Abstract
Mink nursing sickness is a metabolic disorder characterized by hyperglycemia that is similar to the metabolic syndrome associated with type 2, or non-insulin-dependent, diabetes mellitus. This research studied the effects of short-term administration of antidiabetic supplements on the blood glucose concentration in female mink during late lactation. Female mink that had blood glucose levels < 5.5 mmol/L (normoglycemic [NG]) or ≥ 5.5 mmol/L (hyperglycemic [HG]) early in lactation were given daily supplements of various combinations of herring oil (HerO, 3% in diet), chromium picolinate (CrPic, 200 μg), and acetylsalicylic acid (ASA, 100 mg) for 1 wk starting at day 21 post partum. In the NG mink, most of the treatments did not significantly change the blood glucose concentration from day 28 to 42 post partum. However, treatment with ASA alone and treatment with the combination HerO-CrPic-ASA elevated the blood glucose levels when compared with those of the control group, which had received just the basal diet. In the HG mink, all treatment combinations except CrPic alone and ASA alone, reduced the blood glucose concentration. Thus, in lactating mink with hyperglycemia, the blood glucose concentration may be effectively lowered by dietary antidiabetic supplementation; however, because hyperglycemia also occurs before nursing, preventive measures are recommended throughout the year.
Résumé
Chez le vison la maladie de l’allaitement est un désordre métabolique caractérisé par une hyperglycémie et est similaire au syndrome métabolique associé avec le diabète mellitus de type 2, dit non insulino-dépendant. Au cours du présent projet on a étudié les effets d’une administration de courte durée d’un supplément anti-diabète sur la glycémie de visons femelles tard dans la période de lactation. Les visons femelles qui avaient des niveaux de glucose sanguin < 5,5 mmol/L (normoglycémique [NG]) ou ≥ 5,5 mmol/L (hyperglycémique [HG]) tôt dans la période de lactation ont reçu un supplément quotidien de différentes combinaisons d’huile de hareng (HerO; 3 % dans la diète), de picolinate de chrome (CrPic; 200 μg) et d’acide acétylsalicylique (ASA; 100 mg) durant 1 semaine débutant au jour 21 de la lactation. Chez les visons NG, la plupart des traitements n’ont pas modifié de manière significative la glycémie entre les jours 28 à 42 de la lactation. Toutefois, un traitement avec de l’ASA seule et un traitement avec la combinaison HerO-CrPic-ASA ont fait augmenter les niveaux de glucose sanguin comparativement à ceux du groupe témoin, qui n’avaient reçu qu’une diète de base. Chez les visons HG, toutes les combinaisons de traitement excepté celui de CrPic seul et celui d’ASA seule ont réduit la glycémie. Ainsi, chez les visons en lactation avec hyperglycémie, la concentration de glucose sanguin peut être réduite efficacement par une supplémentation alimentaire anti-diabétique; toutefois, étant donné que l’hyperglycémie se produit également avant l’allaitement, des mesures préventives sont recommandées durant toute l’année.
(Traduit par Docteur Serge Messier)
Introduction
The nursing period is a time of critical importance during the reproductive cycle of the mink dam. As lactation progresses, the ability of the mink female to meet increasing energy demands is influenced by various metabolic, nutritional, and environmental factors (1,2). Body reserves are frequently mobilized in order to cover production requirements. Excessive mobilization often leads to nursing sickness (1,3), which is characterized by progressive weight loss, lethargy, loss of appetite, extreme dehydration (4), and high blood glucose and insulin levels (3). Acquired insulin resistance has been proposed as the underlying cause of nursing sickness in the mink female (2).
Within normal pregnancy, insulin resistance develops, favoring glucose supply to the developing fetus and milk production; glucose homeostasis is restored at the cessation of lactation (5). However, obesity or lipodystrophy (deficiency of body fat), deficiency of n-3 polyunsaturated fatty acids (PUFA), oxidative stress, or a combination of these factors may aggravate this metabolic response, resulting in hyperglycemia and possibly acquired insulin resistance in the mink dam (2). Closer examination of glycemic control in the mink dam during lactation may provide further insight into the events leading to high blood glucose levels. The purpose of this research was to develop a more complete understanding of the immediate causative components of hyperglycemia and to evaluate short-term administration of potential antidiabetic treatments for the prevention or reversal of hyperglycemia during late lactation. Oxidative stress was also evaluated in relation to glycemic control and the studied dietary supplements.
