INTRODUCTION
What is Chronic Myeloid Leukaemia?
Chronic Myeloid Leukaemia (CML) is a clonal, myeloproliferative disease that develops when a single, pluripotential, haemopoetic stem cell acquires the Philadelphia chromosome. CML was the first haematological malignancy to be associated with a specific genetic lesion. First recognised in 1845, CML exhibits a consistent chromosomal abnormality in leukaemic cells, identified in 1960 by Nowell and Hungerford, termed the Philadelphia (Ph) chromosome1. The cytogenetic hallmark of CML was identified in 1973 as the reciprocal translocation t(9;22)(q34:11). Furthermore, in 1984, the ABL (Abelson) proto-oncogene was identified as being involved in this translocation. Breakthroughs in cancer biology have led to extensive characterisation of CML and it is now heralded as a ‘model’ of cancer2.
The haemopoietic cell lines are transformed by the chimeric oncogene BCR-ABL. CML is an unusual malignancy in that a single oncogene product is central to its pathology1. CML is capable of expansion in both the myeloid or lymphoid lineages, and may involve myeloid, monocytic, erythroid, megakaryocytic, B-lymphoid and occasionally T-lymphocytic lineages, although expansion is predominantly in the granulocyte compartment of the myeloid lineages in the bone marrow3.
Epidemiology of CML
The incidence of CML is approximately 1-2 per 100,000 population per year. Consistent with this, there are 10-12 new cases of CML in Northern Ireland each year. The median age of presentation is 45 to 55 years, accounting for 20% of leukaemia affecting adults. As with all leukaemias, males are affected more than females in CML, with a 2:1 ratio. CML is more common with Caucasian ethnicity3.
Natural History and Clinical Course
The clinical course of the disease may be divided into three main sections4, (Table I). Signs and symptoms at presentation may include fatigue, weight loss, abdominal fullness, bleeding, purpura, splenomegaly, leukocytosis, anaemia, and thrombocytosis3. In approximately 50% of cases it is an incidental finding.
Table I.
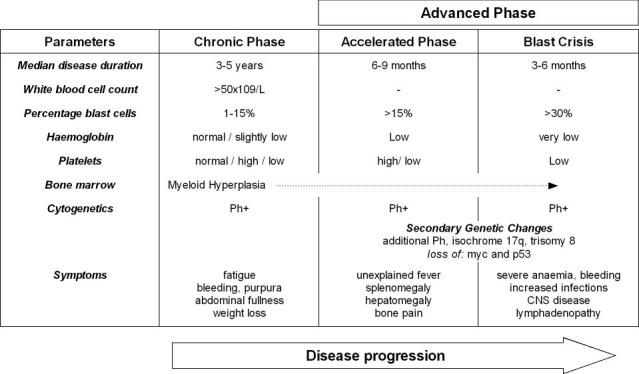 |
The Ph chromosome is present in 95% of patients with classic CML. The impetus for Ph chromosome formation and the time span required for overt disease progression are unknown. It is proposed that CML, similar to many other neoplasms, may be the result of a multistep pathogenetic process. There is very little evidence to support any additional acquired molecular aberrations prior to t(9;22) translocation6. It is generally accepted that the Ph+ clone is susceptible to the acquisition of additional molecular changes that may underlie disease progression. The Ph chromosome is generally the only cytogenetic abnormality present in the chronic phase of disease. Approximately 85% of patients are diagnosed in chronic phase, and this stage of disease responds to therapy4. As the disease progresses through the accelerated phase and into the blast crisis, additional cytogenetic abnormalities become evident (see Table I)7.
MOLECULAR PATHOLOGY
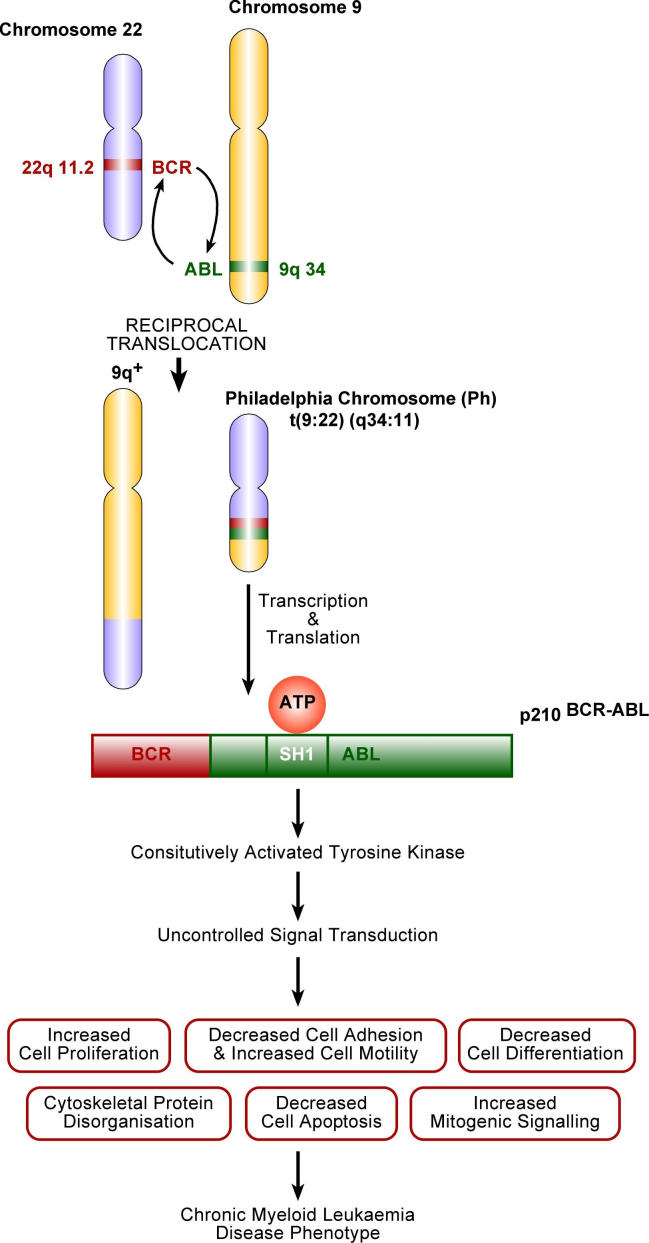
Classic CML is characterised by a reciprocal translocation between chromosomes 9 and 22. This results in juxtaposition of 3′ sequences from the Abl-proto-oncogene on chromosome 9, with the 5′ sequences of the truncated Bcr (breakpoint cluster region) on chromosome 22. Fusion mRNA molecules of different lengths, are produced and subsequently transcribed into chimeric protein products, with varying molecular weights, the most common being p210 BCR-ABL (Fig 1)3.
