Abstract
Diabetic retinopathy remains a major cause of worldwide preventable blindness. Measures to avoid blindness include medical management (control of blood sugar, blood pressure, and serum lipids) and ocular management (laser photocoagulation and pars plana vitrectomy). Adjunctive pharmacologic therapies (intravitreal triamcinolone acetonide and anti-vascular endothelial growth factor agents) have shown early promise in the treatment of both diabetic macular edema and proliferative diabetic retinopathy. Other medications under investigation include the fluocinolone acetonide implantable device, extended-release dexamethasone implant, oral ruboxistaurin, and intravitreal hyaluronidase.
1. INTRODUCTION
Despite advancements in the delivery of ophthalmological care, diabetic retinopathy remains a major cause of preventable blindness [1]. The two most important visual complications of diabetic retinopathy are diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR). Glycemic control [2, 3] and photocoagulation [4, 5] have been standard treatments for both DME and PDR for over 3 decades. Nevertheless, some patients suffer permanent visual loss despite prompt and appropriate therapy.
In recent years, further advances in pharmacotherapy have shown promise in the treatment of diabetic retinopathy. The three major classes of medications currently being studied are corticosteroids, vascular endothelial growth factor (VEGF) antagonists, and miscellaneous agents. Multiple clinical series have been reported (Table 1), and many more are ongoing or being planned (Table 2).
Table 1.
Selected published clinical series evaluating pharmacotherapies for advanced diabetic retinopathy. CMT: central macular thickness (assessed by optical coherence tomography), FA: fluorescein angiography, IVTA: intravitreal triamcinolone acetonide, DME: diabetic macular edema, NV: neovascularization, PDR: proliferative diabetic retinopathy, TA: triamcinolone acetonide, VA: visual acuity, and VH: vitreous hemorrhage.
| Study (reference) | Reported patients | Reported eyes | Medication | Indication and outcome measures | Efficacy |
|
| |||||
| Martidis et al. [9] | 16 | 16 | IVTA | DME, VA, CMT | Favorable |
| Gillies et al. [12] | 43 | 69 | IVTA | DME, VA | Favorable |
| Lam et al. [13] | 63 | 63 | IVTA | DME, VA, CMT | Favorable |
| Tunc et al. [23] | 50 | 60 | Peribulbar TA | DME, VA, FA | Favorable |
| Bakri et al. [24] | 50 | 63 | Peribulbar TA | DME, VA | Favorable |
| Cardillo et al. [25] | 12 | 24 | Peribulbar TA | DME, VA, CMT | Unfavorable |
| Bonini-Filho et al. [26] | 36 | 36 | Peribulbar TA | DME, VA, CMT | Unfavorable |
| Kuppermann et al. [29] | 286 | 286 | Extended-release dexamethasone | DME, VA, CMT | Favorable |
| Cunningham et al. [36] | 172 | 172 | Pegaptanib | DME, VA, CMT, photocoagulation | Favorable |
| Adamis et al. [37] | 16 | 20 | Pegaptanib | PDR, retinal NV | Favorable |
| Spaide et al. [38] | 2 | 2 | Bevacizumab | PDR, retinal NV, VH | Favorable |
| Jorge et al. [39] | 15 | 15 | Bevacizumab | PDR, VA, retinal NV, FA | Favorable |
| Avery et al. [40] | 32 | 45 | Bevacizumab | PDR, VA, iris and retinal NV, FA | Favorable |
| Chun et al. [41] | 10 | 10 | Ranibizumab | DME, VA, CMT | Favorable |
| Nguyen et al. [42] | 10 | 10 | Ranibizumab | DME, VA, CMT | Favorable |
| PKC-DRS [43] | 252 | 504 | Ruboxistaurin | DME, PDR, VA, Photocoagulation | Favorable |
| PKC-DRS2 [44] | 685 | 1370 | Ruboxistaurin | DME, VA, Photocoagulation | Favorable |
| PKC-DMES [45] | 686 | 1372 | Ruboxistaurin | DME, Photocoagulation | Unfavorable |
| Strom et al. [46] | 41 | 55 | Ruboxistaurin | DME, VA, vitreous fluorometry | Somewhat favorable |
| Kuppermann et al. [47] | 1125 | 1125 | Hyaluronidase | VH, anatomic clearance, VA | Favorable |
| DRCR.net et al. [48] | 109 | 129 | Peribulbar TA | DME, VA, CMT | Unfavorable |
Table 2.