Materials and methods
Animals
Forty-eight yearling female mink were randomly selected from the herd at the Canadian Centre for Fur Animal Research, Truro, Nova Scotia. The animals were housed in individual cages, with water provided ad libitum. The experimental procedures and husbandry conditions were approved by the Animal Care and Use Committee of the Nova Scotia Agricultural College and in accordance with the guidelines of the Canadian Council on Animal Care (6).
Sample collection and analysis
On days 10, 21, 28, 35, and 42 post partum, a sample of capillary blood was collected from each dam approximately 90 min post-prandially for measurement of the glucose concentration, and the dam body weight and body condition score (BCS) and the litter size and weight were measured, as previously described (7). At late lactation (on day 42 post partum), an additional capillary blood sample was taken for comet assay, a measure of oxidative stress, as previously described (8).
Experiment
The experiment had a randomized complete block design with a 24 factorial (3 replicates) arrangement of treatments, the main effects of day and treatment being used to evaluate factors associated with blood glucose concentration and oxidative stress. The mink dams were classified by the blood glucose concentrations on days 10 and 21 post partum as normoglycemic (NG; concentration <5.5 mmol/L on both days) or hyperglycemic (HG; concentration ≥ 5.5 mmol/L on either day) and randomly assigned to 1 of 8 dietary treatments, with 3 NG and 3 HG females per treatment. The treatments were based on combinations of established antidiabetic, antioxidative, or anti-inflammatory agents. Starting on day 21 post partum, 1 of the following 8 treatments was administered daily for 1 wk.
Nonsupplemented wet mink-lactation ration, which also served as the basal diet (control diet).
Addition of dietary herring oil (HerO) at a 3% inclusion level.
Supplementation with chromium picolinate (CrPic), 200μg/d.
Supplementation with HerO and CrPic (HerO-CrPic).
Supplementation with acetylsalicylic acid (ASA), 100 mg/d.
Supplementation with HerO and ASA (HerO-ASA).
Supplementation with CrPic and ASA (CrPic-ASA).
Supplementation with HerO, CrPic, and ASA (HerO-CrPic-ASA).
Statistical analysis
Blood glucose data were analyzed by means of the MIXED procedure for repeated measures with SAS, version 8 (SAS Institute, Cary, North Carolina, USA). Blood glucose levels, dam weights, and litter weights at days 10 and 21 and dam color phase were used as covariates. An inverse transformation was applied to the blood glucose data to achieve normality. Results are reported for nontransformed blood glucose data with the use of significance as determined for transformed data. The comet assay score was evaluated with the MIXED procedure, with blood glucose levels, dam weights, and litter weights on days 10 through day 42 being used as covariates. Covariates not found to influence the variables (P >0.15) were not included in the model. Results are reported as least-squares means (with standard error in parenthesis). A statistical significance level of P < 0.05 was used unless otherwise stated.
Results
Blood glucose concentration
A significant interaction effect of HerO-CrPic-ASA supplementation and glycemic status on blood glucose concentration was observed. As shown in Table I, within the control group, NG females had significantly lower mean blood glucose values (P < 0.001) than HG females during weeks 4 to 6 of lactation, at 3.8 (0.8) versus 9.9 (0.8) mmol/L. No other significant differences were observed in blood glucose values from day 28 to 42 post partum between the NG and HG females receiving the same treatment. Dam weights, litter weights, and color type did not affect the blood glucose levels observed at late lactation.
Table I.
Mean (least-squares) blood glucose concentration, averaged over days 28 to 42 of lactation, for mink dams fed an experimental diet for 7 d from day 21
| Dams;a glucose concentration, mmol/L, mean (andstandarderror)
|
|||
|---|---|---|---|
| Dietb | NG | HG | Pc |
| Basal (control) | 3.8 (0.8) | 9.9 (0.8) | < 0.001 |
| HerO | 4.6 (0.7) | 4.6 (1.0) | 0.734 |
| CrPic | 5.0 (1.0) | 6.4 (0.7) | 0.277 |
| HerO-CrPic | 5.0 (0.8) | 5.4 (0.8) | 0.679 |
| ASA | 7.9 (0.8) | 6.5 (0.8) | 0.713 |
| HerO-ASA | 4.2 (0.8) | 5.3 (0.8) | 0.402 |
| CrPic-ASA | 4.8 (0.8) | 5.3 (0.8) | 0.391 |
| HerO-CrPic-ASA | 5.7 (0.8) | 4.8 (0.8) | 0.224 |
Classified by the blood glucose concentrations on days 10 and 21 post partum as normoglycemic (NG; concentration <5.5 mmol/L on both days) or hyperglycemic (HG; concentration ≥ 5.5 mmol/L on either day).