Figure 1.
Molecular events leading to the expression of CML disease phenotype.
The SH1 domain of ABL encodes a non-receptor tyrosine kinase. Protein kinases are enzymes that transfer phosphate groups from ATP to substrate proteins, thereby governing cellular processes such as growth and differentiation. Tight regulation of tyrosine kinase activity is essential, and if not maintained, deregulated kinase activity can lead to transformation and malignancy1.
The portion of ABL responsible for governing regulation of the SH1 domain is lost during the reciprocal translocation. The addition of the BCR sequence constitutively activates the tyrosine kinase activity of the SH1 domain.
Its activity usurps the normal physiological functions of the ABL enzyme, as it interacts with a number of effector proteins7. Thus, the SH1 domain of BCR-ABL is the most crucial for oncogenic transformation.
Cellular Signalling
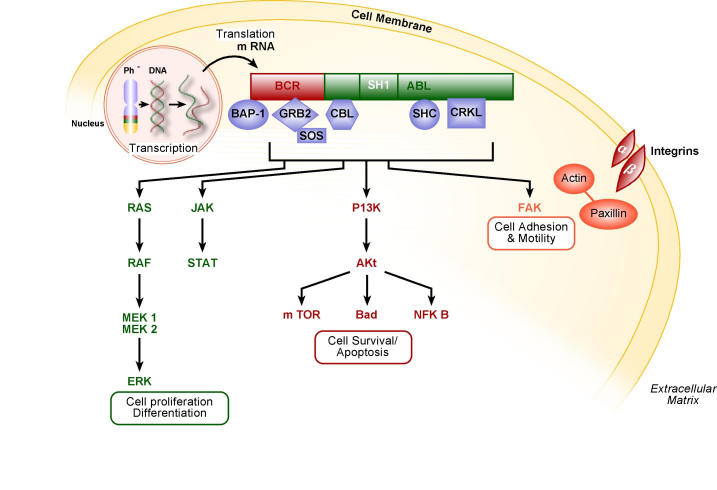
BCR-ABL has several substrates and impacts on key signalling pathways resulting in the CML phenotype6. The net result is deregulated cellular proliferation and development of growth factor independence, decreased adherence of the leukaemic cells to the bone marrow stroma, and a reduced apoptotic response to mutagenic stimuli (Figs 1 and 2)1.
Figure 2.
BCR-ABL signalling pathways.
CONVENTIONAL CYTOGENETICS
Cytogenetics is the genetic analysis of cells and assesses the structural integrity of chromosomes. The Ph chromosome, discovered in 1960, was identified as the smaller of the two chromosomes derived from a reciprocal translocation involving chromosomes 9 and 22. This translocation can be found in more than 95% of CML patients at diagnosis. CML was the first disease in which the cytogenetic abnormality was defined on a molecular basis and such work pioneered the combination of molecular cloning and hybridization techniques to produce fluorescence in situ hybridization (FISH)8,9. FISH uses specific fluorescently tagged DNA probes to map the chromosomal location of genes and identify other genetic anomalies. This technique can be applied in all stages of the cell cycle (interphase cytogenetics). This assay is based on the ability of single stranded DNA to hybridize to complementary DNA. FISH can be performed with substrates such as blood, bone marrow, body fluids, tissue touch preparation and paraffin embedded fixed tissue9.
FISH assays are relevant particularly at diagnosis and in relapse, when a large pool of affected cells are present. This is due to the inherent low levels of sensitivity with FISH; at best, sensitivities are within the range of 1 malignant cell in every 100 normal cells. Bone marrow and peripheral blood samples are used to diagnose CML by the presence of Ph chromosome. It is unacceptable to use FISH to detect minimal residual disease following therapy8,9.
Polymerase chain reaction (PCR) analysis is used at CML diagnosis. PCR is used to detect the m-RNA that encodes for the chimeric BCR-ABL protein in bone marrow and peripheral blood samples. As PCR is more sensitive than FISH it can be used at diagnosis and in monitoring response to treatment9,10.
MOLECULAR DIAGNOSTICS
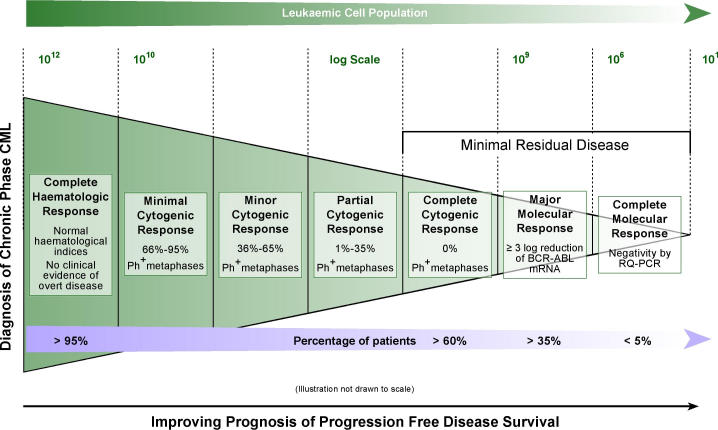
Molecular techniques are used in the diagnosis and monitoring response to therapy. Response to treatment may be defined as occurring at haematologic, cytogenetic, or molecular levels11,12. This is illustrated in Figure 3.
Figure 3.
Defining response to treatment and minimal residual disease, for patients diagnosed with chronic phase CML, treated with imatinib.
Minimal Residual Disease
On current therapeutic regimens a complete cytogenetic response can be achieved for the majority of patients (Fig 3), but a small proportion of these will relapse. Relapse arises from a persistent malignant cellular population present at a low level, below the level of detection by standard techniques. This reservoir of neoplastic cells detected only by sensitive molecular methods is referred to as minimal residual disease (MRD)12. Methods for detecting MRD, should ideally have sensitivity within the 105 to 106 range, be applicable for almost all patients with the disease, provide information on the target, be inexpensive, rapid, readily standardized and disease specific. Additionally, to utilise results effectively good interlaboratory reproducibility and standardisation of reporting is essential. Measuring patient response to imatinib may be achieved by conventional quantitative real-time PCR (RQ-PCR) or nested PCR. Analysis with RQ-PCR detects up to 1 in 104–105 cells and nested PCR 1 malignant cell in 106 normal cells9,10. MRD may be designated as values below 109 to 1010. Clinical observation and experience implies a positive correlation between the improving levels of molecular response and better progression-free disease survival12.