Selected ongoing clinical trials evaluating pharmacotherapies for advanced diabetic retinopathy. DME: diabetic macular edema, DRCR.net: Diabetic Retinopathy Clinical Research network, PF/IVTA: preservative-free intravitreal triamcinolone acetonide, PDR: proliferative diabetic retinopathy, and TA: triamcinolone acetonide.
| Sponsor | Medication | Indication | Status |
|
| |||
| DRCR.net (phase 3) | PF/IVTA | DME | Completed enrollment |
| Bausch & Lomb | Fluocinolone implant | DME | Completed enrollment |
| Allergan (phase 3) | Extended-release dexamethasone | DME | In planning stages |
| OSI/Eyetech | Pegaptanib | DME | Enrolling patients |
| DRCR.net (phase 2) | Bevacizumab | DME | Completed enrollment |
| Genentech | Ranibizumab | DME | Enrolling patients |
| DRCR.net (phase 3) | Ranibizumab or IVTA | DME with no PDR | Enrolling patients |
| DRCR.net (phase 3) | Ranibizumab or IVTA | DME with PDR | Enrolling patients |
2. CORTICOSTEROIDS
Corticosteroids may work through multiple mechanisms of action. In addition to their well-known anti-inflammatory effects, corticosteroids may cause downregulation of VEGF [6, 7]. Intravitreal triamcinolone acetonide (IVTA) is commonly used today as an off-label adjunctive treatment of DME (Figure 1). Other intravitreal corticosteroids under study include a sustained-release device containing fluocinolone acetonide (Retisert; Bausch & Lomb, Rochester, NY) and extended-release dexamethasone in a biodegradable polymer (Posurdex, Allergan, Irvine, Calif).
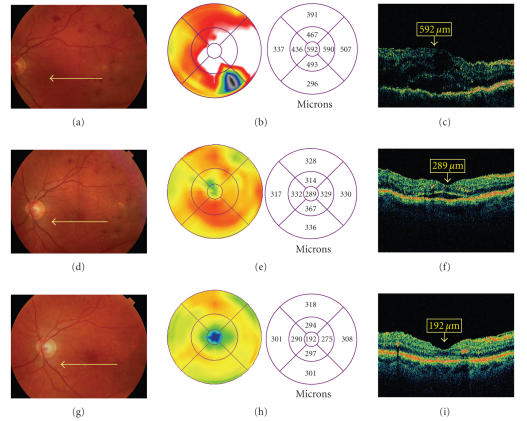
Figure 1.
Intravitreal triamcinolone acetonide for diabetic macular edema. A patient presented with diabetic macular edema, visual acuity 20/60. Fundus photography (a) and optical coherence tomography (OCT) (b, c) are shown. The patient was treated with intravitreal triamcinolone acetonide. One month after treatment, visual acuity improved to 20/40, with improvement of macular edema on photography (d) and OCT (e, f). Four months after treatment, visual acuity improved to 20/20, with further improvement of macular edema on photography (g) and OCT (h, i).
2.1. Triamcinolone acetonide
Although intravitreal triamcinolone acetonide (Kenalog 40, Bristol-Myers Squibb, Princeton, NJ) has been administered for many years [8], its use has become more common since 2002 [9–11]. Recent prospective, randomized clinical trials have demonstrated generally favorable outcomes [12, 13]. The Diabetic Retinopathy Clinical Research network (DRCR.net) has completed enrollment on a three-year, randomized, prospective, multicenter clinical trial comparing two doses (1 mg and 4 mg) of preservative-free IVTA (Allergan, Irvine, Calif) with modified early treatment diabetic retinopathy study (ETDRS) photocoagulation for DME. Furthermore, IVTA may be a useful adjunct to photocoagulation for PDR, perhaps by decreasing the macular edema sometimes worsened by the treatment [14, 15].