Basal diet alone or with supplementary herring oil (HerO, 3% inclusion per day), chromium picolinate (CrPic, 200 μg/d), acetylsalicylic acid (ASA, 100 mg/d), or a combination of supplements.
As determined with transformed data.
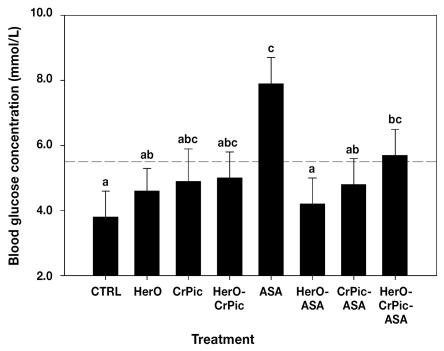
Among the NG females (Figure 1), blood glucose levels were significantly increased from day 28 to 42 post partum in those treated with ASA as compared with the control group (P = 0.001), at 7.9 (0.8) versus 3.8 (0.8) mmol/L. Although HerO and CrPic alone did not significantly alter blood glucose levels, the combination HerO-CrPic-ASA increased the blood glucose concentration to 5.7 (0.8) mmol/L, significantly greater (P = 0.01) than the control mean. No other statistically significant effects on blood glucose levels of NG dams during late lactation were observed among the treatments when compared with the control diet.
Figure 1.
Mean (least-squares) blood glucose levels, with standard errors, averaged over days 28 to 42 of lactation, for normoglycemic mink females fed the basal, control diet (CTRL) or the basal diet plus various combinations of herring oil (HerO), chromium picolinate (CrPic), and acetylsalicylic acid (ASA) for 1 wk from day 21. Means with the same letter(s) are not significantly different, as determined with transformed data (P < 0.05). Broken line indicates the cutoff point for normoglycemia (5.5 mmol/L).
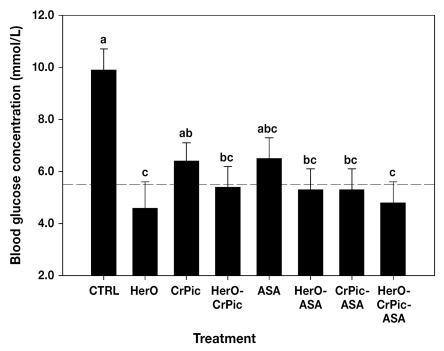
In contrast, HG females (Figure 2 and Table II) in the control group showed significantly higher blood glucose levels from day 28 to 42 post partum than those fed HerO (P = 0.01), HerO-CrPic (P = 0.03), HerO-ASA (P = 0.03), CrPic-ASA (P = 0.003), or HerO-CrPic-ASA (P = 0.003), for whom the mean blood glucose levels were below the initial hyperglycemia criterion of a value of ≥ 5.5 mmol/L on day 10 or 21 post partum.
Figure 2.
Mean blood glucose levels for hyperglycemic mink females receiving the same experimental dietary treatments. Details as for Figure 1.
Table II.
Details for 2 dams exhibiting typical signs of nursing sickness at day 42 post partum
| Variable | Dam A | Dam B |
|---|---|---|
| Dietary group | CrPic-ASA | Control |
| Blood glucose level, mmol/L | ||
| Day 42 | 5.8 | 17.1 |
| Day 43 | 3.4 | 27.9 |
| Day 44 | ||
| am | 3.4 | 6.1 |
| pm | 4.6 | 2.0 |
| Diagnosis | Valvular endocarditis | None |
Oxidative stress
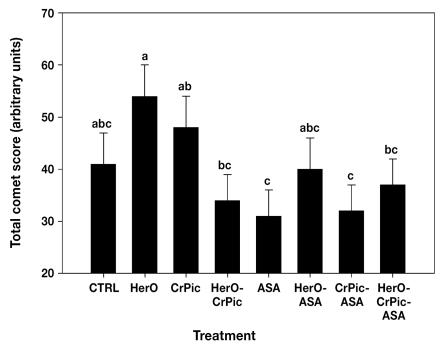
The comet score, a measure of oxidative damage, was not significantly affected by the glycemic status of the mink dam as assessed at day 10 or 21 post partum, nor did the mean score associated with each treatment differ significantly from that for the control group (Figure 3). However, evidence of increased oxidative damage was observed in mink dams administered supplemental HerO alone, whose mean score was 54 (6), significantly more than that of the females treated with HerO-CrPic (33 [5]; P = 0.01), ASA (31 [5]; P = 0.005), CrPic-ASA (32 [5]; P = 0.01), or HerO-CrPic-ASA (37 [5]; P = 0.04). Females given supplementary CrPic alone had a higher mean comet assay score (48 [6]) than those given ASA alone (P = 0.04) or the combination CrPic-ASA (P = 0.05).
