Abstract
The objectives of this study were to describe the incidence of, survival until, and survival after the diagnosis of canine bone tumors by breed, sex, age, and geographic location of residence. Dogs under 10 y old and insured by a Swedish insurance company between 1995 and 2002 were studied. In total, 764 dogs had claims for bone tumors, and the incidence rate was 5.5 cases per 10 000 dog-years at risk (DYAR). At ages 6, 8, and 10 y, the proportions of dogs with bone tumors were 0.13%, 0.30%, and 0.64%. The top 3 breeds at risk were Irish wolfhound, St. Bernard, and leonberger (incidence rates 99, 78, and 53 cases per 10 000 DYAR, respectively). Median survival time after diagnosis was 56 d in the 419 dogs that survived ≥ 1 d. With a Cox regression model controlling for breed and age, females were shown to be at decreased risk of bone tumors, with a hazard ratio of 0.71 (99% confidence interval 0.58 to 0.87).
Résumé
Les objectifs de la présente étude étaient de décrire l’incidence de tumeurs osseuses canines et la survie jusqu’à, ainsi que la survie après, le diagnostic selon l’âge, la race, le sexe et la distribution géographique. L’étude a été menée chez des chiens âgés de moins de 10 ans et assurés par une compagnie d’assurance suédoise entre 1995 et 2002. Au total, des réclamations pour des tumeurs osseuses ont été faites pour 764 chiens, et le taux d’incidence était de 5,5 cas par 10 000 années-chiens à risque (DYAR). À 6, 8 et 10 ans, les proportions de chiens avec des tumeurs osseuses étaient de 0,13 %, 0,30 % et 0,64 %. Les trois races les plus à risque étaient le lévrier irlandais, le St-Bernard et le leonberger (taux d’incidence respectifs de 99, 78 et 53 cas par 10 000 DYAR). La médiane du temps de survie après le diagnostic était de 56 j chez les 419 chiens qui ont survécu ≥ 1 j. Avec un modèle de régression de Cox contrôlant pour la race et l’âge, on a démontré que les femelles avaient un risque diminué de tumeurs osseuses, avec un ratio de chance de 0,71 (intervalle de confiance 99 %, 0,58 à 0,87).
(Traduit par Docteur Serge Messier)
Introduction
Osteosarcoma accounts for more than 85% of all primary malignant bone tumors in the dog and approximately 5% of all canine neoplasms (1). Osteosarcoma occurs primarily in large breeds, and the median age at diagnosis is 7 y (2). The usual sites are in the appendicular skeleton, of which the distal radius and proximal humerus of the forelimbs are the most common. In the hindlimbs, the distal tibia and fibula and the proximal tibia are frequently involved (2). Osteosarcomas are highly malignant. They cause lysis or new bone formation, or both, but rarely spread over joints. Metastases are common: at clinical presentation, up to 90% of the dogs have micrometastases, although only 15% have radiographically visible metastases at that time (2). The lungs are the most commonly affected, but metastases have also been detected in regional lymph nodes, bone, and soft tissue (3,4). Dogs treated with surgery alone (amputation) had a median survival time of 19 wk after surgery in 1 case series (5). The mean survival time has been extended to approximately 1 y with different postoperative chemotherapeutic protocols (2). Pathological microfractures in the tumor and metastatic disease are common reasons for euthanasia.
It has been suggested that canine osteosarcoma mimics pediatric osteosarcoma in humans (6,7). The similarities include large patient size, predilection for appendicular sites, metaphyseal location, idiopathic etiology, documented metastasis in less than 10% of patients at presentation, high-grade histologic features, aneuploidy in 75% of tumors, high metastatic rate with amputation alone, the lung the most common site of metastasis, and improved survival with adjuvant chemotherapy (6). Canine osteosarcoma is an attractive model of spontaneous cancer for comparative studies because the absolute number of cases is believed to be higher than in humans, and dogs have a much shorter life expectancy (1,6). A Swedish animal insurance database (Agria Insurance, Stockholm) has proven useful for the study of overall morbidity and mortality rates in dogs (8), as well as specific diseases (9). The database covers approximately one-third of the Swedish dog population (10).
The objectives of this study were to describe the incidence of, survival to, and survival after bone tumors in dogs insured by Agria in the period 1995 to 2002 by generating crude measures of disease incidence and survival as well as estimates stratified by breed, sex, age, and geographic location of residence.
Materials and methods
Database
There are 2 types of insurance for dogs offered by Agria. Veterinary-care insurance has no age limit and reimburses the owner most of the fee if the dog receives costly veterinary care. Dogs can also be life insured, but only up to 10 y of age. With life insurance the owner will generally be reimbursed if the dog dies or is euthanized. Whether the dog dies or undergoes euthanasia cannot be differentiated in the database. Most insured dogs have both types of insurance. The insurance process has previously been described in detail (8).