The most important complication of IVTA is increased intraocular pressure (IOP) resulting in secondary open-angle glaucoma, which sometimes may be severe [16] and intractable [17, 18]. Elevation of IOP up to 24 mm Hg may occur in about 40% of patients, usually within about 3 months [19]. The second most important complication of IVTA is cataract formation, which may become visually significant in about half of eyes within 1 year [20].
The rates of injection-related endophthalmitis following IVTA have been reported to be in the range of 0.099%–0.87% per injection [21, 22]. The incidence of pseudoendophthalmitis, due to migration of triamcinolone acetonide crystals into the anterior chamber, is probably higher than that of infectious endophthalmitis. Other reported complications of IVTA (and of any intravitreal injection) include retinal detachment, lens trauma, and vitreous hemorrhage.
The use of peribulbar, rather than intravitreal, triamcinolone acetonide offers reduced risks of endophthalmitis and perhaps other complications. Peribulbar triamcinolone acetonide may have some limited efficacy for patients with DME [23, 24] although the bulk of the current literature appears to indicate that IVTA is more effective [25, 26]. DRCR.net has recently published a phase 2 randomized, prospective, multicenter clinical trial comparing peribulbar triamcinolone acetonide with and without photocoagulation. Peribulbar triamcinolone did not significantly improve vision in patients with mild DME [48]. Neither peribulbar triamcinolone nor IVTA appears to offer long-term efficacy for DME, which has led to the investigation of various extended-release corticosteroids.
2.2. Fluocinolone acetonide
The fluocinolone acetonide intravitreal implant (Retisert) is FDA-approved for the treatment of chronic, noninfectious posterior segment uveitis [27]. Although the device was also studied in patients with DME, no specific results has been published in the peer-reviewed literature at this time [28].
2.3. Extended-release dexamethasone
A bioerodable, extended-release dexamethasone implant (Posurdex, Allergan, Irvine, Calif) has shown favorable outcomes in the treatment of macular edema due to various etiologies, including DME, in a recent phase 2 study [29]. A phase 3 trial is underway.
3. VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) INHIBITORS
Vascular endothelial growth factor (VEGF) increases retinal vascular permeability, causes breakdown of the blood-retinal barrier, and results in retinal edema [30]. VEGF is upregulated in diabetic retinopathy [31] and is present in increased levels in the aqueous and vitreous humor of patients with PDR [32, 33]. At least 5 isoforms of VEGF are known [34]. Three currently available anti-VEGF agents are pegaptanib, bevacizumab, and ranibizumab.
3.1. Pegaptanib
Pegaptanib (Macugen, OSI/Eyetech, Melville, NY) is a pegylated aptamer directed against the VEGF-A 165 isoform. It was the first FDA-approved ophthalmologic anti-VEGF agent, for the treatment of choroidal neovascularization from age-related macular degeneration (AMD) [35]. In a phase 2, prospective clinical trial, pegaptanib appeared to improve anatomic and visual outcomes in patients with DME [36]. Retrospective analysis of these data demonstrated some efficacy on retinal neovascularization as well [37]. Phase 3 trials of pegaptanib for DME are currently being conducted.
3.2. Bevacizumab
Bevacizumab (Avastin, Genentech, Inc., South San Francisco, Calif), a full-length recombinant humanized antibody, is active against all isoforms of VEGF-A. It is FDA-approved as an adjunctive systemic treatment for metastatic colorectal cancer [49]. Although off-label systemic bevacizumab has demonstrated some efficacy for exudative AMD [50], the agent has shown greater promise as an intravitreal medication. Case reports and small observational series have been reported using off-label intravitreal bevacizumab to treat exudative AMD [51], macular edema from nonischemic central retinal vein occlusion [52], iris neovascularization [53, 54], pseudophakic cystoid macular edema [55], and other diseases. Small, nonrandomized pilot studies have documented some efficacy against diffuse DME [56] and various complications of PDR [38–40] (Figure 2).