Figure 3.
Mean comet assay score on day 42 of lactation in the entire study group of mink females.
Dam death
At day 42 post partum, 2 HG females displayed typical signs of nursing sickness: sunken eyes, emaciation, and lethargy. Dam A had received the CrPic-ASA treatment, whereas dam B was in the control group. Dam A was unresponsive and appeared to have deteriorated further than dam B. Severe hyperglycemia was observed in dam B but not dam A (Table II). At autopsy, low body fat stores were found in both animals, and their stomachs were contracted and without content.
Discussion
Our data indicate that, within the control group, late-lactation blood glucose levels were significantly higher among mink dams that early in the nursing period had shown elevated blood glucose levels in comparison with females that had normal levels at that time. During gestation and lactation, healthy individuals maintain glucose homeostasis in spite of the adaptive increase in insulin resistance because of a compensatory increase in insulin secretion by pancreatic β-cells (5). Deterioration to postprandial hyperglycemia occurs when insulin resistance increases further, the compensatory insulin-secretion response decreases, or both (9,10). Research suggests that humans experiencing gestational and postpartum insulin resistance have a degree of insulin resistance before their pregnancy, a condition that is partially inherited and partially acquired (5,11). Previous studies have identified genetic transmission of glucose intolerance (12–14) and pregestational obesity (15,16) as determinants for the development of hyperglycemia during gestation. The implication is that hyperglycemia is not a transient condition in nursing female mink and that those experiencing problems with glucose regulation early in lactation may be susceptible to similar problems as lactation progresses.
Although dam body weight and condition and litter weight and size were not found to immediately affect the blood glucose levels observed in the mink dams during late lactation, it is possible that the initial defects in glucose homeostasis occurred earlier in the reproductive cycle. Previous research has shown that autumnal fattening in mink, fed at 120% of the recommended dietary allowance (RDA) of metabolizable energy over a 4-mo period, resulted in higher blood glucose values in males and females and higher insulin levels in males in comparison with those fed the RDA or less (17). In a study by Børsting and Damgaard (18), the increased demand for milk production placed on females nursing 8 kits resulted in higher glucose production than in those nursing only 4 kits. With the development of insulin resistance during pregnancy, a mechanism favoring glucose supply to the growing fetus (5), a similar increase in glucose demand associated with carrying larger litters is to be expected. This increased demand may aggravate underlying insulin resistance in mink females. We suggest that previous nonideal body condition and increased gestational glucose demands may compromise the mink dam’s ability to regulate glucose levels throughout the nursing period. This disruption in glucose homeostasis, when prolonged, may then lead to the development of nursing sickness.
The elevated blood glucose levels observed in NG females treated with ASA were unexpected. Hundal et al (19) reported decreased basal hepatic glucose production, enhanced peripheral insulin sensitivity, and decreased insulin clearance in patients with type 2, or non-insulin-dependent, diabetes mellitus treated for 2 wk with high-dose ASA. Recent data evaluating the effect of ASA on glycemic control in apparently healthy subjects are sparse. However, although numerous studies have identified ASA as an effective agent for improving insulin sensitivity in insulin-resistant patients, in overdosage it has triggered transient hyperglycemia in healthy subjects (20). In healthy men, high-dose ASA treatment impaired glucose metabolism in insulin-sensitive tissues (21–23). Thus, in our study, the selected dose, 100 mg/d, may have interfered with glucose metabolism in the apparently healthy dams and prompted the development of hyperglycemia. The mechanism for this effect is not clear; however, in a case study involving salicylate toxicity in a 5-y-old girl, in whom hypoglycemia was followed by hyperglycemia, it was suggested that metabolic acidosis led to aberrations in oxidative phosphorylation, resulting in hyperglycemia due to increased gluconeogenesis (24).