Data management
The following variables were downloaded from the insurance database and used in the analysis: diagnoses for insurance claims (for both veterinary care and death), dates when dogs entered or left the insurance program, types of insurance for which dogs were enrolled, the dog’s breed, sex, and dates of birth and death, and postal code of the owner.
Breeds were classified according to the Swedish Kennel Club breed-classification system. In the following analysis, some breeds were combined because they were considered to be the same. For example, “dachshund miniature” included all dachshund miniature variants, “dachshund, normal size” included all except long-haired dachshunds, “German pointer” included both smooth-haired and wire-haired German pointers, and “poodle” included miniature and toy poodles. For analyses regarding age-specific incidence rates, the categories were assigned according to the age in years of the dog on January 1 each year of the study (<1, 1 < 2 . . . 9 < 10), as previously described (8).
The owner’s last registered postal code was used to identify where in Sweden the dog lived. Three primary regions were defined: south, middle, and north. Dogs were further recorded as living in either an urban environment or elsewhere, according to whether the owner resided in 1 of the 3 largest cities in Sweden (Stockholm, Göteborg, and Malmö) or elsewhere. These 2 variables were combined into 1 variable, “geography”, with 5 categories: south–urban, south–other, middle–urban, middle–other, and north–other.
The attending veterinarian had assigned diagnostic codes from a hierarchically constructed diagnostic registry with approximately 8000 codes (11) that has previously been described (12). Records of all dogs with a diagnosis of bone tumor were obtained by first retrieving all skeletal codes for tumors (“system” = skeletal; “process” = tumor) in the database. Thereafter, records of dogs with a diagnosis of benign tumors, tumors originating from cartilage, fibroma/fibrosarcoma/hemangiosarcoma, and metastasis of solid tumors to bone were eliminated from the case group.
Population
Dogs were included in the study population if they simultaneously had both insurance for veterinary care and life insurance during the period 1995 to 2002 and were born during the period 1986 to 2002. By definition, all dogs were less than 10 y of age because life-insurance coverage ceases to apply at this age. Each dog was entered at the date of enrolment in the insurance plan or on January 1, 1995, if enrolled before this date, and removed when it had a bone tumor event or left the insurance plan. Dogs that had not been removed before December 31, 2002, were considered withdrawn as of this date. The dogs without a match to geographic region (approximately 800) were omitted. Each animal contributed to the denominator the exact number of dog-years at risk (DYAR) in the database. Descriptive statistics are presented according to the number of dogs and the number of DYAR in the population. An overall mortality rate was also determined.
Descriptive analyses of bone tumor occurrence
Dogs were considered cases from the time of the 1st registered bone tumor diagnosis. Crude, claim-type-specific, diagnosis-specific, and age-specific numbers of bone tumor cases are presented. The recorded diagnoses were also grouped on the basis of anatomic location.
Incidence rates for bone tumors were calculated crudely by breed, sex, age, and geographic location of residence. For each case, the time at risk stopped at the 1st registered bone tumor event. The incidence rates were multiplied by 10 000 for interpretation as the number of dogs with bone tumors per 10 000 DYAR. Breed-specific incidence rates were estimated only for breeds with more than 7000 DYAR or at least 10 cases of bone tumors. Age-category-specific and year-specific incidence rates were constructed for the 1st recorded bone tumor diagnosis. Standard errors for rates were constructed by dividing the root of the number of cases by the DYAR (13), then multiplying by 10 000.Ninety-five percent confidence intervals (95% CI) were constructed (± 1.96 standard error). Age-category-specific rates and CIs were averaged over the study years for presentation purposes.
Survival to diagnosis
The crude and breed-specific proportions of dogs that had insurance claims for bone tumors up to certain ages (6, 8, and 10 y) were calculated for the breeds with at least 18 cases of bone tumors, with use of the baseline survival statement from separate Cox regression models without independent variables.
Survival after diagnosis
The survival after diagnosis of a bone tumor was estimated by the Kaplan–Meyer method. Crude, sex-specific, and age-at-1st-bone-tumor-diagnosis-specific survival rates were determined for all dogs with bone tumors except for those that died the day of 1st diagnosis. The age intervals chosen were less than 5 y, 5 to less than 8 y, and 8 y or more. Breed-specific median survival was similarly estimated for 4 high-risk breeds from data based on at least 20 cases. Median survival was also determined for dogs that survived more than 30 d after the 1st bone tumor diagnosis. Mortality was defined as death with any diagnosis (bone tumor or other) or for dogs that died but did not have a registered life-insurance claim. The log-rank test was used to determine if overall survival varied by sex or age at 1st bone tumor diagnosis.
