obesity during breeding and gestation. Females thin at gestation showed an overall increase in blood glucose concentration during the nursing period, whereas those in optimal condition showed a decrease in these values. Similarly, females classified as obese at gestation had a larger drop in blood glucose concentration during lactation than those scored as having an ideal body condition. The increased variation in blood glucose levels observed among the females in nonideal condition throughout the reproductive cycle indicates impaired ability to regulate glucose homeostasis.
Blood glucose levels at lactation may be influenced by supplementation with CrPic at the onset of nursing. However, as there was evidence of poor glucose regulation in the females before lactation, it appears that preventive measures need to be taken throughout the year. Although genetic and husbandry contributions could not be differentiated, the results of this study suggest that the combination of high-quality n-3 PUFA and high levels of dietary carbohydrate, fed throughout the production year, warrants further investigation as a tool to better enable mink dams to maintain glucose homeostasis and body condition during the critical nursing period.
Acknowledgments
We thank the staff and families of the collaborating mink ranches and Mr. Gerry Russell for assistance in sample collection. We also thank Dr. Gordon Finley and Dr. Leslie MacLaren for their advice and expertise and Dr. Tess Astatkie for help in statistical analysis. This research was supported by the Canada Mink Breeders Association, the Nova Scotia Fur Institute, and the Natural Sciences and Engineering Research Council (NSERC Discovery Grant to Dr. Rouvinen-Watt).
Footnotes
This research was part of the MSc thesis research of Amber M.J. Hynes.
References
- 1.Clausen TN, Olesen CR, Hansen O, Wamberg S. Nursing sickness in lactating mink (Mustela vison). I. Epidemiological and pathological observations. Can J Vet Res. 1992;56:89–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Børsting CF, Gade A. Glucose homeostasis in mink (Mustela vison): a review based on interspecies comparisons. Scientifur. 2000;24:9–18. [Google Scholar]
- 3.Rouvinen-Watt K. Nursing sickness in the mink — A metabolic mystery or a familiar foe? Can J Vet Res. 2003;67:161–168. [PMC free article] [PubMed] [Google Scholar]
- 4.Bell AW, Bauman DE. Adaptation of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol. 1997;2:265–278. doi: 10.1023/a:1026336505343. [DOI] [PubMed] [Google Scholar]
- 5.Di Cianni G, Miccoli R, Volpe L, Lencioni C, Del Prato S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res. 2003;19:259–270. doi: 10.1002/dmrr.390. [DOI] [PubMed] [Google Scholar]
- 6.Rand JS, Fleeman LM, Farrow HA, Appleton DJ, Lederer R. Canine and feline diabetes mellitus: Nature or nurture? J Nutr. 2004;134:2072S–2080S. doi: 10.1093/jn/134.8.2072s. [DOI] [PubMed] [Google Scholar]
- 7.Wamberg S, Clausen TN, Olesen CR, Hansen O. Nursing sickness in lactating mink (Mustela vison). II. Pathophysiology and changes in body fluid composition. Can J Vet Res. 1992;56:95–101. [PMC free article] [PubMed] [Google Scholar]
- 8.Olfert ED, Cross BM, McWilliams AA, editors. Guide to the Care and Use of Experimental Animals. 2. Vol. 1. Ottawa, Ontario: Canadian Council on Animal Care; 1993. [Google Scholar]
- 9.Hynes AM, Rouvinen-Watt K, Armstrong D. Body condition and glycemic control in mink females during reproduction and lactation. Scientifur. 2004;28(3):79–86. [Google Scholar]
- 10.Schneider RR, Hunter DB. Nursing disease in the mink. Scientifur. 1992;16:239–242. [Google Scholar]
- 11.Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol. 1993;264:R1111–1118. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br J Nutr. 2000;84:175–184. [PubMed] [Google Scholar]
- 13.Hearn WE. Interspecific competition and habitat segregation among stream-dwelling trout and salmon: a review. Fisheries. 1987;12:24–31. [Google Scholar]
- 14.Ezaki O, Tsuji E, Momomura K, Kasuga M, Itakura H. Effects of fish and safflower oil feeding on subcellular glucose transporter distributions in rat adipocytes. Am J Physiol. 1992;263:E94–E101. doi: 10.1152/ajpendo.1992.263.1.E94. [DOI] [PubMed] [Google Scholar]
- 15.Long SD, Pekala PH. Regulation of GLUT4 gene expression by arachidonic acid. J Biol Chem. 1996;271:1138–1144. doi: 10.1074/jbc.271.2.1138. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Rizkalla SW, Boillot J, et al. Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J Nutr. 1996;126:1951–1958. doi: 10.1093/jn/126.8.1951. [DOI] [PubMed] [Google Scholar]