RQ-PCR is used to monitor for MRD in patients that have achieved a complete cytogenetic response. This procedure is more amenable to interlaboratory standardisation, and has been introduced as it facilitates rapid and sensitive detection of the fusion gene transcript showing comparable results when simultaneous analysis has been performed on blood and bone marrow specimens, allowing follow up of imatinib treated CML patients9,13,14.
European laboratories from 10 countries have collaborated to establish a standardized protocol for TaqMan-based RQ-PCR, in an effort to analyze the prominent leukaemia-associated fusion genes (including BCR-ABL) within the Europe Against Cancer (EAC) program. The EAC protocol has the potential to provide the basis for an international reference of MRD using RQ-PCR analysis of fusion gene transcripts15. The Department of Haematology at Queens University, Belfast, have been completing analysis of CML patient samples using these set protocols.
DISEASE MANAGEMENT
Allogenic Stem Cell Transplants
Allogenic stem cell transplant (allo-SCT) has been used since the 1970s in the treatment of CML1 and is the only curative therapy for CML, however, it bears a significant mortality risk. Age, disease status, disease duration, recipient-donor gender combinations, degree of histocompatability between donor and recipient and the source of the transplant product have all been identified as significantly influencing long-term survival. Evidence in the pre imatinib era suggests that bone marrow transplant is best performed in the early phase of chronic CML1,16. Using blood or bone marrow derived stem cells from an HLA-identical sibling performed in the chronic phase of the disease offers a 60-80% probability of leukaemia-free survival at 5 years. If performed in the accelerated phase, disease survival decreases by half17.
Conventionally, conditioning treatments are necessary prior to allo-SCT. This involves ‘myeloablative’ doses of chemoradiotherapy, aiming to facilitate engraftment of healthy donor stem cells via permanent elimination of malignant haematopoiesis. This is a rather arduous regimen associated with toxicity and mortality. It is therefore preferably administered to those aged less than 65 years without other co-morbid conditions. Success is generally attributed to an immunologically mediated graft-versus-leukaemia effect7.
Bone marrow transplants have seen recent developments in research. Reduced intensity conditioning treatments (RICT) or non-myeloablative transplants have been proposed. This endeavours to produce graft-versus-leukaemia effects without exposing the patient to the potential toxicity of conditioning treatments. Here, reconstitution of the immune system and associated anti-leukaemia effect of the donor graft, compete against the growth of the malignancy. Preliminary data suggests that this approach may confer benefit, particularly in chronic phase CML16.
Interferon Alpha
Interferon alpha (INFα), is a glycoprotein, of biological origin. It displays antiviral and antiproliferative properties. INFα was the first effective therapy for CML. The drug entered clinical trials in the early 1980s, and remained the treatment of choice for CML patients, until a shift in therapeutic strategy after the arrival of imatinib18. In CML INFα prolongs survival in patients, especially of those who are cytogenetic responders. It is able to induce a cytogenetic response in 35 to 55% of patients, with a longer survival achievable in combination with chemotherapy. With this therapy the level of disease decreased with time, but CML was rarely completely eliminated16.
Imatinib Mesylate
The BCR-ABL protein is an ideal drug target for CML treatment. Unique to leukaemic cells, the BCR-ABL protein is expressed at high levels and its tyrosine kinase activity of the SH1 domain is essential for its ability to induce CML7. The SH1 domain responsible for oncogenic transformation is an extremely attractive target in combating CML.
The most successful synthetic ATP inhibitor designed was imatinib mesylate (STI 571, Gleevec (Glivec), Novartis, Switzerland), approved by the Food and Drug Administration in May 2001 in the United States, later licensed for use in the UK by the European Medicines Evaluation Agency (EMEA) in November 2001 for the treatment of CML6,19. The introduction of this drug has dramatically changed the management of CML20. It is currently considered as the ‘gold standard’ in treating CML, approved for the first line treatment of adult patients with Ph+ CML at all disease stages21,22.
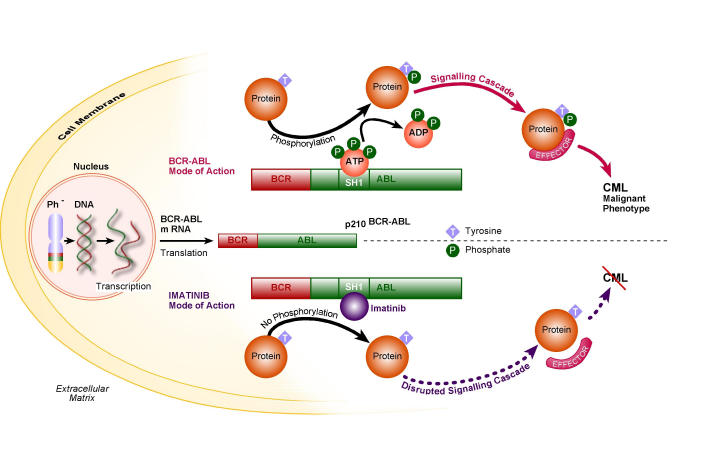
Imatinib functions as a mimic of ATP, in the ATP binding pocket in the BCR-ABL SH1 domain (Fig 4). A further characteristic of imatinib is its striking degree of specificity for the ATP binding pocket, as its effect on other cellular tyrosine kinases is negligible19,23.
Fig 4.
Comparing the mode of action of BCR-ABL and imatinib in CML pathogenesis.
In the treatment of chronic phase CML, imatinib produces a superior and sustainable response compared to INFα. The IRIS study (International Randomised Study of Interferon and STI571), a Phase III clinical trial, compared the use of imatinib and conventional drugs used in the treatment of patients with newly diagnosed CML. Conventional drugs included recombinant INFα, and low dose cytarabine having demonstrated superior rates of cytogenetic response and survival than interferon monotherapy. The results of this trial concluded that the haematologic and cytogenetic responses in terms of tolerability and likelihood of progression to accelerated or blast phase CML, provided superior results with imatinib24–26.
Imatinib has produced a sustained cytogenetic response in the majority of patients and it is clinically well tolerated. The advantages of imatinib therapy have lead to the revision of allo-SCT protocol, even in patients who may be good allo-SCT candidates. Clinicians are currently recommending that all newly diagnosed patients are treated with imatinib. Only upon failure to respond satisfactorily on imatinib will allo-SCT be considered in suitable candidates.
Imatinib Resistance
Despite its remarkable efficacy in treating CML, secondary resistance is emerging in a minority of patients. This involves the emergence of a resistant leukaemic clone after regular drug administration27–29.
Primary or intrinsic resistance differs, and is relatively less common in its incidence. It may be defined by a lack of haematologic or cytogenetic response, treatment having had negligible effects since initiation. It is uncommon in chronic phase CML, as is secondary resistance. In accelerated phase of CML primary resistance is relatively common, whilst in accelerated or indeed blast phase it is the rule, as is acquired resistance29–31.