Multivariable analysis
A multivariable Cox regression model was developed for a reduced dataset consisting of the data for 50 breeds with more than 7000 DYAR and the breeds with at least 10 cases of bone tumors, the total being 56 breeds. The outcome was time to the 1st bone tumor event. Sex and geographic location of residence were entered as fixed effects. The proportional-hazards assumption was investigated by plotting the natural logarithm of the cumulative hazard stratified by sex and geographic location of residence (log–log plots from Cox regressions without covariates) against the log of the year of exit. A frailty term (random effects in the survival analysis) was entered for breed. Time at entry was the age at the start of observation; time at exit was the age at exit, either when a bone tumor was diagnosed or at the end of the study. The rationale was to test all possible 2-way interactions between the variables that stayed in the model after backward main-effect reduction based on Wald’s criterion. A P-value of 0.01 was considered significant in the final multivariable model, and the interactions were added at P < 0.01. The overall model fit was evaluated with the use of Cox–Snell residuals for the model without frailties (14). Model fit was also inspected by means of plots of Martingale and deviance residuals against DYAR and against covariates. Confidence intervals presented for hazard ratios are 99% CIs. The statistical software program STATA 9.1 (Stata Corporation, College Station, Texas, USA) was used for multivariable analysis; the procedure STCOX was used for Cox proportional-hazards regression. Data handling was performed by means of SAS version 9.1 (SAS Institute, Cary, North Carolina, USA).
Results
Population
During 1995 to 2002, 394 061 dogs contributed to a total denominator of 1 386 293 DYAR. At the end of 2002, 185 862 dogs were still alive. The dogs were of 338 breed designations, of which the 50 most common are listed in Table I. Each dog contributed < 0.01 to 8 DYAR (median, 3.2; mean, 3.5). Overall, 40 840 dogs died with an assigned diagnosis. The overall mortality rate was 295 deaths per 10 000 DYAR (95% CI, 292 to 297).
Table I.
The 50 breeds with more than 7000 dog-years at risk (DYAR) represented in the Swedish study populationa
| American cocker spaniel | Irish setter |
| Beagle | Jack Russell terrier |
| Bearded collie | Jämthund |
| Bernese mountain dog | Labrador retriever |
| Bichon frisée | Miniature schnauzer |
| Border collie | Mongrel |
| Border terrier | Münsterländer |
| Boxer | Newfoundland |
| Cairn terrier | Nova Scotia duck tolling retriever |
| Cavalier King Charles spaniel | Papillon |
| Collie, roughhaired | Petite Basset Griffon |
| Dachshund, miniature | Poodle |
| Dachshund, normal size | Rottweiler |
| Dalmatian | Samoyed |
| Doberman | Shetland sheepdog |
| Drever | Shih-tzu |
| Elkhound | Soft-coated wheathen terrier |
| English cocker spaniel | Standard poodle |
| English springer spaniel | Standard schnauzer |
| Finnish hound | Swedish hound |
| Finnish spitz | Tervuerense |
| Flat-coated retriever | Tibetian spaniel |
| German pointer | Wachteldog |
| German shepherd | West Highland white terrier |
| Golden retriever | Yorkshire terrier |
Data source (for each table): Agria Insurance, Stockholm, Sweden.
Descriptive analyses of bone tumor occurrence
Of the 764 dogs with either a veterinary-care claim or a life-insurance claim related to a diagnosis of malignant bone tumor, 330 (43%) had a specific osteosarcoma diagnosis; the 2 categories were considered the same in the presented analyses. Of the 764 dogs, 179 (23%) had only veterinary-care claims, 256 (34%) only life-insurance claims, and 329 (43%) claims of both insurance types. The median age at the 1st claim was 7.8 y; the 10th and 90th percentiles were 3.7 and 9.5 y. Among the 764 dogs, 83 different diagnoses were used for the tumors. Table II shows the anatomic location of the tumors as recorded in the database.
Table II.
Anatomic location of malignant bone tumors diagnosed between 1995 and 2002 in the same population
| Location | No. (and %)a of dogs (n = 764) |
|---|---|
| Appendicular | |
| Radius/ulna | 112 (15) |
| Humerus | 109 (14) |
| Femur | 78 (10) |
| Tibia/fibula | 66 (9) |
| Phalangeal | 60 (8) |
| Scapula | 13 (2) |
| Tarsal | 13 (2) |
| Carpal | 11 (1) |
| Metacarpal | 10 (1) |
| Metatarsal | 5 (1) |
| Patella | 4 (1) |
| Subtotal | 475 (62) |
| Axial | |
| Spine | 72 (9) |
| Mandible | 46 (6) |
| Skull (incl. maxilla) | 45 (6) |
| Ribs | 25 (3) |
| Pelvis | 24 (3) |
| Subtotal | 211 (28) |
| Unspecified/multiple bones | 97 (13) |
Each location is represented once per affected dog. The numbers add up to more than the subtotals and total, and the percentages to more than 100, because a few dogs had tumors in more than 1 location.