- 17.Kahn BB. Dietary regulation of glucose transporter gene expression: tissue specific effects in adipose cells and muscle. J Nutr. 1994;124:1289S–1295S. doi: 10.1093/jn/124.suppl_8.1289S. [DOI] [PubMed] [Google Scholar]
- 18.Khayat ZA, Patel N, Klip A. Exercise and insulin stimulated muscle glucose transport: distinct mechanisms of regulation. Can J Appl Physiol. 2002;27:129–151. doi: 10.1139/h02-010. [DOI] [PubMed] [Google Scholar]
- 19.Wood S, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 20.Delarue J, LeFoll C, Corporeau C, Lucas D. N-3 long chain polyunsaturated fatty acids: A nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity? Reprod Nutr Dev. 2004;44:289–299. doi: 10.1051/rnd:2004033. [DOI] [PubMed] [Google Scholar]
- 21.Fink R, Børsting CF. Quantitative glucose metabolism in lactating mink (Mustela vison) — effects of dietary levels of protein, fat and carbohydrates. Acta Agric Scand Sect A Anim Sci. 2002;52:34–42. [Google Scholar]
- 22.Fink R, Børsting CF, Damgaard BM, Rosted AKL. Glucose metabolism and regulation in lactating mink (Mustela vison) — effects of low dietary protein supply. Arch Anim Nutr. 2001;56:155–166. doi: 10.1080/00039420214183. [DOI] [PubMed] [Google Scholar]
- 23.Hansen BK, Berg P. Mink dam weight changes during the lactation period. I. Genetic and environmental effects. Acta Agric Scand Sect A Anim Sci. 1998;48:49–57. [Google Scholar]
- 24.Pölönen I, Scott R, Oldfield J. Mink diet energy during preweaning and early post-weaning periods. Scientifur. 1993;17:47–51. [Google Scholar]
- 25.Fink R, Tauson AH, Chwalibog A, Hansen NE, Kristensen NB, Wamberg S. Effects of substitution of dietary protein with carbohydrate on lactation performance in the mink (Mustela vison) J Anim Feed Sci. 2004;13:647–664. [Google Scholar]
- 26.Wamberg S, Olesen CR, Hansen HO. Influence of dietary fat sources on lipid synthesis in the mink (Mustela vison) mammary tissue. Comp Biochem Physiol. 1992;103A:199–204. doi: 10.1016/0300-9629(92)90263-p. [DOI] [PubMed] [Google Scholar]
- 27.Hansen MU, Lassén M, Tauson A-H, Sørensen H, Clausen T. Different ratio between n-6 and n-3 fatty acids in diets for lactating mink (Mustela vison) dams — effect on milk and kit tissue fatty acid composition. Scientifur. 2004;28:103–108. [Google Scholar]
- 28.Fiorotto ML, Burrin DG, Perez M, Reeds PJ. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol. 1991;260:1104–1113. doi: 10.1152/ajpregu.1991.260.6.R1104. [DOI] [PubMed] [Google Scholar]
- 29.Fink R, Tauson A-H, Hansen KB, Wamberg S, Kristensen NB. Energy intake and milk production in mink (Mustela vison) — effect of litter size. Arch Anim Nutr. 2001;55:221–242. doi: 10.1080/17450390109386194. [DOI] [PubMed] [Google Scholar]
- 30.Volk A, Renn W, Overkamp D, et al. Insulin action and secretion in healthy, glucose tolerant first degree relatives of patients with type 2 diabetes mellitus. Influence of body weight. Exp Clin Endocrinol Diabetes. 1999;107:140–147. doi: 10.1055/s-0029-1212089. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson JW, Smith U, Waagstein F, Wysocki M, Jansson P-A. Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients. Is cellular insulin resistance a secondary phenomenon? Diabetes. 1999;48:1572–1578. doi: 10.2337/diabetes.48.8.1572. [DOI] [PubMed] [Google Scholar]
- 32.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 33.Weijers RNM, Bekedam DJ, Smulders YM. Determinants of mild gestational hyperglycemia and gestational diabetes mellitus in a large Dutch multiethnic cohort. Diabetes Care. 2002;25:72–77. doi: 10.2337/diacare.25.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Holemans K, Aerts L, Van Assche FA. Evidence for an insulin resistance in the adult offspring of pregnant streptozotocin- diabetic rats. Diabetologia. 1991;34(2):81–85. doi: 10.1007/BF00500377. [DOI] [PubMed] [Google Scholar]
- 35.Okauchi N, Mizuno A, Zhu M, et al. Effects of obesity and inheritance on the development of non-insulin-dependent diabetes mellitus in Otsuka-Long-Evans-Tokushima fatty rats. Diabetes Res Clin Pract. 1995;29(1):1–10. doi: 10.1016/0168-8227(95)01114-s. [DOI] [PubMed] [Google Scholar]
- 36.Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes. 2002;51:1499–1506. doi: 10.2337/diabetes.51.5.1499. [DOI] [PubMed] [Google Scholar]
- 37.Holemans K, Caluwaerts A, Poston L, Van Assche FA. Diet-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:858–865. doi: 10.1016/j.ajog.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Shiba Y, Yamasaki Y, Kubota M, et al. Increased hepatic glucose production and decreased hepatic glucose uptake at the pre-diabetic phase in the Otsuka Long-Evans Tokushima fatty rat model. Metabolism. 1998;47:908–914. doi: 10.1016/s0026-0495(98)90343-2. [DOI] [PubMed] [Google Scholar]