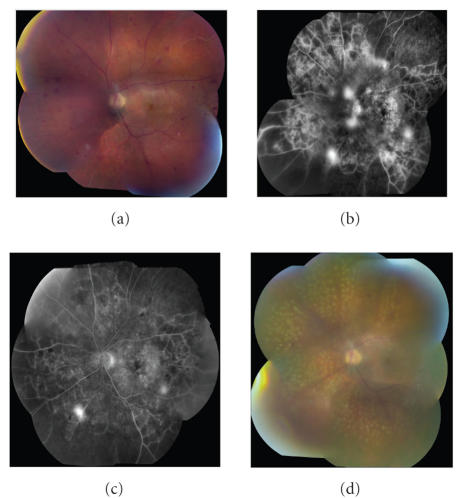
Figure 2.
Intravitreal bevacizumab for proliferative diabetic retinopathy. A patient presented with proliferative diabetic retinopathy. Fundus photography (a) and fluorescein angiography (b) are shown. The patient was treated with intravitreal bevacizumab. Followup fluorescein angiography demonstrated improvement in angiographic leakage (c). Panretinal photocoagulation was then applied (d). (Case courtesy of Geeta Lalwani, MD, and Carmen A. Puliafito, MD, MBA.)
DRCR.net has completed enrollment on a phase 2, prospective, randomized, multicenter clinical trial to determine the safety and possible benefits of this agent. Plans for a phase 3 trial of two doses of an intravitreal anti-VEGF agent versus modified ETDRS grid laser photocoagulation for DME are under discussion.
3.3. Ranibizumab
Ranibizumab (Lucentis, Genentech, Inc., South San Francisco, Calif), a recombinant humanized antibody fragment, is active against all isoforms of VEGF-A. Intravitreal ranibizumab is FDA-approved for the treatment of exudative AMD [57, 58]. Two pilot studies of ranibizumab demonstrated some efficacy in the treatment of DME [41, 42]. DRCR.net is planning two phase 3, prospective, randomized, multicenter trials comparing patients. In the first trial, patients with DME and no PDR will be randomized to: (1) modified ETDRS grid laser photocoagulation; (2) photocoagulation before ranibizumab; (3) photocoagulation plus IVTA; or (4) ranibizumab before photocoagulation. In the second trial, patients with DME and PDR will be randomized to: (1) modified ETDRS grid laser photocoagulation plus scatter photocoagulation; (2) modified ETDRS grid laser photocoagulation plus scatter photocoagulation plus ranibizumab; or (3) modified ETDRS grid photocoagulation plus scatter photocoagulation plus IVTA.
The risk of injection-related endophthalmitis with the anti-VEGF agents is variable, but appears to be lower in more recent studies. Major prospective clinical trials of pegaptanib and ranibizumab reported rates between 0.7%–1.6% per eye [35, 57–59]. Most eyes in these reports received a series of injections, and a recent observational case series reported an incidence of 0.014% per injection for bevacizumab [60].
4. OTHER AGENTS
4.1. Ruboxistaurin
The enzyme protein kinase C β (PKC β) is activated by VEGF and appears to increase various systemic complications, including diabetic retinopathy [61]. Ruboxistaurin (Arxxant, Eli Lilly and Company, Indianapolis, Ind), an orally administered PKC β inhibitor, has shown efficacy against DME in two separate phase 3 trials [43, 44], although a recent study reported that treatment did not delay disease progression over a 30-month followup [45]. A smaller study noted that ruboxistaurin treatment was associated with a reduction in retinal vascular leakage, as measured by vitreous fluorometry, but visual acuity was not affected [46]. Although Lilly received an approvable letter from the FDA on August 18, 2006, the FDA requested an additional, 3-year, Phase 3 clinical trial to collect additional efficacy data in spite of an appeal with additional data.
4.2. Hyaluronidase
In an attempt at pharmacologic vitreolysis, intravitreal purified ovine hyaluronidase (Vitrase, ISTA Pharmaceuticals, Irvine, Calif) was proposed to accelerate clearance of vitreous hemorrhage from PDR and other causes. A recent phase 3 prospective clinical trial showed some favorable efficacy [47] and safety [62] outcomes for the clearance of vitreous hemorrhage due to all causes, but this agent is not currently FDA-approved for this indication.