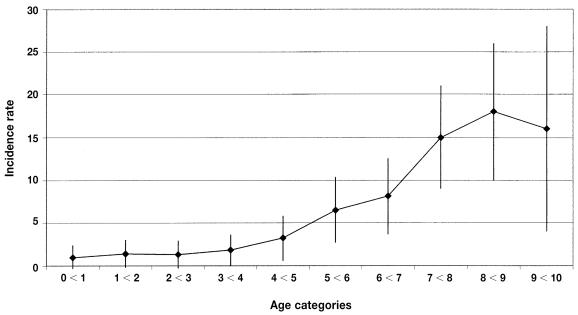
The incidence rate of bone tumors was 5.5 cases per 10 000 DYAR (95% CI, 5.1 to 5.9). Table III demonstrates that the CIs most often overlapped when the rates were stratified by sex or geographic location of residence. However, south–other and north–other had nonoverlapping CIs, and the CIs for sex just intersected. Table IV presents breed-specific incidence rates for the 15 breeds with the highest risk, all above the average incidence rate, among the 56 breeds with at least 10 cases of bone tumor or more than 7000 DYAR. The overall incidence rate in this 56-breed group was 5.8 cases per 10 000 DYAR (95% CI, 5.4 to 6.3; 661 cases; 1 130 501 DYAR). No cases of bone tumor were registered in 4 breeds: the Finnish spitz, the petite basset griffon, the American cocker spaniel, and the shih-tzu. Figure 1 shows the age-specific incidence rates of bone tumors for the entire study population.
Table III.
Incidence rates per 10 000 DYAR of malignant bone tumors in the same population, by sex and geographic location of residence
| Variable | DYAR | No. of dogs | Rate (95% CI) |
|---|---|---|---|
| Sex | |||
| Male | 686 039 | 416 | 6.1 (5.5–6.6) |
| Female | 700 255 | 348 | 5.0 (4.4–5.5) |
| Residence | |||
| South–urban | 146 792 | 90 | 6.1 (4.9–7.4) |
| South–other | 432 611 | 256 | 5.9 (5.1–6.6) |
| Middle–urban | 181 934 | 113 | 6.2 (5.1–7.4) |
| Middle–other | 454 888 | 243 | 5.3 (4.7–6.0) |
| North–other | 170 068 | 62 | 3.6 (2.7–4.6) |
CI — confidence interval.
Table IV.
Incidence rates per 10 000 DYAR of malignant bone tumors in the 15 breeds at highest risk out of the 56 breeds with more than 7000 DYAR or at least 10 cases of malignant bone tumors
| Breed | DYAR | No. of dogs | Rate (95% CI) | Ranka | Median age (y)b |
|---|---|---|---|---|---|
| Irish wolfhound | 2316 | 23 | 99 (59–140) | 1 | 6.6 |
| St. Bernard | 2437 | 19 | 78 (43–113) | 2 | 7.3 |
| Leonberger | 6843 | 36 | 53 (35–70) | 4 | 7.2 |
| Great Dane | 4236 | 19 | 45 (25–65) | 3 | 7.2 |
| Rottweiler | 23 392 | 85 | 36 (29–44) | 5 | 7.9 |
| Flat-coated retriever | 21 483 | 75 | 35 (27–43) | 7 | 8.6 |
| Greyhound | 4635 | 14 | 30 (14–46) | 6 | 6.2 |
| Hovawart | 4363 | 12 | 28 (12–43) | 9 | 6.9 |
| Bernese mountain dog | 12 707 | 33 | 26 (17–35) | 8 | 7.3 |
| Doberman | 7484 | 18 | 24 (13–35) | 10 | 7.5 |
| Newfoundland | 7825 | 17 | 22 (11–32) | 11 | 8.2 |
| Boxer | 12 913 | 17 | 13 (7–19) | 12 | 8.2 |
| Standard schnauzer | 8203 | 7 | 9 (2–15) | 13 | 9.0 |
| Irish setter | 10 508 | 8 | 8 (2–13) | 14 | 8.6 |
| Golden retriever | 86 955 | 49 | 6 (4–7) | 20 | 7.1 |
Frailty rank from Cox regression.
At the time of the 1st insurance claim related to the tumor.
Figure 1.
Age-specific incidence rates (number of cases per 10 000 dog-years at risk), with 95% confidence intervals, for bone tumors in a Swedish population of dogs with insurance for veterinary care and life insurance during 1995 to 2002. Data source: Agria Insurance, Stockholm, Sweden.
Survival to diagnosis
By the ages of 6, 8, and 10 y, 0.13% (95% CI, 0.11 to 0.14), 0.30% (95% CI, 0.27 to 0.32), and 0.64% (95% CI, 0.60 to 0.69), respectively, of the study population had a diagnosis of a malignant bone tumor. Table V shows the proportions by sex and by breed for the 9 breeds with at least 18 recorded cases of bone tumors.
Table V.