- 39.Rouvinen-Watt K, Murphy JP, Chan C. Effect of feeding intensity on body condition and glycemic control in mink Mustela vison. Scientifur. 2004;28:129–135. [Google Scholar]
- 40.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmet P, Hodge A, Nicolson M, et al. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. Br Med J. 1996;313:965–969. doi: 10.1136/bmj.313.7063.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forouhi NG, Jenkinson G, Thomas EL, et al. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia. 1999;42:932–935. doi: 10.1007/s001250051250. [DOI] [PubMed] [Google Scholar]
- 43.Plotnick AN, Greco DS. Diagnosis of diabetes mellitus in dogs and cats: contrasts and comparisons. Vet Clin North Am Small Anim Pract. 1995;25:563–570. doi: 10.1016/s0195-5616(95)50053-1. [DOI] [PubMed] [Google Scholar]
- 44.Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc. 1998;212:1725–1731. [PubMed] [Google Scholar]
- 45.Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001;60:375–380. doi: 10.1079/pns200195. [DOI] [PubMed] [Google Scholar]
- 46.Kautzky-Willer A, Prager R, Waldhausl W, et al. Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care. 1997;20:1717–1723. doi: 10.2337/diacare.20.11.1717. [DOI] [PubMed] [Google Scholar]
- 47.Farmer G, Russell G, Hamilton-Nicol DR, et al. The influence of maternal glucose metabolism on fetal growth, development and morbidity in 917 singleton pregnancies in nondiabetic women. Diabetologia. 1988;31:134–141. doi: 10.1007/BF00276845. [DOI] [PubMed] [Google Scholar]
- 48.Vambergue A, Nuttens MC, Verier-Mine O, Dognin C, Cappoen JP, Fontaine P. Is mild gestational hyperglycemia associated with maternal and neonatal complications? The Diagest Study. Diabetic Med. 2000;17:203–208. doi: 10.1046/j.1464-5491.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 49.Børsting C, Damgaard B. The intermediate glucose metabolism in the nursing period of the mink. Presented at NJF [Nordic Association of Agricultural Scientists] Seminar 253; 1995 Oct 4–6; Gothenburg, Sweden. [Google Scholar]
- 50.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 51.Aerts L, Van Assche FA. Intrauterine transmission of disease. Placenta. 2003;24:905–911. doi: 10.1016/s0143-4004(03)00115-2. [DOI] [PubMed] [Google Scholar]
- 52.Jovanovic L, Gutierrez M, Peterson CM. Chromium supplementation for women with gestational diabetes mellitus. J Trace Elem Exp Med. 1999;12:91–97. [Google Scholar]
- 53.Anderson RA, Cheng N, Bryden NA, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 54.Morris BW, MacNeil S, Hardisty CA, Heller S, Burgin C, Gray TA. Chromium homeostasis in patients with type II (NIDDM) diabetes. J Trace Elem Med Biol. 1999;13:57–61. doi: 10.1016/S0946-672X(99)80024-8. [DOI] [PubMed] [Google Scholar]
- 55.Vincent JB. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 56.Vincent JB. Mechanisms of chromium action: low-molecular- weight chromium-binding substance. J Am Coll Nutr. 1999;18(1):6–12. doi: 10.1080/07315724.1999.10718821. [DOI] [PubMed] [Google Scholar]
- 57.Cheng HH, Lai MH, Hou WC, Huang CL. Antioxidant effects of chromium supplementation with type 2 diabetes mellitus and euglycemic subjects. J Agric Food Chem. 2004;52:1385–1389. doi: 10.1021/jf035074j. [DOI] [PubMed] [Google Scholar]
- 58.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 59.Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr. 1998;17:548–555. doi: 10.1080/07315724.1998.10718802. [DOI] [PubMed] [Google Scholar]
- 60.Kim DS, Kim TW, Park IK, Kang JS, Om AS. Effects of chromium picolinate supplementation on insulin sensitivity, serum lipids, and body weight in dexamethasone-treated rats. Metab Clin Exp. 2002;51:589–594. doi: 10.1053/meta.2002.31985. [DOI] [PubMed] [Google Scholar]
- 61.Jen K-LC, Buison A, Pellizzon M, Ordiz F, Jr, Santa Ana L, Brown J. Differential effects of fatty acids and exercise on body weight regulation and metabolism in female Wistar rats. Exp Biol Med. 2003;228:843–849. doi: 10.1177/15353702-0322807-10. [DOI] [PubMed] [Google Scholar]
- 62.Korhonen H, Harri M, Mononen J. Regulation of weight loss in male farm mink. Comp Biochem Physiol. 1989;92A:355–357. doi: 10.1016/0300-9629(89)90575-6. [DOI] [PubMed] [Google Scholar]
- 63.Rouvinen K, Kiiskinen T. Influence of dietary fat source on the body fat composition of mink (Mustela vison) and blue fox (Alopex lagopus) Acta Agric Scand. 1989;39:279–288. [Google Scholar]