Acquired resistance to imatinib therapy is caused most commonly by mutations in the BCR-ABL kinase domain, thus preventing imatinib binding sucessfully. A frequent mutation in this domain, conferring a particularly poor prognosis, is in the ATP phosphate binding loop (P-loop). This is a highly conserved domain involved in ATP binding32. Further mechanisms of secondary resistance involve over expression of BCR-ABL; acquired additional mutations, clonal evolution, that is the addition of novel chromosomal aberrations, and pharmacological mechanisms, resulting in a reduction in the quantity of available unbound imatinib, resulting in suboptimal levels of imatinib for effect27,31.
Monitoring treatment response
The advent of imatinib therapy has added significantly to the cohort of patients in whom a complete cytogenetic response is achieved. It would therefore be logical to utilize molecular assays in monitoring treatment response. Indeed, molecular monitoring has become routine in CML management33. The aim of monitoring therapy is to identify sub-optimal responders to imatinib therapy and to consider alternative approaches to management in an effort to prolong progression-free disease survival16.
Studies using RQ-PCR have shown that an early reduction of BCR-ABL gene transcript levels can predict a subsequent cytogenetic response in CML26,34. Once patients achieve MRD status (Fig 3), it is important to continue monitoring closely. The determination of the trend in the quantitative numbers of residual BCR-ABL positive cells is considered to provide important therapeutic information in the follow up of CML patients, providing key prognostic information allowing treatment optimization15.
Branford, et al.35, concluded from their research that a more than two fold rise in BCR-ABL levels by RQ-PCR identified 97% of patients with BCR-ABL domain kinase mutations. Therefore, monitoring levels of BCR-ABL could potentially serve as an early indicator or predictor of relapse and precipitant for reassessment of therapeutic management, identifying patients for whom imatinib may not be the best form of long term treatment1,2.
Additionally, it has been documented that a few CML patients are beginning to exhibit clonal karyotypic abnormalities in Phnegative cells whilst completing imatinib therapy. Emergence of such events strongly elude that there is a requirement for intermittent bone marrow cytogenetic analysis9,36.
This prompts the question of how patients with CML should be monitored. Principle laboratory tests used in monitoring CML drug therapy are peripheral blood counts, cytogenetic analysis, RQ-PCR, and assessment of ABL kinase domain mutations. It is accepted that early treatment of disease relapse should translate into a greater response rate2,9,37,38. Use of such an approach will require multicentre standardisation of RQ-PCR and mutation analysis2. Provisional recommendations in this area have been made. These include proposals for implementing internationally standardised methodologies for measuring and recording BCR-ABL transcript levels in patients currently undergoing treatment using RQ-PCR; and reporting and detecting BCR-ABL kinase domain mutations36.
Molecular mutations can be used to monitor treatment response and disease progression. To date haemopoietic stem cell transplantation is the only proven cure16. Of the third of CML patients in whom this therapy is both feasible and appropriate, a majority achieve the status of molecular remission. The remainder of patients may have residual but stable levels of BCR-ABL transcripts. If we are comparing non transplant therapy with allotransplant, the endpoint for each must also be directly comparable, thus molecular remissions must be the goal. This further emphasises the necessity for standardisation of methodology and reporting in monitoring CML treatment response33.
Allo-immunity may be a factor in preventing disease relapse in allo-SCT. Imatinib confers no such benefit in its subjects treated to MRD or molecular response, and so cannot guarantee that it can maintain patients in this state indefinitely. However with the excellent response of newly diagnosed patients to imatinib, there has been a reluctance to consider allo-SCT treatment7. It is therefore essential that emerging resistance is recognised early, permitting timely consideration of transplant options if appropriate, before overt progression of CML30,35,38,39. It would therefore be prudent to set conservative targets for therapeutic achievements to facilitate prompt reassessment of suboptimal therapy. A modest strategy has been proposed, suggesting; complete haematologic response at 3 months, minor cytogenetic response at 6 months, major cytogenetic response at 12 months, and a complete cytogenetic response at 18 months11. Failure to meet these criteria would warrant a subsequent re-assessment of disease management.
Strategies to Overcome Imatinib Resistance
Imatinib resistance has been postulated to develop more rapidly and uniformly than other examples of cytotoxic drugs because of its high specificity for its target20. Several strategies have been proposed to overcome imatinib resistance.
Firstly, early treatment with imatinib upon diagnosis is considered crucial. Patients who are treated with imatinib within four years of initial diagnosis of CML, have a better prognosis and a significantly lower incidence of mutations than those treated outside the four year time frame. In addition to prompt administration of imatinib an adequate dose is necessary. The lowest approved dose is 400mg daily in chronic phase CML, in advanced stage 600mg daily14. A second strategy is imatinib dose escalation31,40.
Thirdly, combination therapy may be considered. Despite the excellent results achievable with imatinib, only 5-10% of such patients achieve a molecular remission, that is, undetected BCR-ABL transcripts. There is therefore a rationale for combining therapies effective against CML to try and improve the efficacy of therapy. Conceivably, resistance to imatinib may be caused by more than one mechanism in each cell41,42.
By targeting CML cells with combination therapies cross resistance would presumably be prevented and therapeutic performance improved as disease would be tackled by a number of different means.
The two best non transplant therapies approved for use in CML are INFα and imatinib. It would be reasonable to combine both agents to assess if response rates could be improved. One such study that considered the merits of combining imatinib with pegylated interferon was the PISCES trial (PEGIntron and Imatinib Combination Evaluation Study). In this Phase I/II study preliminary results showed that this dual therapy had improved activity over imatinib alone and was clinically well tolerated. Unfortunately, myelosupression was common. Further data would be necessary to confirm these findings, requiring a large, prospective, randomised study7.
The SPIRIT trial (STI571 Prospective International Randomised Trial) is currently underway. This Phase III study will compare the administration of imatinib at escalated doses of 400 mg/day, 800mg/day and imatinib at 400mg/day with interferon and low dose cytarabine, involving patients who have chronic phase CML, having been diagnosed within a three month time span7.
Second generation ABL kinase inhibitors
Imatinib has had unprecedented success in the treatment of CML. Despite its capability to achieve clinical remission, disease has progressed in a small minority. Progression made in IRIS is very slow and it is no longer a randomised control study. Few patients remain on the control arm of the study; IRIS follow-up may now be considered a long term imatinib follow-up study. Relapsing patients require alternative therapies, and with time the net number of such patients will increase. Whilst imatinib has proven efficacious, alternatives are now required in some patients. Figure 3 demonstrates a minority of patients will achieve a molecular response with imatinib. The remaining majority of patients still have an existing pool of approximately 106-107 leukaemic cells, from which relapse is a possibility, even in controlled disease43,44.