The HerO-CrPic-ASA-treated females in our study showed increased blood glucose levels when compared with those in the control and HerO-ASA treatment groups. The differences observed between the HerO-ASA and HerO-CrPic-ASA treatment groups indicate an additive effect of CrPic on the combined effects of HerO and ASA on blood glucose, effectively increasing the blood glucose levels in apparently healthy mink dams during lactation. Most scientific evidence indicates that chromium supplementation has no significant effect in healthy individuals with good glucose tolerance (25–27). To the best of our knowledge, no data describe interactions between chromium and fish oil on blood glucose regulation; however, ASA has been found to enhance chromium absorption (28). The influence of chromium on the control of blood glucose is through increased insulin receptor number and increased insulin phosphorylation (26,29,30). However, with multiple mechanisms of action of anti-diabetic agents on glucose metabolism through pancreatic, hepatic, and peripheral effects (31), it is possible that in combination the studied treatments acted at multiple sites to induce abnormalities in glucose metabolism. In light of potential disruptions in glucose homeostasis associated with ASA and HerO-CrPic-ASA, we recommend against their use in apparently healthy mink dams. Overall, our results indicate no beneficial effects of the applied treatments on blood glucose levels during late lactation in female mink that are NG early in the nursing period.
In contrast to the increases in blood glucose concentration observed in NG dams treated with ASA alone and HerO-CrPic-ASA, several of the experimental treatments reduced blood glucose levels during late lactation in the dams that had exhibited elevated blood glucose levels early in the nursing period. Furthermore, blood glucose levels in these treatment groups were lowered to below the initial hyperglycemia criterion. Supplemental HerO, fed alone or in any combination with CrPic and ASA, decreased blood glucose levels in HG dams during lactation in comparison with the control diet. Fish oils high in n-3 PUFA have had beneficial effects on insulin-stimulated glucose transport and metabolism in peripheral tissues (32–35). If supplemental n-3 PUFA can improve blood glucose levels in female mink during late lactation, then we should consider how the dietary fatty acid profile could be changed throughout the production year. A small increase in dietary n-3 PUFA could have a beneficial effect on glycemic regulation in the mink female throughout the reproductive cycle.
When administered individually, CrPic and ASA did not significantly alter blood glucose levels in HG females in comparison with the control diet. However, the 2 agents in combination significantly reduced blood glucose levels during late lactation. In diabetic individuals, vulnerability to oxidative damage might be partly attributed to a lower status of antioxidative micronutrients, including trace elements (36). Conditions that increase circulating insulin and glucose concentrations, including pregnancy (37) and obesity, also increase urinary chromium output (29,38,39), and chromium deficiency may be an aggravating factor in the progression of diabetes (36,40). Davis et al (28) showed that ASA increased absorption, tissue retention, and urinary excretion of chromium in adult female rats. Although the mechanism has not been clarified, it was proposed that chromium absorption was increased through ASA’s inhibitory effect on prostaglandin synthesis (28). The cumulative effect observed may have resulted from interactions among the insulin-sensitizing effects of chromium (25,26,29,30) and the anti-inflammatory (19,41–43) and antioxidant (44,45) properties of ASA.
Hyperglycemia induces the overproduction of reactive oxygen species, particularly superoxide anion, by the mitochondrial electron-transport chain (9), which in turn damages cellular DNA. However, in the current study, females that were NG early in the nursing period did not have significantly different comet assay scores than females that were HG at that time. It is suggested that oxidative damage is a late event, occurring as an endpoint of hyperglycemia-dependent cellular changes (46,47).
When evaluating the effect of short-term supplementation in nursing mink females, we found that none of the dietary groups differed significantly from the control group in the degree of oxidative damage observed. Elevated comet assay scores were observed in the blood of the HerO-treated females in comparison with those in the HerO-CrPic, CrPic-ASA, ASA, and HerO-CrPic-ASA treatment groups. When exposed to oxidative stress, PUFA can be attacked by free radicals and oxidized into lipid peroxides (48). High doses of fish oil fed over a short period have increased susceptibility to oxidative stress, expressed as lipid peroxidation, in rat erythrocytes (49). Cho et al (50) suggested that lipid peroxidation may lead to DNA damage, as they detected higher levels of 8-hydroxydeoxyguanosine, a marker of DNA oxidation, in the liver of fish-oil-fed rats receiving low levels of the antioxidant vitamin E compared with those receiving moderate or high levels. Although the dietary requirement of antioxidants for a PUFA-rich diet has not been defined, data indicate that in order to prevent increased susceptibility of fish oil to lipid peroxidation, greater amounts of antioxidants than those needed to stabilize the oil may be required.