5. CLINICAL GUIDELINES
Although none of the pharmacologic agents discussed above is FDA-approved for treatment of patients with diabetic retinopathy, off-label treatment can be considered for patients unresponsive to traditional standard care. These guidelines are summarized in Table 3.
Table 3.
Guidelines for pharmacologic treatment of advanced diabetic retinopathy.
-
Clinically significant macular edema (CSME)
-
Evaluation
-
Initial diagnosis
Complete eye examination
Fundus photography, fluorescein angiography, optical coherence tomography (OCT)
-
Followup
Clinical examination
OCT
-
-
Treatment
-
First-line therapy
Focal or modified ETDRS grid photocoagulation for focal or diffuse CSME
Intravitreal pharmacotherapies ± photocoagulation for more advanced, diffuse CSME
-
For persistent or recurrent CSME (visual acuity <20/40)
Repeat photocoagulation
Intravitreal triamcinolone acetonide or intravitreal antivascular endothelial growth factor (VEGF) agent
-
For CSME refractory to photocoagulation and intravitreal pharmacotherapies, consider pars plana vitrectomy (PPV)
No traction: PPV with internal limiting membrane (ILM) peeling
Taut posterior hyaloid face or vitreomacular traction syndrome: PPV and ILM peeling
-
-
-
Proliferative diabetic retinopathy (PDR)
-
Evaluation
-
Initial diagnosis
Complete eye examination
Fundus photography, fluorescein angiography (sometimes), optical coherence tomography (OCT), echography (if necessary)
-
Followup
Clinical examination
OCT for evaluation of macular disease
-
-
Treatment
-
First-line therapy
In eyes with clear media: panretinal photocoagulation (PRP)
In eyes with vitreous hemorrhage and no retinal detachment: consider intravitreal anti-VEGF agent, with PRP after clearing
In eyes with traction retinal detachment, consider intravitreal anti-VEGF agent before pars plana vitrectomy to reduce vascularity
In eyes with attached posterior hyaloid, consider use of intravitreal triamcinolone acetonide to assist in hyaloid removal
-
For combined PDR/CSME
-
Consider medical options
Intravitreal anti-VEGF agent or hyaluronidase for vitreous hemorrhage
Standard focal or modified grid and PRP
-
Consider standard surgical options for more advanced disease
Nonclearing vitreous hemorrhage
Advanced traction retinal detachment
-
-
-
In patients with diabetic macular edema not responsive to photocoagulation, either IVTA or an intravitreal anti-VEGF agent may be considered as second-line treatments. At this time, there are no published head-to-head comparisons of IVTA versus the anti-VEGF agents for this disease, although the pending DRCR.net trials may provide useful guidelines in this regard. Triamcinolone may be relatively more efficacious for DME, while the anti-VEGF agents appear more efficacious for PDR. Triamcinolone is considerably less expensive than the anti-VEGF agents, but is associated with risks of elevated IOP, cataract, and pseudoendophthalmitis.
In patients with complications of PDR not amenable to photocoagulation, intravitreal anti-VEGF agents may produce short-term stabilization or regression of iris and/or retinal neovascularization. In most patients, however, photocoagulation will eventually be necessary.
Intravitreal anti-VEGF agents may be helpful in patients with dense vitreous hemorrhage and patients with glaucoma secondary to neovascularization. If B-scan echography shows no evidence of retinal detachment, these agents may provide useful short-term anatomic improvement, until definitive photocoagulation can be given, or to reduce intraoperative bleeding in eyes with neovascular glaucoma.
6. SUMMARY
Clinical experience with pharmacologic treatment for diabetic retinopathy continues to increase and reported outcomes in observational case series are promising. At this time, improved metabolic control and local ocular treatments (photocoagulation and vitrectomy) remain the proven treatments, through evidence-based medicine. As prospective randomized clinical trials accumulate data, the role of pharmacologic treatments will become clearer.
ACKNOWLEDGMENTS
This work is partially supported by NIH center Grant P30-EY014801, and by an unrestricted grant to the University of Miami from Research to Prevent Blindness, New York. Stephen G. Schwartz, MD, has received research funding from Genentech and has served as a consultant to Novartis. Harry W. Flynn, Jr., MD, has served as a consultant to Alcon, Eli Lilly, Genentech, Novartis, OptiMedica, and Pfizer.