Crude, sex-specific, and breed-specific proportions with a diagnosis of malignant bone tumor at certain ages among the 9 breeds with at least 18 dogs with bone tumors
| Age (y); proportion (and 95% CI)
|
|||
|---|---|---|---|
| 6 | 8 | 10 | |
| All | 0.13 (0.11–0.14) | 0.30 (0.27–0.32) | 0.64 (0.60–0.69) |
| Male | 0.14 (0.11–0.17) | 0.33 (0.29–0.38) | 0.72 (0.65–0.79) |
| Female | 0.11 (0.09–0.14) | 0.26 (0.22–0.30) | 0.57 (0.51–0.63) |
| Irish wolfhound | 3.2 (1.1–5.2) | 6.8 (3.6–9.9) | 12.3 (6.6–17.6) |
| St. Bernard | 2.1 (0.4–3.7) | 4.0 (1.6–6.3) | 8.2 (4.5–11.8) |
| Leonberger | 0.9 (0.2–1.5) | 3.4 (2.1–4.8) | 7.7 (4.8–10.5) |
| Great Dane | 0.8 (0–1.6) | 4.6 (2.1–7.0) | 8.5 (4.4–12.4) |
| Rottweiler | 0.5 (0.2–0.8) | 2.2 (1.6–2.9) | 5.5 (4.3–6.7) |
| Flat-coated retriever | 0.3 (0.1–0.6) | 1.1 (0.7–1.5) | 4.1 (3.2–5.0) |
| Bernese mountain dog | 0.7 (0.3–1.1) | 1.8 (1.1–2.6) | 3.6 (2.2–5.0) |
| Doberman | 0.9 (0.2–1.5) | 1.6 (0.7–2.5) | 3.0 (1.5–4.5) |
| Golden retriever | 0.2 (0.1–0.3) | 0.3 (0.2–0.4) | 0.6 (0.4–0.7) |
Survival after diagnosis
Of the 764 cases of malignant bone tumor, death was registered within the study period in 636 (83%), 585 of which had a life-insurance claim related to the bone tumor, 47 a life-insurance claim not related to the tumor, and 4 no claim. For the 419 dogs that survived at least 1 d, the median survival time from the 1st bone tumor diagnosis was 56 d (95% CI, 38 to 69 d; 294 deaths), 58 (95% CI, 36 to 102) d for males and 45 (95% CI, 35 to 67) d for females. The P-value for the log-rank test was 0.78, indicating that this apparent difference in survival between the sexes lacked statistical significance. For the 244 dogs that survived more than 30 d after the 1st bone tumor diagnosis, the median survival time was 274 d (95% CI, 180 d to 2.4 y; 126 deaths). The median survival time for the 82 dogs with a 1st bone tumor diagnosis at less than 5 y of age (52 deaths) was 86 d (95% CI, 28 d to 2.2 y), for the 154 dogs 5 to less than 8 y of age (118 deaths) 36 d (95% CI, 23 to 56 d), and for the 183 dogs aged 8 y or more (124 deaths) 69 d (95% CI, 49 to 104 d). The P-value for the overall log-rank test was 0.02, demonstrating a significant difference in survival among the age groups. Pairwise comparisons demonstrated that the survival in the middle age group was lower than that in the youngest group (P = 0.009).
The breed-specific median survival times for the 4 selected high-risk breeds were as follows: rottweiler, 17 d (95% CI, 12 to 39 d; no. of dogs, 45; no. of deaths, 38); the flat-coated retriever, 60 d (95% CI, 17 to 104 d; no. of dogs, 43; no. of deaths, 32); the Bernese mountain dog, 17 d (95% CI, 7 to 34 d; no. of dogs, 21; no. of deaths, 16); and the golden retriever, 69 d (95% CI, 20 d to 6.4 y; no. of dogs, 30; no. of deaths, 19).
Multivariable analysis
The dataset used for the multivariable model consisted of 316 864 dogs of 56 breeds, of which 661 had a recorded claim for malignant bone tumor. The log–log plots demonstrated that the proportional-hazards assumption was valid for sex and geographic location of residence. Sex was the only fixed covariate that had a significant effect on the hazard of bone tumors and therefore remained in the reduced model (P < 0.0001). A protective effect against bone tumors of −0.339 (standard error, 0.078) was estimated for females, yielding a hazard ratio of 0.71 (99% CI, 0.58 to 0.87). The frailty effect was significant (P < 0.0001), judging by the likelihood-ratio test of theta. The ranking of the survival frailties, adjusted for sex and age, are shown for the 15 breeds with the highest risk in Table IV. Plots of Martingale and deviance residuals against DYAR and covariates were judged to be satisfactory. There was a tendency towards larger negative residuals for males than for females. The Cox–Snell plot from the model without frailties provided a straight line, indicating a good fit.
Discussion
We decided to include cases with diagnoses explicitly stated as osteosarcoma, as well as cases that were defined only as malignant bone tumors, in the study sample because it is well accepted that at least 85% of all malignant bone tumors in dogs are osteosarcomas (1,15). The tumor locations and relative frequencies were in agreement with those in earlier reports (2,15,16). At first it can seem strange that the n for certain locations is high; however, tumors in, for example, the tarsus, patella, and carpal bones account for 1% to 2% each of the total and, hence, are still considered rare.