Female mink treated with CrPic had a higher mean comet assay score than those treated with ASA and CrPic-ASA. Long-term treatment with ASA is associated with a reduction of superoxide production in normotensive and hypertensive rats (51). The reduction in oxidative damage associated with CrPic when combined with ASA may involve the antioxidant properties of the latter (44,45). Dietary supplementation with CrPic-ASA had neither positive nor negative effects on the total comet assay score, as a measure of oxidative stress, in mink dams at late lactation. In light of our results, evaluating the degree of oxidative damage among nursing and non-nursing mink females would be of interest.
Valvular endocarditis was diagnosed at autopsy in dam A, in the HG CrPic-ASA treatment group. Culture showed growth of Staphylococcus intermedius, an organism that may originate from disrupted oral, gastrointestinal, or urogenital mucosal surfaces or from any other localized source of infection (52); this was a significant finding, as death was unrelated to other observations. Although HG was demonstrated early in lactation, no blatant abnormalities in glycemic control were observed after the treatment period. On the other hand, dam B, in the HG control group, experienced severe HG on days 42 and 43 of lactation. Metabolic diseases are almost impossible to diagnose at autopsy, and although there was no diagnosis in this case, it was clear that this female was unable to regulate blood glucose throughout the nursing period and had an acute failure in late lactation. The observed rapid drop in the blood glucose concentration when the animal was terminally ill (on day 44) may suggest poor diagnostic value for glucose levels at the time of necropsy. Dam B gave birth to 13 kits and was nursing a litter of 7. An increased gestational demand for glucose may have exacerbated underlying insulin resistance, resulting in further disruptions in glucose homeostasis throughout lactation.
Conclusions
We conclude that in apparently healthy, NG female mink, there are no advantageous effects on the blood glucose concentration at late lactation of short-term dietary supplementation with HerO, CrPic, and ASA, alone or in any combination, at the levels given in this study. In addition, these agents had neither positive nor negative effects on the degree of oxidative damage measured at late lactation. However, in dams demonstrating problems with glycemic control early in the nursing period, several of the treatments reduced blood glucose levels during late lactation, suggesting that a combined approach of blood glucose monitoring and dietary supplementation may help improve glucose regulation in mink females. With evidence of poor glucose regulation in mink females before lactation, it appears that preventive measures need to be taken throughout the year. Further research should target long-term dietary strategies that aid mink females in blood glucose regulation during the fall and help maintain normoglycemia through pregnancy and lactation.
Acknowledgments
We thank the staff of the Canadian Centre for Fur Animal Research, Rena Currie and Tanya Morse, and Shelli Meleck for their assistance in sample collection, and Renée Garbes for carrying out the comet assay. We also acknowledge Dr. Tess Astatkie for help in statistical analysis and Dr. Gordon Finley and Dr. Leslie MacLaren for their advice and expertise. This research was supported by the Canada Mink Breeders Association, the Nova Scotia Fur Institute, and the Natural Sciences and Engineering Research Council (NSERC Discovery Grant to Dr. Rouvinen-Watt).
Footnotes
This research was part of the MSc thesis research of Amber M.J. Hynes.