Imatinib is now the keystone of disease management, and a model upon which future drug development is based, largely due to the contribution that structural biology has made in understanding imatinib resistance. This has aided the design of new kinase-inhibitors43, leading to two alternative types of compound.
Nilotinib (AMN107)
Strategy one involved the modification of imatinib structure. Nilotinib (developed by Novartis) is similar to its cousin imatinib as they both bind to an inactive conformation of the ABL kinase domain and function as an ATP inhibitor. There are a number of ways in which they differ. Nilotinib is capable of binding more tightly to BCR-ABL protein to enhance drug efficacy and sensitivity. Most BCR-ABL mutants are 20-fold more sensitive to nilotinib43–45. The exception to this rule is the mutant T315I46,47. Furthermore, with its superior topographical fit to the ABL protein, nilotinib proves to be more potent than imatinib.
A Phase I clinical trial with nilotinib demonstrated rates of complete haematologic response in imatinib resistant patients to be 92% in chronic phase, 75% in accelerated phase, 39% in blast phase. Cytogenetic responses were 35%, 55% and 27%, respectively48. Phase II studies are ongoing. With success in refractory CML recognised, further study should be focussed to evaluate if nilotinib has therapeutic potential at all stages of disease49.
Dasatinib (BMS-354825)
Strategy two involved preparing a compound with a completely different chemical structure to imatinib. This was based upon a drug originally synthesised as a primary Src family inhibitor. Dasatinib (developed by Bristol-Myers Squibb) was observed to inhibit wild type BCR-ABL and most resistant imatinib mutations43.
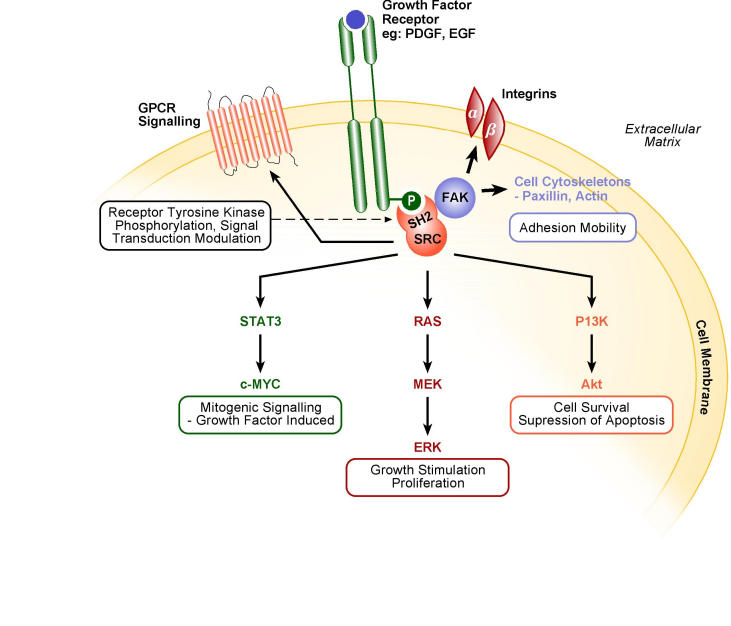
Src is a non-receptor tyrosine kinase that has a plethora of roles in cell signalling including cellular adhesion, motility and growth. Many substrates that Src is capable of phosphorylating with its kinase domain form part of intracellular signalling cascades (Fig 5)50,51. The deregulated activity of Src has already been recognised in neoplastic cells, such as colon and breast cancer. Due to such properties and activity, Src has been considered as a target in drug development, alongside other protein kinases50.
Fig 5.
Src signalling pathways.
The Src protein has three functioning molecular domains. SH2 (SRC homology 2) and SH3 are involved in protein-protein interactions. The third, SH1 is a kinase catalytic domain. Src can transfer from inactive to active state through control of its phosphorylation state, or via protein-protein interactions. FAK (focal adhesion kinase) and PDGF (platelet derived growth factor) are capable of rendering Src active by binding to its SH2 domain50.
GPCR: G-protein coupled receptors EGF: epidermal growth factor
Dasatinib is therefore a dual Src/ABL kinase inhibitor. It differs from imatinib in a number of ways. Unlike imatinib, dasatinib is capable of binding to both the inactive and active forms of BCR-ABL. Thus, dasatinib can bind to a more structurally conserved area between ABL and Src kinase than is present in the inactive conformation52. It is also more flexible in binding to differing conformations of BCR-ABL and is able to recognise multiple states of BCR-ABL. This confers enhanced binding affinity due largely to dasatinib's less rigid conformational demands on the kinase structure53. Although dasatinib is the most potent ABL kinase inhibitor to date, it is not the most specific, its target profile expanding to include other Src family members54.
Phase I clinical trials have demonstrated that, similar to its colleague nilotinib, dasatinib too is incapable of overcoming T315I mutations. Dasatinib demonstrated complete cytogenetic responses in chronic phase, accelerated and blast phase CML of 92%, 45%, 35%; with major cytogenetic response of 45%, 27% and 35%, respectively. Clinical activity was also noted in patients who received poor or no cytogenetic benefit from imatinib. This may have implications for patients who have received a suboptimal response from imatinib although not displaying frank resistance55,56.
NOVEL THERAPIES
Hommoharringtonine
Hommoharringtonine (HHT) is a novel plant alkaloid derived from a Chinese evergreen tree. An anticancer agent, it has recognised activity in acute myeloid leukaemia (AML), having been incorporated into the treatment regimen for AML and CML57,58. HHT is thought to conduct its anti-leukaemia effect through the inhibition of protein synthesis. HHT displays pronounced activity upon CML, in the past it has been used as salvage therapy in patients who became refractory to INFα59. Studies have investigated the consequences of HHT in combination with INFα or low dose cytarabine. When in dual therapy or in triple combination therapy, complete haematologic and complete cytogenetic responses equivalent to or superior to HHT single therapy have been shown, suggesting improved survival rates compared to HHT alone58–60. Shortly after such studies imatinib was introduced. In vitro HHT functions synergistically with imatinib, to decrease BCR-ABL protein expression. Research has shown imatinib and HHT to display synergistic cytotoxicity throughout different stages of disease progression. In chronic phase the duo demonstrated properties of dose dependant apoptosis and growth inhibition7,16. Additional examination of the potential therapeutic effects of HHT as a single therapy or as dual regimen with imatinib is warranted.