The overall incidence rate of bone tumors in this study was 5.5 cases per 10 000 DYAR, similar to the standardized incidence rate of 5.7 cases per 10 000 DYAR that was recently found in a British study based on insurance data (17). Dorn et al (18) found an incidence rate of 7.9 per 100 000 dogs, but both the period of study and the methodology were strikingly different from ours. We found that the incidence of bone tumors increased with age, as have others (19). However, our study had the limitation of ending at 10 y of age. Ru et al (19) reported that 829 of 3040 cases (27%) were in dogs over 10 y of age but that the odds ratio of bone tumors for dogs of this age was similar to that for dogs 8 to 10 y old. The result of using the studied population is that the overall incidence is not directly comparable to the incidence rates reported from most other studies. However, the age-stratified measure should be readily comparable to age-stratified measures from other studies.
The breeds with the highest risk of bone tumors in this study were similar to what has been found by others. However, we found a few high-risk breeds that were not included in the top-8 list of Ru et al (19): leonberger, flat-coated retriever, greyhound, hovawart, Bernese mountain dog, and Newfoundland. Misdorp and Hart (16) found an increased risk in large dogs, the most frequently encountered breeds being boxers, German shepherds, rottweilers, and Great Danes. We also calculated the proportion of dogs within a breed in which bone tumors developed up to 10 y of age; the proportion approached 10% in the Irish wolfhound and St. Bernard. However, in our experience, these estimates should be viewed with some caution, as they tend to slightly overestimate the diseased proportion when numerators are small, possibly by a few percentage points at age 10.Accordingly, the actual proportion of affected golden retrievers (with 49 cases) is more correct than the actual proportion of Irish wolfhounds (23 cases). The incidence of bone tumors in Labrador retrievers and German shepherds, 2 relatively large and high-denominator breeds, as well as the average incidence across all breeds, was close to that in the golden retriever. However, the list of high-risk breeds was cut at the golden retriever; hence, these data were not shown.
Sex was borderline significant in the univariable analysis, as judged by the 95% CIs. In the multivariable analysis, in which the effects of breed and age were taken into account, the hazard of bone tumors was significantly lower among females. The hazard ratio for being female in this study population was 0.71, meaning that at any given point in time the rate at which bone tumors were occurring was 29% lower in females than in males. Also, there was a significant difference between the sexes in the survival to bone tumor diagnosis at the age of 10 y. In most studies, males are considered overrepresented in cases of canine bone tumors, with a ratio of 1.5:1 (1,2,16,20), similar to the sex distribution of osteosarcoma in humans (21). Not all reports on dogs support the male predominance, however (19,22). Cooley et al (22) reported a 4-fold increase in the risk of osteosarcoma in gonadectomized rottweilers; the study included only 31% intact animals in the normal population (n = 597) and had a total of 86 osteosarcomas for both sexes. The authors suggested that, since sex steroids are essential for skeletal homeostasis, the alterations in circulating concentrations of gonadal hormones might influence skeletal oncogenesis. Jeusette et al (23,24) recently reported that neutering can lead to excessive body weight gain, in part because of hormonal alterations that affect both basal metabolism and appetite regulation. This might explain the apparent increased risk of bone tumors among gonadectomized dogs. Traditionally, explanations for increased risk of osteosarcoma are height and greater body weight (16,19), both of which are considered to be male phenotype characteristics. A general view is that osteosarcomas predominate in large breeds in weight-bearing bones and adjacent to late-closing physes. Multiple episodes of minor trauma can lead to proliferation of cells in the physeal regions and hence induce mitogenic signals and promote clonal expansion of mutant lineages (1). A limitation of the current study is the lack of documentation on neuter status. However, relatively few Swedish dogs are neutered (7% of females and 4% of males) (10), and neutering is often done late in life, as a consequence of disease.
Univariable analysis showed a slightly lower incidence rate in dogs living in the north–other area compared with the other 4 areas. However, this variable dropped out of the multivariable analysis. The reason for this observed difference might be the variation in breed distribution between regions of Sweden.
We found that the median survival time after the 1st recorded diagnosis of malignant bone tumor was almost 2 mo in the 419 dogs that survived at least 1 d. This estimate cannot be directly compared with similar figures obtained from clinical trials or case series data because the correctness of the diagnosis has not been validated and because it is not known what kind of treatment each dog received, if any. Furthermore, many dogs die or are euthanized shortly after the diagnosis is made. Taking these factors into consideration, the survival figure relates to how long this specific population survives after a diagnosis and should be regarded in that light.