References
- 1.The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinpathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology. 1991;98(supplement 5):766–785. [PubMed] [Google Scholar]
- 6.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. European Journal of Pharmacology. 1998;341(2-3):309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 7.Nauck M, Roth M, Tamm M, et al. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. American Journal of Respiratory Cell and Molecular Biology. 1997;16(4):398–406. doi: 10.1165/ajrcmb.16.4.9115750. [DOI] [PubMed] [Google Scholar]
- 8.McCuen BW, II, Bessler M, Tano Y, Chandler D, Machemer R. The lack of toxicity of intravitreally administered triamcinolone acetonide. American Journal of Ophthalmology. 1981;91(6):785–788. doi: 10.1016/0002-9394(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 9.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109(5):920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 10.Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Archives of Ophthalmology. 2003;121(1):57–61. [PubMed] [Google Scholar]
- 11.Chieh JJ, Roth DB, Liu M, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 2005;25(7):828–834. doi: 10.1097/00006982-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gillies MC, Sutter FKP, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113(9):1533–1538. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 13.Lam DS, Chan CK, Mohamed S, et al. A prospective randomised trial of different doses of intravitreal triamcinolone for diabetic macular oedema. British Journal of Ophthalmology. 2007;91(2):199–203. doi: 10.1136/bjo.2006.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandello F, Polito A, Pognuz DR, Monaco P, Dimastrogiovanni A, Paissios J. Triamcinolone as adjunctive treatment to laser panretinal photocoagulation for proliferative diabetic retinopathy. Archives of Ophthalmology. 2006;124(5):643–650. doi: 10.1001/archopht.124.5.643. [DOI] [PubMed] [Google Scholar]
- 15.Zacks DN, Johnson MW. Combined intravitreal injection of triamcinolone acetonide and panretinal photocoagulation for concomitant diabetic macular edema and proliferative diabetic retinopathy. Retina. 2005;25(2):135–140. doi: 10.1097/00006982-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Quiram PA, Gonzales CR, Schwartz SD. Severe steroid-induced glaucoma following intravitreal injection of triamcinolone acetonide. American Journal of Ophthalmology. 2006;141(3):580–582. doi: 10.1016/j.ajo.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Kaushik S, Gupta V, Gupta A, Dogra MR, Singh R. Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. American Journal of Ophthalmology. 2004;137(4):758–760. doi: 10.1016/j.ajo.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Viola F, Morescalchi F, Staurenghi G. Argon laser trabeculoplasty for intractable glaucoma following intravitreal triamcinolone. Archives of Ophthalmology. 2006;124(1):133–134. doi: 10.1001/archopht.124.1.133. [DOI] [PubMed] [Google Scholar]
- 19.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. American Journal of Ophthalmology. 2004;138(5):740–743. doi: 10.1016/j.ajo.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JT. Cataract formation and other complications of intravitreal triamcinolone for macular edema. American Journal of Ophthalmology. 2006;141(4):629–637. doi: 10.1016/j.ajo.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Westfall AC, Osborn A, Kuhl D, Benz MS, Mieler WF, Holz ER. Acute endophthalmitis incidence: intravitreal triamcinolone. Archives of Ophthalmology. 2005;123(8):1075–1077. doi: 10.1001/archopht.123.8.1075. [DOI] [PubMed] [Google Scholar]
- 22.Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. American Journal of Ophthalmology. 2003;136(5):791–796. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 23.Tunc M, Onder HI, Kaya M. Posterior sub-Tenon's capsule triamcinolone injection combined with focal laser photocoagulation for diabetic macular edema. Ophthalmology. 2005;112(6):1086–1091. doi: 10.1016/j.ophtha.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Bakri SJ, Kaiser PK. Posterior sub-Tenon triamcinolone acetonide for refractory diabetic macular edema. American Journal of Ophthalmology. 2005;139(2):290–294. doi: 10.1016/j.ajo.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 25.Cardillo JA, Melo LAS, Jr, Costa RA, et al. Comparison of intravitreal versus posterior sub-Tenon's capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005;112(9):1557–1563. doi: 10.1016/j.ophtha.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Bonini-Filho MA, Jorge R, Barbosa JC, Calucci D, Cardillo JA, Costa RA. Intravitreal injection versus sub-Tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Investigative Ophthalmology and Visual Science. 2005;46(10):3845–3849. doi: 10.1167/iovs.05-0297. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113(6):1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Fluocinolone acetonide ophthalmic - Bausch & Lomb: fluocinolone acetonide envision TD implant. Drugs in R and D. 2005;6(2):116–119. doi: 10.2165/00126839-200506020-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Archives of Ophthalmology. 2007;125(3):309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 30.Aiello LP, Bursell S-E, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform-selective inhibitor. Diabetes. 1997;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 31.Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histology and Histopathology. 1997;12(1):99–109. [PubMed] [Google Scholar]