Arsenic Trioxide
Arsenic trioxide (As2O3), an older therapy for CML, has been re-investigated. With the evolution of safer forms of arsenicals and efficacy of As2O3 in acute promyelocytic leukaemia recently identified, interest of its potential use in CML was rekindled59. It is not certain how As2O3 exerts its anti-CML effects. Its ability to promote apoptosis has been suggested61. Studies have shown dose dependant growth inhibition and a pro-apoptotic effect when CML cells were treated with clinically tolerable levels of As2O3. A significant decline in BCR-ABL protein levels was also noted, and did not coincide with reduction in any other cellular proteins, suggesting specificity of this treatment. CML cell lines studies with As2O3 and imatinib have described a synergistic relationship between the two drugs, providing growth reduction and induction of apoptosis59,62.
Other Novel therapies
Proteasome inhibition has been a further area of interest in CML therapy. The ubiquitin-proteasome pathway is responsible for the degradation of cellular proteins. Proteasome have a dual role of maintenance (disposal of damaged proteins) and regulation (degradation of proteins involved in cell cycle regulation and neoplastic growth) within the cell. Due particularly to its latter property, proteasome inhibitors are being investigated as a new cancer therapy59. The inactivation of NF-κB is pertinent to its action. Although the mechanism has not been established by which decreased expression of BCR-ABL protein is mediated when CML cells are treated with proteasome inhibitors; caspase activation and apoptosis were recognised. The proteasome inhibitor PS-341 has shown significant effect upon growth inhibition and apoptosis of several cell lines. These have included both imatinib resistant and sensitive BCR-ABL positive cell lines7. Again, clinical studies in imatinib resistant patients are ongoing in this field59.
Further examples of a therapeutic target in CML are farnesyl transferase inhibitors. They predominantly mediate post translational modification to activate Ras G-protein. The Ras pathway is a well characterised downstream signalling cascade attributed to the tyrosine kinase activity of BCR-ABL. Thus, inhibiting Ras via farnesyl transferase inhibitors would potentially prevent expression of CML phenotype7. Presently, three such compounds present themselves as anti-leukaemic candidates. The most studied is SCH6636. When combined with imatinib SCH6636 is capable of suppressing the growth of CML progenitor cells in vitro, including imatinib resistant cells, with the possibility that it is capable of sensitizing imatinib resistant cells to imatinib-induced apoptosis59.
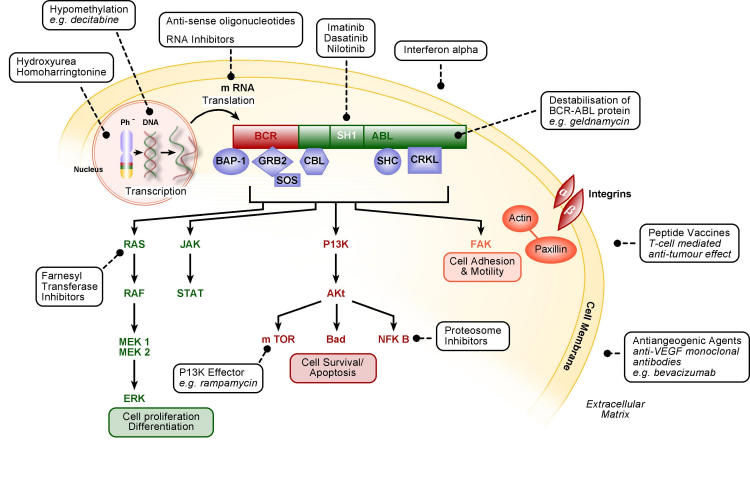
Other novel agents have been illustrated on Fig 6. They include antiangiogenic agents; peptide vaccines; TNF (tumour necrosis factor) related induction of apoptosis; DNA hypomethylation; antisense oligonucleotides and RNA inhibitors; P13K effectors; destabilisation of BCR-ABL protein7,59. Many of the agents listed are in preclinical development.
Figure 6.
Targets for CML therapy.
CONCLUSION
Imatinib is the first line agent for treatment of CML. We have examined the aims of imatinib therapy in terms of monitoring and defining disease response to treatment. Fig 7 is a suggested therapeutic algorithm for management of CML upon consideration and appraisal of the current literature. It is not however an ideal, as CML management strategies must be directed by an objective approach due to disease heterogeneity, where various subpopulations of patients may differ in their response to therapeutic regimens.
Figure 7.
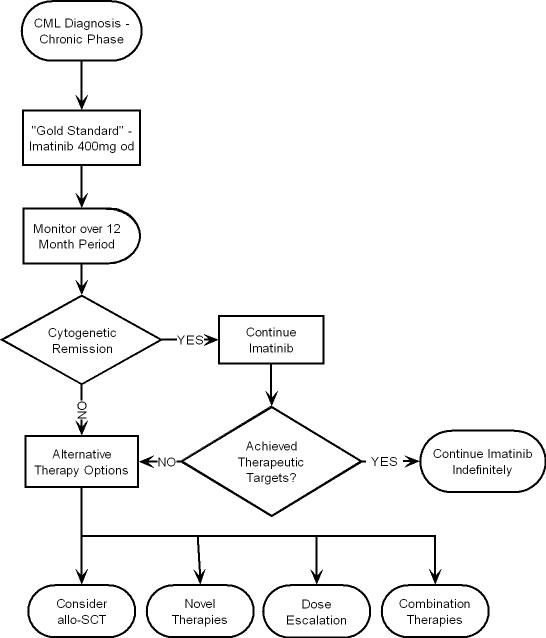
CML therapeutic algorithm.
Imatinib saw the dawn of a new era for CML management. Its success demonstrated the power and efficacy of genomic medicine and set precedents for future therapy. However, emergence of resistance remains a problem. Novel therapies appear at an impressive pace, promising to strengthen the therapeutic regimen for CML. The management of CML in the 21st century is exciting and challenging, as it seems that cure of CML is a possibility, but still just out of reach.