Different treatment options are offered for dogs with osteosarcoma. The median survival time is reported to be 19 to 25 wk with amputation alone in appendicular osteosarcoma (5,25,26). The overall survival time can be markedly increased with the combination of surgery and a chemotherapeutic protocol, reaching a median of 1 year (1,27). In Sweden, the main treatment of choice after a confirmed diagnosis of osteosarcoma has been short-term palliation with analgesics only. This could explain the shorter survival reported in this study. However, the median survival was 274 d among the animals that did not die within the 1st 30 d after the 1st claim for bone tumor. This probably reflects a treatment effect in dogs that went through more invasive treatment. However, the applied treatment in each case was not included in the information available from the insurance database. Treatment outcome was not analyzed in this study, but the survival data further stress the severity of the diagnosis.
The population insured at Agria is mostly representative of the total Swedish dog population (10). The main exception is that older dogs and mongrels are less often insured than other dogs. The results from the current study can therefore likely be extrapolated to the general Swedish dog population, but only for dogs less than 10 y of age.
Each time this or any other secondary database is used to study a specific diagnosis, the validity of the diagnoses must be considered. Correctness can be defined as the proportion of recorded cases in which the animal actually has the disease in question; completeness can be viewed as the proportion of subjects with the disease of interest registered in the database (28). Overall, 84% of the recorded diagnoses in the Agria database were found to be correct when compared with the clinical records (29). With malignant bone tumors, veterinarians likely make a correct diagnosis, given that the animal is taken for examination. Further, most malignant bone tumors will be detected because the disease is severe, and the affected animals are likely to become seriously ill and die from metastatic disease if not treated. Therefore, we assumed that both correctness and completeness of the information regarding malignant bone tumors were good in the insurance database. We also assumed that the 1st recorded diagnosis of malignant bone tumor in the database was actually the 1st time the diagnosis had been made for a given dog, and we believe this to be accurate in many cases. Given that a veterinarian uses radiography, the cost of a visit will exceed the deductible, and the diagnosis will get registered in the database if a claim is submitted by the owner. The owner might have taken the dog for examination before this, but malignant bone tumor will probably not have been diagnosed, because radiography is the most common way to verify the diagnosis.
The Center for Cancer Research of the US National Cancer Institute recently launched its Comparative Oncology Program (ccr.nci.nih.gov/resources/cop/announcement.asp) to assist in clinical research on improving treatment outcomes for human cancers based on results in pet animal models. Everything from clinical appearance to biologic aspects will be investigated in canine osteosarcoma. A vital part of this multidisciplinary effort is to uniformly characterize the cancer phenotype in dogs and to narrow down the genetic diversity. A valuable tool is a reliable cancer epidemiology model that selects high-risk breeds in an unbiased way in relation to all breeds at risk. Few large epidemiologic studies of canine osteosarcoma have been performed (17,19), and conclusions are often drawn from studies performed at single veterinary teaching hospitals or from tumors induced in beagles (30,31). We used an insurance database to quantify the problem of malignant bone tumors in dogs, generating tumor risks from data for a large number of both cases and healthy dogs in a fairly unbiased base population. The most obvious limitations are lack of data on neuter status and that the dogs in the study sample were only up to 10 y of age.
Acknowledgments
This work was supported by grants from the Foundation for Research, Agria Insurance. We also thank Agria for allowing access to the insurance database.
References
- 1.Dernell WS, Straw RC, Withrow SJ. Tumors of the skeletal system. In: Withrow SJ, MacEwan EG, editors. Small Animal Clinical Oncology. 3. Philadelphia: WB Saunders; 2001. pp. 378–417. [Google Scholar]
- 2.Dernell WS. Tumours of the skeletal system. In: Dobson JM, Lascelles BD, editors. BSAVA Manual of Canine and Feline Oncology. 2. Quedgeley, England: British Small Animal Veterinary Association; 2003. pp. 179–195. [Google Scholar]
- 3.Dickerson ME, Page RL, LaDue TA, et al. Retrospective analysis of axial skeleton osteosarcoma in 22 large-breed dogs. J Vet Intern Med. 2001;15:120–124. doi: 10.1892/0891-6640(2001)015<0120:raoaso>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Hillers KR, Dernell WS, Lafferty MH, Withrow SJ, Lana SE. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986–2003) J Am Vet Med Assoc. 2005;226:1364–1367. doi: 10.2460/javma.2005.226.1364. [DOI] [PubMed] [Google Scholar]