- 32.Adamis AP, Miller JW, Bernal M-T, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. American Journal of Ophthalmology. 1994;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 33.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New England Journal of Medicine. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. Journal of Cellular Biochemistry. 1991;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 35.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. New England Journal of Medicine. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham ET, Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Adamis AP, Altaweel M, Bressler NM, et al. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113(1):23–28. doi: 10.1016/j.ophtha.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26(3):275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study) Retina. 2006;26(9):1006–1013. doi: 10.1097/01.iae.0000246884.76018.63. [DOI] [PubMed] [Google Scholar]
- 40.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695–1705.e6. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 41.Chun DW, Heier JS, Topping TM, Duker JS, Bankert JM. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006;113(10):1706–1712. doi: 10.1016/j.ophtha.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. American Journal of Ophthalmology. 2006;142(6):961–969.e4. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 43.The PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the protein kinase C β inhibitor diabetic retinopathy study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54(7):2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 44.PKC-DRS2 Group. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113(12):2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 45.The PKC-DMES Study Group. Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Archives of Ophthalmology. 2007;125(3):318–324. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 46.Strøm C, Sander B, Klemp K, Aiello LP, Lund-Andersen H, Larsen M. Effect of ruboxistaurin on blood-retinal barrier permeability in relation to severity of leakage in diabetic macular edema. Investigative Ophthalmology and Visual Science. 2005;46(10):3855–3858. doi: 10.1167/iovs.05-0096. [DOI] [PubMed] [Google Scholar]
- 47.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR, Vitrase for Vitreous Hemorrhage Study Groups Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Avastin®) for the management of vitreous hemorrhage. American Journal of Ophthalmology. 2005;140(4):573–584. doi: 10.1016/j.ajo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Diabetic Retinopathy Clinical Research Network, Chew E, Strauber S, Beck R, et al. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. American Journal of Ophthalmology. 2007;114(6):1190–1196. doi: 10.1016/j.ophtha.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New England Journal of Medicine. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112(6):1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin®) for neovascular age-related macular degeneration. Ophthalmic Surgery Lasers and Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 52.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin®) for macular edema from central retinal vein occlusion. Ophthalmic Surgery Lasers and Imaging. 2005;36(4):336–339. [PubMed] [Google Scholar]
- 53.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26(3):352–354. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz SG, Hickey M, Puliafito CA. Bilateral CRAO and CRVO from thrombotic thrombocytopenic purpura: OCT findings and treatment with triamcinolone acetonide and bevacizumab. Ophthalmic Surgery Lasers and Imaging. 2006;37(5):420–422. doi: 10.3928/15428877-20060901-10. [DOI] [PubMed] [Google Scholar]
- 55.Mason JO, III, Albert MA, Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006;26(3):356–357. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 56.Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26(9):999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 58.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. New England Journal of Medicine. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 59.Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration. A phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113(4):633–642.e4. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 60.Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: using the internet to assess drug safety worldwide. British Journal of Ophthalmology. 2006;90(11):1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aiello LP, Bursell S-E, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform-selective inhibitor. Diabetes. 1997;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 62.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR, Vitrase for Vitreous Hemorrhage Study Groups Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Avastin®) for the management of vitreous hemorrhage. American Journal of Ophthalmology. 2005;140(4):585.e1–585.e15. doi: 10.1016/j.ajo.2005.06.022. [DOI] [PubMed] [Google Scholar]