Conflict of interest: none declared
REFERENCES
- 1.Goldman JM, Melo JV. Chronic myeloid leukemia - advances In biology and new approaches to treatment. New Engl J Med. 2003;349(15):1451–64. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien S, Tefferi A, Valent P. Chronic myelogenous leukemia and myeloproliferative disease. Hematology. 2004;(1):146–62. doi: 10.1182/asheducation-2004.1.146. [DOI] [PubMed] [Google Scholar]
- 3.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. New Engl J Med. 1999;341(3):164–72. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 4.Cortes J. Natural history and staging of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18(3):569–84. doi: 10.1016/j.hoc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New Engl J Med. 2001;344(14):1084–6. doi: 10.1056/NEJM200104053441409. [DOI] [PubMed] [Google Scholar]
- 6.Deiniger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–56. [PubMed] [Google Scholar]
- 7.Druker BJ, O'Brien SG, Cortes J, Radich J. Chronic myelogenous leukemia. Hematology. 2002;(1):111–35. doi: 10.1182/asheducation-2002.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Reid T. Cytogenetics - in colour and digitized. New Engl J Med. 2004;350(16):1597–600. doi: 10.1056/NEJMp038242. [DOI] [PubMed] [Google Scholar]
- 9.Braziel RM, Shipp MA, Feldman AL, Espina V, Winters W, Jaffe ES, et al. Molecular diagnostics. Hematology. 2003;(1):279–93. doi: 10.1182/asheducation-2003.1.279. [DOI] [PubMed] [Google Scholar]
- 10.Schoch C, Schnittgner S, Bursch S, Gerstner D, Hochhaus A, Berger U, et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study in 350 cases. Leukemia. 2002;16(1):53–9. doi: 10.1038/sj.leu.2402329. [DOI] [PubMed] [Google Scholar]
- 11.Deininger MW. Management of early stage disease. Hematology. 2005;(1):174–82. doi: 10.1182/asheducation-2005.1.174. [DOI] [PubMed] [Google Scholar]
- 12.Lowenberg B. Minimal residual disease in chronic myeloid leukemia. New Engl J Med. 2003;349(15):1399–401. doi: 10.1056/NEJMp038130. [DOI] [PubMed] [Google Scholar]
- 13.Beillard E, Pallisgaard N, van der Velden V.J., Bi W, Dee R, van der Schoot E., et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using, real-time' quantitative reverse transcriptase polymerase chain reaction (RQ-PCR)- a Europe against cancer program. Leukemia. 2003;17(12):2474–86. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 14.Hochhaus A, Reiter A, Saussele S, Reichert A, Emig M, Kaeda J, et al. Molecular heterogeneity in complete cytogenetic responders after interferon-alpha therapy for chronic myelogenous leukemia: low levels of minimal residual disease are associated with continuing remission. German CML Study Group and the UK MRC CML Study Group. Blood. 2000;95(1):62–6. [PubMed] [Google Scholar]
- 15.Gabert J, Beillard E, van der Velden V.H.J., Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of, real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia- a Europe Against Caner Program. Leukemia. 2003;17(12):2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 16.Melo JV, Hughes TP, Apperley JF. Chronic myeloid leukemia. Hematology. 2003;17(1):132–52. doi: 10.1182/asheducation-2003.1.132. [DOI] [PubMed] [Google Scholar]
- 17.Mughal TI, Goldman JM. Encyclopedia of life sciences [electronic resources] Basingstoke: Nature Publishing Group; 2002. Chronic myeloid leukaemia. Available from: http://www.mrw.interscience.wiley.com/emrw/970470015902/els/article/a0002181/current/pdf. Accessed November 2006. [Google Scholar]
- 18.Tsao AS, Kantarjian H, Talpaz M. STI-571 in chronic myelogenous leukaemia. Br J Haematol. 2002;119(1):15–24. doi: 10.1046/j.1365-2141.2002.03899.x. [DOI] [PubMed] [Google Scholar]
- 19.Druker BJ, Moshe T, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New Engl J Med. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 20.Deininger MW, McCreevey L, Willis S, Bainbridge TM, Druker BJ, Heinrich MC. Detection of ABL kinase domain mutations with denaturing high-performance liquid chromatography. Leukemia. 2004;18(4):864–71. doi: 10.1038/sj.leu.2403307. [DOI] [PubMed] [Google Scholar]
- 21.Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist. 2004;9(3):271–81. doi: 10.1634/theoncologist.9-3-271. [DOI] [PubMed] [Google Scholar]
- 22.Peggs K, Mackinnon S. Imatinib mesylate - the new gold standard for treatment of chronic myeloid leukemia. New Engl J Med. 2003;348(11):104–50. doi: 10.1056/NEJMe030009. [DOI] [PubMed] [Google Scholar]
- 23.Savage DG, Antman KH. Imatinib mesylate - a new oral targeted therapy. New Engl J Med. 2002;346(9):683–93. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. New Engl J Med. 2003;34(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 25.Stone RM. Optimizing treatment of chronic myeloid leukemia: a rational approach. Oncologist. 2004;9(3):259–70. doi: 10.1634/theoncologist.9-3-259. [DOI] [PubMed] [Google Scholar]
- 26.Merx K, Muller MC, Kreil S, Lahaye T, Paschka P, Schoch C, et al. Early reduction of BCR-ABL mRNA transcript levels predicts cytogenetic response in chronic phase CML patients treated with imatinib after failure of interferon alpha. Leukemia. 2002;16(9):1579–83. doi: 10.1038/sj.leu.2402680. [DOI] [PubMed] [Google Scholar]
- 27.von Bubnoff N, Peschel C, Duyster J. Resistance of Philadelphia-chromosome positive leukemia towards the kinase inhibitor imatinib (STI571, Glivec): a targeted oncoprotein strikes back. Leukemia. 2003;17(5):829–38. doi: 10.1038/sj.leu.2402889. [DOI] [PubMed] [Google Scholar]
- 28.Tauchi T, Ohyashiki K. Molecular mechanisms of resistance to imatinib mesylate. Leuk Res. 2004;28(Suppl 1):S39–S45. doi: 10.1016/j.leukres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 29.O'Dwyer ME, Mauro MJ, Blasdel CB, Farnsworth M, Kurilik G, Hsieh YC, et al. Clonal evolution and lack of cytogenetic response are adverse prognostic factors for hematologic relapse of chronic phase CML patients treated with imatinib mesylate. Blood. 2004;103(2):451–455. doi: 10.1182/blood-2003-02-0371. [Epub. 2003 Sep 25] [DOI] [PubMed] [Google Scholar]
- 30.Hochhaus A, Hughes TP. Clinical resistance to imatinib: mechanisms and implications. Hematol Oncol Clin North Am. 2004;18(3):641–56. doi: 10.1016/j.hoc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Hochhaus A, La Rosee P. Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistence. Leukemia. 2004;18(8):1321–31. doi: 10.1038/sj.leu.2403426. [DOI] [PubMed] [Google Scholar]
- 32.Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of the BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102(1):276–83. doi: 10.1182/blood-2002-09-2896. [Epub 2003 Mar 6] [DOI] [PubMed] [Google Scholar]