- 5.Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988) J Am Vet Med Assoc. 1992;200:995–999. [PubMed] [Google Scholar]
- 6.Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. 1991;270:159–168. [PubMed] [Google Scholar]
- 7.Khanna C, Wan X, Bose S, et al. The membrane–cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 8.Egenvall A, Bonnett BN, Olson P, Hedhammar Å. Gender, age, breed and geographic pattern of morbidity and mortality in insured dogs during 1995 and 1996. Vet Rec. 2000;146:519–525. doi: 10.1136/vr.146.18.519. [DOI] [PubMed] [Google Scholar]
- 9.Egenvall A, Bonnett BN, Öhagen P, Olson P, Hedhammar Å, von Euler H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995–2002. Prev Vet Med. 2005;69:109–127. doi: 10.1016/j.prevetmed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Egenvall A, Hedhammar Å, Bonnett BN, Olson P. Survey of the Swedish dog population: age, gender, breed, location and enrollment in animal insurance. Acta Vet Scand. 1999;40:231–240. doi: 10.1186/BF03547021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenska djursjukhusföreningen (Swedish Animal Hospital Organisation) Diagnosregister för häst, hund och katt (Diagnostic Registry for the Horse, the Dog and the Cat) Taberg, Sweden: Tabergs Tryckeri; 1993. [Google Scholar]
- 12.Egenvall A, Bonnett BN, Olson P, Hedhammar Å. Gender, age and breed pattern of diagnoses for veterinary care events in insured dogs during 1996. Vet Rec. 2000;146:551–557. doi: 10.1136/vr.146.19.551. [DOI] [PubMed] [Google Scholar]
- 13.Breslow NE, Day NE, editors. Statistical Methods in Cancer Research. Vol II. The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. Rates and rate standardisation; pp. 48–79. [Google Scholar]
- 14.Dohoo I, Wayne M, Stryhn H, editors. Veterinary Epidemiologic Research. Charlottetown, PEI: AVC; 2003. Modelling survival data; pp. 409–457. [Google Scholar]
- 15.Jongeward SJ. Primary bone tumors. Vet Clin North Am Small Anim Pract. 1985;15:609–641. doi: 10.1016/s0195-5616(85)50061-3. [DOI] [PubMed] [Google Scholar]
- 16.Misdorp W, Hart AA. Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst. 1979;62:537–545. doi: 10.1093/jnci/62.3.537. [DOI] [PubMed] [Google Scholar]
- 17.Dobson JM, Samuel S, Milstein H, Rogers K, Wood JLN. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–246. doi: 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 18.Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318. [PubMed] [Google Scholar]
- 19.Ru G, Terracini B, Glickman LT. Host related risk factors for canine osteosarcoma. Vet J. 1998;156:31–39. doi: 10.1016/s1090-0233(98)80059-2. [DOI] [PubMed] [Google Scholar]
- 20.Brodey RS, Abt DA. Results of surgical treatment in 65 dogs with osteosarcoma. J Am Vet Med Assoc. 1976;168:1032–1035. [PubMed] [Google Scholar]
- 21.Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286–291. doi: 10.1097/01.blo.0000145991.65770.e6. [DOI] [PubMed] [Google Scholar]
- 22.Cooley DM, Beranek BC, Schlittler DL, Glickman NW, Glickman LT, Waters DJ. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1434–1440. [PubMed] [Google Scholar]
- 23.Jeusette I, Detilleux J, Cuvelier C, Istasse L, Diez M. Ad libitum feeding following ovariectomy in female beagle dogs: effect on maintenance energy requirement and on blood metabolites. J Anim Physiol Anim Nutr. 2004;88:117–121. doi: 10.1111/j.1439-0396.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeusette I, Daminet S, Nguyen P, et al. Effect of ovariectomy and ad libitum feeding on body composition, thyroid status, ghrelin and leptin plasma concentrations in female dogs. J Anim Physiol Anim Nutr. 2006;90:12–18. doi: 10.1111/j.1439-0396.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990) J Am Vet Med Assoc. 1992;200:531–533. [PubMed] [Google Scholar]
- 26.Mauldin GN, Matus RE, Withrow SJ, Patnaik AK. Canine osteosarcoma. Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J Vet Intern Med. 1988;2:177–180. doi: 10.1111/j.1939-1676.1988.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 27.Lascelles BD, Dernell WS, Correa MT, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. 2005;12:1073–1083. doi: 10.1245/ASO.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Jordan K, Porcheret M, Croft P. Quality of morbidity coding in general practice computerized medical records: a systematic review. Fam Pract. 2004;21:396–412. doi: 10.1093/fampra/cmh409. [DOI] [PubMed] [Google Scholar]
- 29.Egenvall A, Bonnett BN, Olson P, Hedhammar Å. Validation of computerized Swedish dog and cat insurance data against veterinary practice records. Prev Vet Med. 1998;36:51–65. doi: 10.1016/s0167-5877(98)00073-7. [DOI] [PubMed] [Google Scholar]
- 30.Shyr LJ, Muggenburg BA. A comparison of the predicted risks of developing osteosarcoma for dogs exposed to 238PuO2 based on average bone dose or endosteal cell dose. Radiat Res. 1992;132:13–18. [PubMed] [Google Scholar]
- 31.Langenbach A, Anderson MA, Dambach DM, Sorenmo KU, Shofer FD. Extraskeletal osteosarcomas in dogs: a retrospective study of 169 cases (1986–1996) J Am Anim Hosp Assoc. 1998;34:113–120. doi: 10.5326/15473317-34-2-113. [DOI] [PubMed] [Google Scholar]