References
- 1.Clausen TN, Olesen CR, Hansen O, Wamberg S. Nursing sickness in lactating mink (Mustela vison). I. Epidemiological and pathological observations. Can J Vet Res. 1992;56:89–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Rouvinen-Watt K. Nursing sickness in the mink — A metabolic mystery or a familiar foe? Can J Vet Res. 2003;67:161–168. [PMC free article] [PubMed] [Google Scholar]
- 3.Wamberg S, Clausen TN, Olesen CR, Hansen O. Nursing sickness in lactating mink (Mustela vison). II. Pathophysiology and changes in body fluid composition. Can J Vet Res. 1992;56:95–101. [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider RR, Hunter DB. Nursing disease in the mink. Scientifur. 1992;16:239–242. [Google Scholar]
- 5.Di Cianni G, Miccoli R, Volpe L, Lencioni C, Del Prato S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res. 2003;19:259–270. doi: 10.1002/dmrr.390. [DOI] [PubMed] [Google Scholar]
- 6.Olfert ED, Cross BM, McWilliams AA, editors. Guide to the Care and Use of Experimental Animals. 2. Vol. 1. Ottawa, Ontario: Canadian Council on Animal Care; 1993. [Google Scholar]
- 7.Hynes AMJ, Rouvinen-Watt K. Monitoring blood glucose levels in female mink during the reproductive cycle: 1. Prevention of hyperglycemia during the nursing period. Can J Vet Res. 2007;71:241–248. [PMC free article] [PubMed] [Google Scholar]
- 8.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 9.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson JW, Smith U, Waagstein F, Wysocki M, Jansson P-A. Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients. Is cellular insulin resistance a secondary phenomenon? Diabetes. 1999;48:1572–1578. doi: 10.2337/diabetes.48.8.1572. [DOI] [PubMed] [Google Scholar]
- 13.Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes. 2002;51:1499–1506. doi: 10.2337/diabetes.51.5.1499. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrin Metab. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 15.Volk A, Renn W, Overkamp D, et al. Insulin action and secretion in healthy, glucose tolerant first degree relatives of patients with type 2 diabetes mellitus. Influence of body weight. Exp Clin Endocrinol Diabetes. 1999;107:140–147. doi: 10.1055/s-0029-1212089. [DOI] [PubMed] [Google Scholar]
- 16.Weijers RNM, Bekedam DJ, Smulders YM. Determinants of mild gestational hyperglycemia and gestational diabetes mellitus in a large Dutch multiethnic cohort. Diabetes Care. 2002;25:72–77. doi: 10.2337/diacare.25.1.72. [DOI] [PubMed] [Google Scholar]
- 17.Rouvinen-Watt K, Murphy JP, Chan C. Effect of feeding intensity on body condition and glycemic control in mink Mustela vison. Scientifur. 2004;28(3):129–135. [Google Scholar]
- 18.Børsting C, Damgaard B. The intermediate glucose metabolism in the nursing period of the mink. Presented at NJF [Nordic Association of Agricultural Scientists] Seminar 253; 1995 Oct 4–6; Gothenburg, Sweden. [Google Scholar]
- 19.Hundal RS, Petersen KF, Mayerson AB, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferner RE. Drug-induced diabetes. Baillieres Clin Endocrinol Metab. 1992;6:849–866. doi: 10.1016/s0950-351x(05)80170-3. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano D, Sacca L, Scognamiglio G, Ungaro B, Torella R. Influence of acetylsalicylic acid on glucose turnover in normal man. Diabetes Metab. 1982;8:279–282. [PubMed] [Google Scholar]
- 22.Newman WP, Brodows RG. Aspirin causes tissue insensitivity to insulin in normal man. J Clin Endocrinol Metab. 1983;57:1102–1106. doi: 10.1210/jcem-57-6-1102. [DOI] [PubMed] [Google Scholar]
- 23.Bratusch-Marrain PR, Vierhapper H, Komjati M, Waldhausl WK. Acetyl-salicylic acid impairs insulin-mediated glucose utilization and reduces insulin clearance in healthy and non-insulin- dependent diabetic man. Diabetologia. 1985;28:671–676. doi: 10.1007/BF00291974. [DOI] [PubMed] [Google Scholar]
- 24.Peña-Alonso YR, Montoya-Cabrera MA, Bustos-Córdoba E, Marroquín-Yáñez L, Olivar-López V. Aspirin intoxication in a child associated with myocardial necrosis: Is this a drug-relation lesion? Pediatr Dev Pathol. 2003;6:342–347. doi: 10.1007/s10024-001-0127-x. [DOI] [PubMed] [Google Scholar]
- 25.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 26.Kim DS, Kim TW, Park IK, Kang JS, Om AS. Effects of chromium picolinate supplementation on insulin sensitivity, serum lipids, and body weight in dexamethasone-treated rats. Metab Clin Exp. 2002;51:589–594. doi: 10.1053/meta.2002.31985. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JB. Recent advances in the biochemistry of chromium (III) J Trace Elem Exp Med. 2003;16:227–236. [Google Scholar]