- 33.Crossman L, O'Brien SG. Imatinib therapy in chronic myeloid leukemia. Hematol Oncol Clin North Am. 2004;18(3):605–17. doi: 10.1016/j.hoc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Pearson K, Fergusion JE, Clark RE. The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. 2003;120(6):990–9. doi: 10.1046/j.1365-2141.2003.04200.x. [DOI] [PubMed] [Google Scholar]
- 35.Branford S, Rudzki Z, Parkinson I, Grigg A, Taylor K, Seymour JF, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify imatinib-treated patients with CML who have BCR-ABL kinase domain mutations. Blood. 2004;104(9):2926–32. doi: 10.1182/blood-2004-03-1134. [DOI] [PubMed] [Google Scholar]
- 36.Hughes T, Deininger MW, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors - review and recommendations for harmonising current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radich JP, Gooley T, Byrant E, Chauncey T, Clift R, Beppu L, et al. The significance of BCR-ABL molecular detection in chronic myeloid leukemia patients “late”, 18 months or more after transplantation. Blood. 2001;98(6):1701–7. doi: 10.1182/blood.v98.6.1701. [DOI] [PubMed] [Google Scholar]
- 38.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. New Engl J Med. 2002;346(9):645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 39.Kiss TL, Abdolell M, Jamal N, Minden MD, Lipton JH, Messner HA. Long term medical outcomes and quality of life assessment of patients with chronic myeloid leukemia followed at least 10 years after allogenic bone marrow transplantation. J Clin Oncol. 2002;20(9):2334–43. doi: 10.1200/JCO.2002.06.077. [DOI] [PubMed] [Google Scholar]
- 40.Mauro MJ, O'Dwyer M, Heinrich MC, Druker BJ. STI571: a paradigm of new agents for cancer therapeutics. J Clin Oncol. 2002;20(1):325–34. doi: 10.1200/JCO.2002.20.1.325. [DOI] [PubMed] [Google Scholar]
- 41.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao N, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 42.Muller MC, Gatterman N, Lahaye T, Deininger W.N., Berndt A, Fruehauf S, et al. Dynamics of BCR-ABL mRNA expression in first-line therapy of chronic myelogenous leukemia patients with imatinib or interferon alpha/ara-c. Leukemia. 2003;17(12):2392–400. doi: 10.1038/sj.leu.2403157. [DOI] [PubMed] [Google Scholar]
- 43.Druker BJ. Circumventing resistance to Kinase-inhibitor therapy. New Engl J Med. 2006;354(24):2594–6. doi: 10.1056/NEJMe068073. [DOI] [PubMed] [Google Scholar]
- 44.O'Hare T, Corbin A, Druker BJ. Targeted CML therapy: controlling resistance, seeking cure. Curr Opin Genet Dev. 2006;16(1):92–9. doi: 10.1016/j.gde.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 45.O'Hare T, Walters DK, Deiniger MW, Druker BJ. AMN107: tightening the grip of imatinib. Cancer Cell. 2005;7(2):117–9. doi: 10.1016/j.ccr.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Weisenberg E, Manley P, Cowan-Jacob S, Ray A, Griffin JD. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Brit J Canc. 2006;94(12):1765–9. doi: 10.1038/sj.bjc.6603170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Bubnoff N, Manley PW, Mestan J, Sanger J, Peschel C, Duyster J. BCR-ABL resistance screening predicts a limited spectrum of point mutations to be associated with clinical resistance to the Abl kinase inhibitor nilotinib (AMN107) Blood. 2006;108(4):1328–33. doi: 10.1182/blood-2005-12-010132. [DOI] [PubMed] [Google Scholar]
- 48.Kantarjian HM, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib resistant CML and and Philadelphia chromosome positive ALL. New Engl J Med. 2006;354(24):2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 49.Manley P, Cowan-Jacob S, Mestan J. Advances in the structural biology, design and clinical development of BCR-ABL kinase inhibitors for the treatment of chronic myeloid leukemia. Biochim Biophys Acta. 2005;1754(1-2):3–13. doi: 10.1016/j.bbapap.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 50.Frame MC. Src in cancer: degregulation and consequences for cell behviour. Biochim Biophys Acta. 2002;1602(2):114–30. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 51.Schlessinger J. Cell signalling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 52.Shah NP, Tran C, Lee FY, Chen N, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 53.Walz C, Sattler M. Novel targeted therapies to overcome imatinib mesylate resistance in chronic myeloid leukemia (CML) Crit Rev Oncol Hematol. 2006;57(2):145–64. doi: 10.1016/j.critrevonc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Martinelli S, Soverini S, Rosti G, Baccarani M. Dual tyrosine kinase inhibitors in chronic myeloid leukemia. Leukemia. 2005;19(11):1872–9. doi: 10.1038/sj.leu.2403950. [DOI] [PubMed] [Google Scholar]
- 55.Talpaz M, Shah NP, Kantarjian HM, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib resistant Philadelphia chromosome positive leukemias. New Engl J Med. 2006;354(24):2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 56.Hampton T. Looking beyond imatinib - next line of targeted drugs for CML shows promise. JAMA. 2006;295(4):369–70. doi: 10.1001/jama.295.4.369. [DOI] [PubMed] [Google Scholar]
- 57.Kano Y, Akutsu M, Tsunoda S, Mano H, Sato Y, Honma Y, et al. In vitro cytotoxic effects of a tyrosine kinase inhibitor STI571 in combination with commonly used anti-leukemic agents. Blood. 2001;97(7):1999–2007. doi: 10.1182/blood.v97.7.1999. [DOI] [PubMed] [Google Scholar]
- 58.O'Brien S, Kantarjian H, Koller C, Feldman E, Beran M, Andreef M, et al. Sequential homoharringtonine and interferon-alpha in the treatment of early chronic phase chronic myeloid leukemia. Blood. 1999;93(12):4149–53. [PubMed] [Google Scholar]
- 59.Cortes J, O'Brien SG, Giles F, Alvarez RH, Talpaz M, Kantarjian H. Investigational strategies in chronic myelegenous leukemia. Hematol Oncol Clin North Am. 2004;18(3):619–639. doi: 10.1016/j.hoc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Kantarjian HM, Talpaz M, Smith TL, Cortes J, Giles FJ, Rios MB, et al. Homoharringtonine and low dose cytarabine in the management of late chronic phase chronic myelogenous leukemia. J Clin Oncol. 2000;18(20):3513–21. doi: 10.1200/JCO.2000.18.20.3513. [DOI] [PubMed] [Google Scholar]
- 61.Du Y, Wang K, Fang H, Li J, Xiao D, Zheng P, et al. Coordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemia. Blood. 2006;107(4):582–90. doi: 10.1182/blood-2005-06-2318. [DOI] [PubMed] [Google Scholar]
- 62.Perkins C, Kim CN, Fang G, Bhalla K. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express BCR-ABL or overexpress MDR, MRP, Bcl-2, or Bcl-xL. Blood. 2000;95(3):14–22. [PubMed] [Google Scholar]