- 28.Davis ML, Seaborn CD, Stoecker BJ. Effects of over-the-counter drugs on 51chromium retention and urinary excretion in rats. Nutr Res. 1995;15:201–210. [Google Scholar]
- 29.Anderson RA, Cheng N, Bryden NA, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr. 1998;17:548–555. doi: 10.1080/07315724.1998.10718802. [DOI] [PubMed] [Google Scholar]
- 31.Pandit M, Burke J, Gustafson A, Minocha A, Peiris A. Drug-induced disorders of glucose tolerance. Ann Intern Med. 1993;118:529–539. doi: 10.7326/0003-4819-118-7-199304010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Ezaki O, Tsuji E, Momomura K, Kasuga M, Itakura H. Effects of fish and safflower oil feeding on subcellular glucose transporter distributions in rat adipocytes. Am J Physiol. 1992;263:E94–E101. doi: 10.1152/ajpendo.1992.263.1.E94. [DOI] [PubMed] [Google Scholar]
- 33.Long SD, Pekala PH. Regulation of GLUT4 gene expression by arachidonic acid. J Biol Chem. 1996;271:1138–1144. doi: 10.1074/jbc.271.2.1138. [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Rizkalla SW, Boillot J, et al. Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J Nutr. 1996;126:1951–1958. doi: 10.1093/jn/126.8.1951. [DOI] [PubMed] [Google Scholar]
- 35.Jen K-LC, Buison A, Pellizzon M, Ordiz F, Jr, Santa Ana L, Brown J. Differential effects of fatty acids and exercise on body weight regulation and metabolism in female Wistar rats. Exp Biol Med. 2003;228:843–849. doi: 10.1177/15353702-0322807-10. [DOI] [PubMed] [Google Scholar]
- 36.Cheng HH, Lai MH, Hou WC, Huang CL. Antioxidant effects of chromium supplementation with type 2 diabetes mellitus and euglycemic subjects. J Agric Food Chem. 2004;52:1385–1389. doi: 10.1021/jf035074j. [DOI] [PubMed] [Google Scholar]
- 37.Jovanovic L, Gutierrez M, Peterson CM. Chromium supplementation for women with gestational diabetes mellitus. J Trace Elem Exp Med. 1999;12:91–97. [Google Scholar]
- 38.Morris BW, MacNeil S, Hardisty CA, Heller S, Burgin C, Gray TA. Chromium homeostasis in patients with type II (NIDDM) diabetes. J Trace Elem Med Biol. 1999;13:57–61. doi: 10.1016/S0946-672X(99)80024-8. [DOI] [PubMed] [Google Scholar]
- 39.Vincent JB. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 40.Vincent JB. Mechanisms of chromium action: low-molecular-weight chromium-binding substance. J Am Coll Nutr. 1999;18(1):6–12. doi: 10.1080/07315724.1999.10718821. [DOI] [PubMed] [Google Scholar]
- 41.Yin M-J, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of Iκ B kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 42.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 43.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- 44.Sobal G, Menzel JE, Sinzinger H. Influence of acetylsalicylic acid on oxidation of native and glycated low-density lipoprotein. Life Sci. 2000;66:1987–1998. doi: 10.1016/s0024-3205(00)00524-5. [DOI] [PubMed] [Google Scholar]
- 45.El Midaoui A, Wu R, de Champlain J. Prevention of hypertension, hyperglycemia and vascular oxidative stress by aspirin treatment in chronically glucose-fed rats. J Hypertens. 2002;20:1407–1412. doi: 10.1097/00004872-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 46.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed AK, Bierhaus A, Schiekofer S, Tritschler, Ziegler R, Nawroth PP. The role of oxidative stress and NF-kappaB activation in late diabetic complications. BioFactors. 1999;10:157–167. doi: 10.1002/biof.5520100211. [DOI] [PubMed] [Google Scholar]
- 48.Eritsland J. Safety considerations of polyunsaturated fatty acids. Am J Clin Nutr. 2000;71(Suppl):197S–201S. doi: 10.1093/ajcn/71.1.197S. [DOI] [PubMed] [Google Scholar]
- 49.Garrido A, Garrido F, Guerra R, Valenzuela A. Ingestion of high doses of fish oil increases the susceptibility of cellular membranes to the induction of oxidative stress. Lipids. 1989;24:833–835. doi: 10.1007/BF02544593. [DOI] [PubMed] [Google Scholar]
- 50.Cho SH, Im JG, Choi YS, Son YS, Chung MH. Lipid peroxidation and 8-hydroxyguanine formation in rats fed fish oil with different levels of vitamin E. J Nutr Sci Vitaminol. 1995;41:61–72. doi: 10.3177/jnsv.41.61. [DOI] [PubMed] [Google Scholar]
- 51.Wu LL, Chiuan-Chian C, Chang P-Y, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Brown VA. Aortic valvular endocarditis in a dog. Can Vet J. 2004;45:682–684. [PMC free article] [PubMed] [Google Scholar]